Abstract
Four phylogenetically independent lineages of frogs are currently known to sequester lipid-soluble skin alkaloids for which a dietary source has been demonstrated. We report here a remarkable fifth such instance, in Eleutherodactylus iberia and Eleutherodactylus orientalis, two species of miniaturized frogs of the family Eleutherodactylidae from Cuba. Six pumiliotoxins and two indolizidines were found in E. iberia, one of the smallest frogs in the world and characterized by a contrasting colour pattern for which we hypothesize an aposematic function. Analyses of stomach content indicated a numerical prevalence of mites with an important proportion of oribatids—a group of arthropods known to contain one of the pumiliotoxins detected in E. iberia. This suggests that miniaturization and specialization to small prey may have favoured the acquisition of dietary skin alkaloids in these amphibians.
Keywords: amphibia, Eleutherodactylidae, Eleutherodactylus iberia, Eleutherodactylus orientalis, Cuba, pumiliotoxins
1. Introduction
Amphibians accumulate numerous compounds into their skin glands, many of which act as a defence against micro-organisms and predators [1,2]. These include amines, peptides, proteins and steroids that appear to be largely produced de novo by the amphibians [3,4], thereby differing from alkaloids, another class of toxins present in some amphibians. Only the steroidal samandarine alkaloids of European salamanders are apparently biosynthesized by them [3,5], while the origin of water-soluble alkaloids (tetrodotoxins; present in some bufonid and brachycephalid toads, at least one dendrobatid frog, and some newts) remains unclarified although symbiotic micro-organisms might be involved [4,6]. On the contrary, lipid-soluble alkaloids are taken up to an overwhelming extent with prey and then sequestered into specialized skin glands [6]. Lipid-soluble alkaloids in amphibians occur in a wide variety, with over 800 compounds identified so far [7], but their occurrence is phylogenetically restricted. They have been reported from one species-rich lineage of Neotropical poison-dart frogs (Dendrobatidae), one genus of bufonid toads (Melanophryniscus), one genus of Madagascan mantellid frogs (Mantella) and one genus of Australian myobatrachid toadlets (Pseudophryne). Minor quantities of alkaloids have also been found in a ranid of the genus Limnonectes [8], but it is unclear whether this points to a regular occurrence of alkaloids in these Asian frogs. The alkaloid-containing frog lineages are phylogenetically unrelated (see electronic supplementary material) occur in a variety of habitats including tropical rainforest (most dendrobatids, Mantella), subtropical shrubland (some Melanophryniscus) or highland bogs (some Pseudophryne), and all or almost all species within these lineages have been found to contain alkaloids. In addition, the great majority of alkaloid-containing amphibians are characterized by vivid colours of probable aposematic function, often covering the entire body although sometimes restricted to the ventral or dorsal side. Representatives of more than 80 other genera of frogs screened contained no alkaloids [3,7,8], confirming that these compounds are restricted to only a small number of lineages.
Frogs of the family Eleutherodactylidae are part of a large clade of direct-developing anurans, the Terrarana, occurring in South and Central America, and the Caribbean [9]. A negative alkaloid screening for the genus Eleutherodactylus has been reported [3], probably referring to South or Central American species of the Terrarana clade that today are considered to belong to other genera [10]. Eleutherodactylids includes two subfamilies, four genera and five subgenera, and their distribution is centred in the Caribbean region. The most species-rich eleutherodactylid genus is Eleutherodactylus with currently 186 species [9,10]. Most frogs on the Caribbean islands belong to this genus, and on the largest of these islands, Cuba, 52 out of 62 frogs are assigned to Eleutherodactylus [11,12]. They radiated into a wide variety of ecological niches and comprise specialized cave-dwelling, riparian and arboreal species, as well as one endemic Cuban clade of highly miniaturized species, the limbatus group [9]. One representative of these dwarf frogs, Eletherodactylus iberia, has been flagged as the smallest frog in the world, competing with Brachycephalus didactylus from Brazil and Stumpffia pygmaea and S. tridactyla from Madagascar. Adults of these species measure only around 10 mm from snout to vent [9,13]. However, little information on the ecology and biology of this and other Cuban dwarf frogs has become available [11,12].
