Abstract
Animals titrate their behaviour against the level of risk and an individual's conspicuousness should influence decisions such as when to flee and for how long to hide. Conspicuousness will vary with variation in substrate colour. Since hermit crabs frequently change the shells they occupy, shell colour will also influence conspicuousness and to be aware of their conspicuousness would require information on both of these factors to be integrated. Reduced boldness in high-contrast shell and substrate combinations compared with situations of low contrast indicates that hermit crabs are aware of current conspicuousness. Differences between individuals remained consistent across conspicuousness levels indicating the presence of animal personalities.
Keywords: hermit crab, animal personality, conspicuousness, startle response
1. Introduction
When faced with a dangerous situation such as the presence of a predator, several factors will influence the level of risk and hence the optimal course of action. This could involve the timing of a decision to flee (e.g. [1–3]) or for animals that occupy shelters, such as hermit crabs inhabiting empty gastropod shells, the duration of a ‘startle response’ of rapidly withdrawing into the shelter before re-emerging [4]. In animals that flee to avoid attack, the decision might be influenced by the proximity and prowess of the predator [1–3]. For shelter-dwelling animals, the decision is influenced by the quality of their shelter [5]. In both animals that flee and animals that withdraw into shelters, an additional factor that might influence the level of risk is an individual's conspicuousness.
Evidence that animals can use information on their own coloration to adjust risk avoidance is provided by studies of broad-headed skinks, Eumeces laticeps [6] and rock lizards, Iberolacerta cyreni [3]. While camouflaged (‘cryptic’) coloration can reduce conspicuousness, this may still vary owing to movement over heterogeneous substrates. Therefore, the ability to make decisions that reduce risk would be enhanced if individuals are aware of their current conspicuousness. This would require the integration of information on (i) the individual's own coloration and (ii) properties of the background.
In European hermit crabs, Pagurus bernhardus, frequent changes of gastropod shell, in addition to heterogeneous substrates, can influence conspicuousness. Littorina obtusata shells occupied by intertidal P. bernhardus occur in a range of colours but 90 per cent express one of two colour morphs, ‘dark reticulata’ or ‘citrina’ [7]. These dark brown and bright yellow shells are thought to be associated with a stable feeding polymorphism of L. obtusata, of grazing on the upper (reticulata) or lower (citrina) side of the seaweed frond [7]. Hermit crabs occupying reticulata shells are more conspicuous against a light substrate than a dark substrate whereas crabs in citrina shells are more conspicuous against a dark substrate than a light substrate ([8]; see electronic supplementary material, S1 and figure 1b). In order to titrate the duration of startle responses against current conspicuousness, hermit crabs would therefore need to use information on both background colour and shell colour.
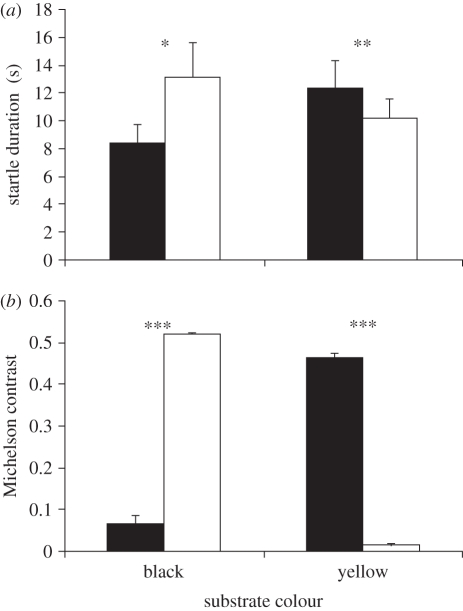
Figure 1.
The interaction between shell type and substrate colour on (a) the duration of startle responses and (b) the Michelson contrast value between the shell and substrate. Significance levels: *p < 0.05, **p < 0.02, ***p < 0.0001. Error bars denote standard errors. Black bars, dark reticulata; white bars, citrina.
In a previous study where risk was manipulated via the presence and the absence of a predator, startle responses were shorter under the low-risk situation when the predator was absent and longest under the high-risk situation when the predator was present [9]. Thus, if hermit crabs are aware of their conspicuousness, we would expect them to show shorter startle responses under shell–substrate combinations that provide low conspicuousness than under combinations that produce high conspicuousness. In addition to such behavioural plasticity, relative differences between individuals may persist across risk levels [9]. ‘Behavioural correlation across situations’ is a key marker for the presence of ‘animal personalities’ ([10]; see [11] for a taxonomic review) and latency to emerge from a refuge has been used as a measure of ‘boldness’ (e.g. [9,12,13]). We aim to determine (i) whether hermit crabs are capable of integrating information about the colour of the substrate and their gastropod shell and (ii) whether between-individual differences in startle responses are robust across situations that vary in two factors.
2. Material and methods
Hermit crabs occupying L. obtusata shells were collected from Hannafore point, Cornwall, UK during September–November 2009. At the laboratory, they were transferred to 125 l tanks containing aerated 15°C sea water and fed ad libitum on white fish. Crabs were removed from their shells (by cracking in a vice), sexed, examined for obvious parasites and loss of appendages and weighed. Only male crabs free from parasites or appendage loss were used. Each crab was placed into a 12 cm dish containing sea water as above and supplied with a new reticulata or citrina shell, of the preferred shell weight for the weight of the crab, established from previous shell selection experiments [14]. There was no variation between crabs in shell fit. The dish contained a 1 cm layer of yellow or black aquarium substrate (Aquatic Gravel, Pettex Ltd, UK). After a 24 h acclimation period, a startle response was induced by removing the crab by hand, inverting for 5 s and returning to the substrate with the aperture facing upwards, enabling us to time the duration of the startle response [8]. Crabs were moved into a new dish containing the alternative substrate colour and the procedure, including the 24 h settling period was repeated. Each crab was then removed again from its shell and supplied with a new shell of the alternative colour, and the process was repeated. Responses were obtained for each crab under four combinations: reticulata shell + black substrate (RB), reticulata shell + yellow substrate (RY), citrina shell + black substrate (CB) and citrina shell + yellow substrate (CY) (see electronic supplementary material, S1). By using crabs from the same size class (occupying L. obtusata shells: 0.1–0.37 g), we controlled for the effects of crab size on startle duration [5,9]. To confirm this, we calculated the Pearson correlation between crab weight and the average startle response for each crab (r38 = 0.12, p = 0.46). All crabs were held in the laboratory for the same amount of time prior to and during the experiment. To avoid order effects, crabs were allocated to four groups (A to D, see electronic supplementary material, S2) with different treatment orders. To minimize stress associated with removing the crabs from shells each crab experienced only one change of shell during the experiment. Since this did not allow inclusion of all possible treatment orders, ‘group’ was included in the analysis. The visual systems of hermit crabs and their crustacean predators are sensitive to luminance (‘lightness’) differences [15] so we collected data to confirm that luminance contrast (‘Michleson contrast’, see Briffa et al. [8] for details) varied between treatment combinations. For five shell–substrate pairs of each combination, we obtained luminance of the wet shell surface (average of two readings taken from the darkest and lightest areas judged by eye) and a wet substrate particle, using a Konica-Minolta CM-2600d portable spectrophotometer (3 mm aperture, 2° viewing angle, D65/SCI illumination) and calculated the contrast for each pair. To determine the effect of shell colour, substrate colour and group on startle duration a two within, one between repeated measures ANOVA was performed. Within subject factors were ‘shell colour’ (reticulata, citrina) and ‘substrate colour’ (black, yellow) and the between subjects factor was ‘group’ (A to D). Post hoc tests are only possible for main effects, so paired t-tests were used to investigate significant interactions. Data were not normally distributed so were Log10 transformed prior to analysis. To determine the consistency of between-individual differences in startle responses we calculated Kendall's coefficient of concordance.