During recent fieldwork in the distribution areas of two Cuban dwarf Eleutherodactylus, E. iberia and E. orientalis, the odour of dissected specimens reminded us of alkaloid-containing dendrobatid and mantellid species. Further analyses provided conclusive evidence that in fact these frogs contain lipophilic alkaloids of the same compound classes previously reported in other alkaloid-containing frog lineages. Here, we report on this first discovery of skin alkaloids in a representative of the Terrarana, provide evidence for their specialization to prey likely to contain alkaloids, and discuss the relevance of miniaturization for the evolution of skin alkaloid-sequestration in amphibians.
2. Material and methods
Specimens of E. iberia were collected in October 2009 and in January 2010 at Bahía de Taco, and specimens of E. orientalis at the Yunque de Baracoa, Guantánamo province, eastern Cuba (see electronic supplementary material for extended methods with precise data on localities and sampling). Although occurring in close geographic proximity, these two frog species are not known to occur sympatrically. Stomach contents of a subset of the specimens collected were analysed using a stereomicroscope. Special attention was given to identifying oribatid mites to the family level, because of their known importance as an alkaloid source for poison frogs [14].
Gas chromatography–mass spectrometry (GC–MS) analyses were carried out on an Agilent 7890A GC system connected to an Agilent 5975C inert mass detector fitted with a HP-5MS-fused silica capillary column (30 m, 0.25 mm i.d., 0.25 µm film; J&W Scientific, USA). The conditions were as follows—inlet pressure: 77.1 kPa, 23.3 ml He min−1; injection volume: 1 µl; transfer line: 300°C; electron energy: 70 eV. GC program: 1 min at 50°C, increasing with 10°C min−1 to 320°C, operated in splitless mode (60 s valve time). Single skins of the frogs were extracted with methanol. This extract was either directly used for GC–MS analysis or the alkaloids were separated from neutrals according to a described procedure [3]. Alkaloids were identified by comparison of mass spectra and gas chromatographic retention times with those of reference samples and are named with codes consisting of the nominal molecular weight and an identifying letter, both in bold face [3,7].
3. Results and discussion
An initial screening of ethanol-preserved skins, collected in October 2009, of three males of E. iberia (voucher nos. AR685–687) and one of E. orientalis (AR670) revealed the probable presence of lipophilic alkaloids despite the inappropriate preservation of these skins. In E. orientalis, minor amounts of pumiliotoxin (PTX) 267C were detected, whereas the skin of E. iberia contained trace amounts of PTX 323A.
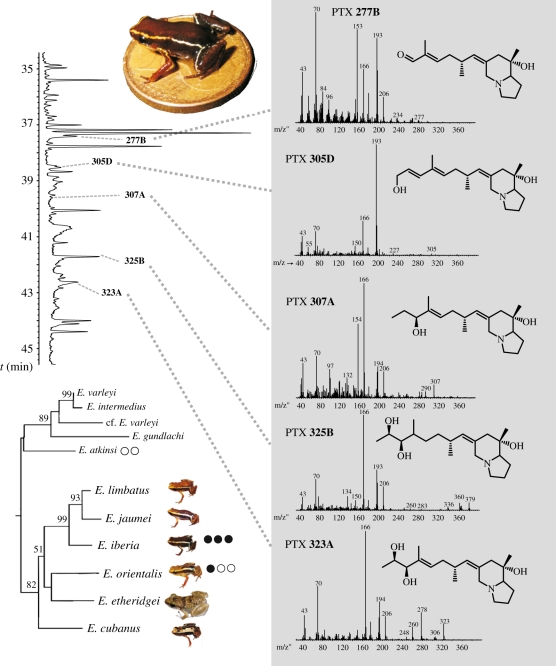
Subsequent analysis of the methanol-preserved skins collected in January 2010 revealed neither alkaloids in two specimens of E. orientalis (AR730–731) nor in one specimen of the sympatric E. atkinsi (AR754). On the contrary, the two specimens of E. iberia had significant amounts of various alkaloids (table 1). PTX 323A was the major alkaloid in both individuals, and several related compounds were present. In addition, two isomeric alkaloids of unknown structure were present in substantial amounts, here named 267 (1) and 267 (2) (see electronic supplementary material). Their mass spectra indicate that these two alkaloids have an indolizidine core structure [7]. Several other alkaloids were present in trace amounts insufficient for complete identification. The mass spectra of the major identified compounds are shown in figure 1. Additional data are included in the electronic supplementary material. Several of the compounds had previously been detected in other frog lineages: 307A in dendrobatids and mantellids, and 323A in dendrobatids, mantellids, bufonids and myobatrachids [15,16].
Table 1.
Alkaloids identified in the skins of two male Eleutherodactylus iberia (identified by their field numbers). Alkaloid designation follows a previous study [7]. +++, major amount; ++, minor amount; +, trace component; ?, unknown structure. Specimen numbers are field numbers of A.R.
| compound | AR722 | AR721 |
|---|---|---|
| PTX 277B | ++ | ++ |
| PTX 305D | ++ | |
| PTX 307A | + | |
| PTX 321A | + | |
| PTX 323A | +++ | +++ |
| PTX 325B | ++ | |
| indolizidine 267 (1)? | + | ++ |
| indolizidine 267 (2)? | ++ | +++ |
Figure 1.
Gas chromatogram of a skin extract of Eleutherodactylus iberia (inset photo) with the peaks corresponding to five pumiliotoxins, and their mass spectra. The mass spectrum of PTX 325B is actually that of the dimethylsiloxane derivative formed during gas chromatography of vicinal diols on polymethylsilyloxy-derived GC phases. The tree shows the phylogenetic relationships of dwarf frogs of the Eleutherodactylus limbatus group as taken from the literature [9] and the contrasted, possibly aposematic colour in E. iberia, E. limbatus, E. jaumei and E. orientalis. Filled dots indicate the number of alkaloid-containing (black) or no alkaloid-containing (white) samples in the GC–MS analyses performed. Numbers at nodes of tree indicate values > 50% of a bootstrap analysis [9]. (Online version in colour.)
Stomach content analyses of a total of seven adult specimens of E. iberia and E. orientalis identified mites as the major prey items of these miniaturized frogs. Oribatid mites, known as alkaloid sources for frogs in Central America [14], were well represented in the diet of these two species (table 2). Numerically, mites made up 66 per cent of the 35 prey items of E. orientalis, and 71 per cent of the 62 prey items of E. iberia. Other arthropod groups were found in much lower proportions: in E. iberia, the third and fourth most common prey items were springtails (Collembola; numerical proportion 10%) and ants (6%).
Table 2.
Prey items identified in the stomach contents of four adult males of Eleutherodactylus iberia and three adult males of E. orientalis and their numerical proportion. Data for mites (Acari) are given separately for orders/suborders, and for families within the Oribatida. Asterisks denote families of oribatids that were present in alkaloid-containing samples of a previous study in Costa Rica and Panama [14]. Frog specimens were labelled with field numbers of A.R.
|
E. iberia |
E. orientalis |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| AR 720 | AR 721 | AR 723 | AR 724 | per cent | AR 729 | AR 731 | AR 732 | per cent | |
| Acari total | 10 | 10 | 7 | 20 | 71.0 | 11 | 4 | 9 | 65.7 |
| Oribatida | 8 | 4 | 5 | 3 | 33.9 | 10 | 3 | 8 | 60.0 |
| Euphthiracaridae | 1 | 0 | 0 | 0 | 1.6 | 1 | 0 | 0 | 2.9 |
| Galumnidae* | 0 | 0 | 0 | 2 | 3.2 | 0 | 0 | 5 | 14.3 |
| Hermanniellidae | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 11.4 |
| Lohmanniidae* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.9 |
| Nothridae | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2.9 |
| Oppiidae* | 3 | 1 | 0 | 0 | 6.5 | 3 | 1 | 1 | 14.3 |
| Oribotritiidae* | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 5.7 |
| Scheloribatidae* | 1 | 0 | 0 | 0 | 4.8 | 0 | 0 | 0 | 0 |
| Stegnacaridae | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2.9 |
| Trhypochthoniidae* | 3 | 1 | 2 | 0 | 9.7 | 0 | 0 | 0 | 0 |
| unknown | 0 | 2 | 2 | 1 | 8.1 | 1 | 0 | 0 | 2.9 |
| Mesostigmata | 4 | 3 | 2 | 14 | 37.1 | 0 | 0 | 2 | 5.7 |
| Araneae | 3 | 0 | 1 | 0 | 6.5 | 1 | 1 | 0 | 5.7 |
| Pseudoscorpiones | 1 | 1 | 1 | 0 | 4.8 | 1 | 0 | 0 | 2.9 |
| Coleoptera (larvae) | 0 | 1 | 0 | 0 | 1.6 | 0 | 1 | 1 | 5.7 |
| Diptera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2.9 |
| Hymenoptera (ants) | 1 | 1 | 1 | 0 | 4.8 | 2 | 0 | 0 | 5.7 |