3. Results
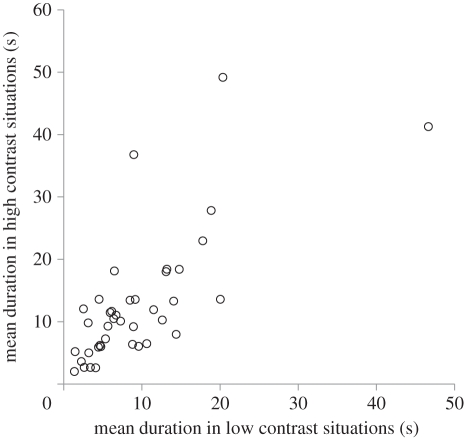
There was no three-factor interaction (F3,36 = 0.7, p = 0.54) but there was a significant interaction between shell and substrate colour (F1,36 = 16.5, p = 0.0003); on the black substrate startle responses were longer in citrina shells (t39 = 2.43, p = 0.019) whereas on the yellow substrate startle responses were longer in reticulata shells (t39 = 2.39, p = 0.022; figure 1a). An interaction between shell colour and group (F2,36 = 4.3, p = 0.012) was caused by crabs in groups A and B showing short responses in reticulata shells, whereas crabs in groups C and D showed shorter responses in citrina shells (see electronic supplementary material, S3). A near-significant interaction between substrate colour and group (F2,36 = 2.6, p = 0.06) resulted from shorter startle responses on black than on yellow sand by crabs in group C. There were no main effects (shell: F1,36 = 0.07, p = 0.83, substrate: F1,36 = 1.5, p = 0.23, group: F3,36 = 1.4, p = 0.25). An interaction (F1,16 = 1079.4, p < 0.0001) between shell and substrate colour indicated that citrina shells contrasted more with black substrate than did reticulata shells (t4 = 23.5, p < 0.0001), whereas reticulata shells contrasted more with yellow substrate than did citrina shells (t4 = 29.7, p < 0.0001; figure 1b). Significant concordance (W = 0.61, p < 0.0001) indicated that differences between individuals persisted across the four situations. To illustrate this we calculated the Pearson correlation between mean responses in the two low and high-contrast situations (r38 = 0.76, p < 0.0001; figure 2).
Figure 2.
The correlation between the mean duration of startle responses in low and high conspicuousness situations.
4. Discussion
Startle responses were shorter when crabs were less conspicuous. Crabs also gave shorter responses in the shells they received first (groups A and B, reticulata; groups C and D, citrina). This was most marked in groups that experienced high conspicuousness first (group B, reticulata-yellow combination; group D, citrina-black combination). These differences could be caused by a cumulative stress response to being removed from their shell twice and there may also be carryover effects between different levels of conspicuousness that were experienced. These interactions, however, were weak compared with the interaction between the substrate and shell colour, indicating that the main driver for startle responses was the current level of conspicuousness.
Several studies show that animals adjust decisions according to their coloration (e.g. [3,6]). In some animals, physiological colour change maintains crypsis against background colours that vary spatially (e.g. chameleons Chamaeleo chamaeleon, [16]) or with season (e.g. mountain hares Lepus timidus, [17]). Similarly, hermit crabs can change their shell to provide a better match with the background but this is constrained by availability and exposure while changing [8]. Therefore, hermit crabs encounter situations where there is a mismatch between shell and substrate colour. In addition to using information on predators [9] and shell size [5], hermit crabs adjust their behaviour (figure 1a) using information on both of these variables (figure 1b). This plasticity, however, is nested within a pattern of consistent differences between individuals. While average responses were greater when crabs were more conspicuous, they were consistent relative to other individuals.
Thus, hermit crabs appear to be aware of their current level of conspicuousness. Crabs may remember information about the visual properties of their shells gained prior to their decision to occupy the shell. Alternatively, the stalked eyes may allow the crabs to see their current shell in addition to the substrate. The capacities of crustaceans for information processing and decision making have been described in a range of species, and hermit crabs are noted for the abilities demonstrated when assessing the morphology of gastropod shells [18]. Here, we show that similar capabilities extend to information on conspicuousness. Further, it appears that animal personalities, i.e. between-individual differences in behaviour that are consistent across situations, in this species are robust across situations that vary in two factors.
Acknowledgements
We are grateful to Victor Kudi for advice on luminance measurements, Kath Sloman and four referees for their comments on the manuscript.
References
- 1.Broom M., Ruxton G. D. 2005. You can run or you can hide: optimal strategies for cryptic prey against pursuit predators. Behav. Ecol. 16, 534–540 10.1093/beheco/ari024 (doi:10.1093/beheco/ari024) [DOI] [Google Scholar]
- 2.Ydenberg R. C., Dill L. M. 1986. The economics of fleeing from predators. Adv. Stud. Behav. 16, 229–249 10.1016/S0065-3454(08)60192-8 (doi:10.1016/S0065-3454(08)60192-8) [DOI] [Google Scholar]
- 3.Martín J., López P., Polo V. 2009. Temporal patterns of predation risk affect antipredator behaviour allocation by Iberian rock lizards. Anim. Behav. 77, 1261–1266 10.1016/j.anbehav.2009.02.004 (doi:10.1016/j.anbehav.2009.02.004) [DOI] [Google Scholar]
- 4.Briffa M., Elwood R. W. 2001. Motivational change during shell fights in the hermit crab Pagurus bernhardus. Anim. Behav. 62, 505–510 10.1006/anbe.2001.1764 (doi:10.1006/anbe.2001.1764) [DOI] [Google Scholar]
- 5.Briffa M., Bibost A.-L. 2009. Effects of shell size on behavioural consistency and flexibility in hermit crabs. Can. J. Zool. 87, 597–603 10.1139/Z09-047 (doi:10.1139/Z09-047) [DOI] [Google Scholar]
- 6.Cooper W. E., Jr 1998. Effects of refuge and conspicuousness on escape behaviour by the broadheaded skink (Eumeces laticeps). Amphibia Reptilia 19, 103–108 10.1163/156853898X00386 (doi:10.1163/156853898X00386) [DOI] [Google Scholar]
- 7.Reimchen T. E. 1979. Substratum heterogeneity, crypsis and colour polymorphism in an intertidal snail (Littorina). Can. J. Zool. 57, 1070–1085 10.1139/z79-135 (doi:10.1139/z79-135) [DOI] [Google Scholar]
- 8.Briffa M., Haskell P., Wilding C. 2008. Behavioural colour change in the hermit crab Pagurus bernhardus: reduced crypticity when the threat of predation is high. Behaviour 145, 915–929 10.1163/156853908784089261 (doi:10.1163/156853908784089261) [DOI] [Google Scholar]
- 9.Briffa M., Rundle S. D., Fryer A. 2008. Comparing the strength of behavioural plasticity and consistency across situations: animal personalities in the hermit crab Pagurus bernhardus. Proc. R. Soc. B 275, 1305–1311 10.1098/rspb.2008.0025 (doi:10.1098/rspb.2008.0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sih A., Bell A., Johnson J. C. 2004. Behavioural syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–377 10.1016/j.tree.2004.04.009 (doi:10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 11.Gosling S. 2001. From mice to men: what we can learn about personality from animal research. Psychol. Bull. 127, 45–86 10.1037/0033-2909.127.1.45 (doi:10.1037/0033-2909.127.1.45) [DOI] [PubMed] [Google Scholar]
- 12.Brown C., Jones F., Braithwaite V. 2005. In situ examination of boldness-shyness traits in the tropical poeciliid, Brachyraphis episcope. Anim. Behav. 70, 1003–1009 10.1016/j.anbehav.2004.12.022 (doi:10.1016/j.anbehav.2004.12.022) [DOI] [Google Scholar]
- 13.Sih A., Kats L. B., Maurer E. F. 2003. Behavioural correlations across situations and the evolution of antipredator behaviour in a sunfish–salamander system. Anim. Behav. 65, 29–44 10.1006/anbe.2002.2025 (doi:10.1006/anbe.2002.2025) [DOI] [Google Scholar]
- 14.Briffa M., Elwood R. W. 2007. Monoamines and decision making during contests in the hermit crab Pagurus bernhardus. Anim. Behav. 73, 605–612 10.1016/j.anbehav.2006.06.008 (doi:10.1016/j.anbehav.2006.06.008) [DOI] [Google Scholar]
- 15.Horridge G. A. 1966. Perception of edges versus areas by the crab Carcinus. J. Exp. Biol. 44, 247–254 [DOI] [PubMed] [Google Scholar]
- 16.Cuadrado M., Martín J., López P. 2001. Camouflage and escape decisions in the common chameleon Chamaeleo chamaeleon. Biol. J. Linn. Soc. 72, 547–554 10.1111/j.1095-8312.2001.tb01337.x (doi:10.1111/j.1095-8312.2001.tb01337.x) [DOI] [Google Scholar]
- 17.Stoner C. J., Bininda-Emonds O. R. P., Caro T. 2003. The adaptive significance of coloration in lagomorphs. Biol. J. Linn. Soc. 79, 309–328 10.1046/j.1095-8312.2003.00190.x (doi:10.1046/j.1095-8312.2003.00190.x) [DOI] [Google Scholar]
- 18.Elwood R. W., Briffa M. 2001. Information gathering and communication during agonistic encounters: a case study of hermit crabs. Adv. Stud. Behav. 30, 53–97 10.1016/S0065-3454(01)80005-X (doi:10.1016/S0065-3454(01)80005-X) [DOI] [Google Scholar]