| Lepidoptera | 0 | 0 | 1 | 0 | 1.6 | 2 | 0 | 0 | 5.7 |
| Collembola | 3 | 1 | 2 | 0 | 9.7 | 0 | 0 | 0 | 0 |
| Isopoda | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 5.7 |
| total items | 20 | 11 | 14 | 17 | 16 | 7 | 12 | ||
Whether alkaloid sequestering is characteristic for all populations of E. iberia and whether it regularly occurs in E. orientalis and other related species of the E. limbatus group cannot be clarified without extensive additional datasets. However, the discovery that at least some of these frogs sequester lipid-soluble alkaloids may contribute to the understanding of the evolutionary pathway to alkaloid-sequestration and aposematism in amphibians. Various authors have noted that in frogs the evolution of skin alkaloid sequestering appears to be correlated with other traits such as specialization to small prey, diurnality, aposematic colour and small body size, usually assuming that these frogs specialize in ants and that these are the most important source of dietary alkaloids [15,17–19]. However, more recently, evidence has accumulated for the major importance of oribatid mites [13] which are a common prey, especially for miniaturized frogs [20], including the dwarf Eleutherodactylus studied here (table 2). Two alkaloids of E. iberia have previously been detected in arthropods, 307A in mites and ants, and 323A in ants, suggesting a dietary source for these compounds [13,14].
Further evidence for the importance of miniaturization came from the phylogenetic position of Wakea madinika, an enigmatic dwarf frog from Madagascar, as the sister group of the alkaloid-containing genus Mantella, which might indicate that the ancestor of Mantella was miniaturized as well [21]. This favours a scenario in which miniaturization with dietary specialization to small-sized prey, including ants but especially mites, was a first evolutionary step, followed by evolution of alkaloid-sequestering, aposematism and diurnality. Cuban dwarf Eleutherodactylus are largely diurnal, and E. iberia is chocolate brown with dorsolateral yellow–white lines, a coloration similar to various highly toxic dendrobatids of the genus Phyllobates and thus indicative of aposematism. Other species of the same clade, however, have a much less contrasted coloration (E. atkinsi, E. cubanus and E. etheridgei; see figure 1), suggesting that comparative studies of dietary specialization, alkaloid content and degree of diurnality of these frogs may yield further insights in the evolutionary history of this complex of correlated characters.
Acknowledgements
We thank Jose Antonio Rodríguez (Baracoa) for invaluable help during fieldwork and Raúl Matos for logistic support, Josmaily Loriga Piñeiro and Gaby Schlitt for their help and patience during fieldwork, and Thomas F. Spande and H. Martin Garraffo who provided helpful information about methods and data interpretation. Mercedes Reyes from the IES kindly identified the mites. The Cuban authorities from Centro de Inspección y Control Ambiental (CICA) granted collection permit (no. 19/2010). Financial support was obtained from the Deutsche Gesellschaft für Herpetologie und Terrarienkunde (to A.R. and M.V.) and the Zukunftsfonds/Programmpauschale of the Deutsche Forschungsgemeinschaft/TU Braunschweig (to M.V. and S.S.).
References
- 1.Erspamer V. 1994. Bioactive secretions of the amphibian integument. In Amphibian biology (eds Heatwole G., Barthalmus T., Heatwole A. Y.), pp. 178–350 Chipping Norton: Surrey Beatty & Sons [Google Scholar]
- 2.Clarke B. T. 1997. The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol. Rev. 72, 365–379 10.1017/S0006323197005045 (doi:10.1017/S0006323197005045) [DOI] [PubMed] [Google Scholar]
- 3.Daly J. W., Myers C. W., Whittaker N. 1987. Further classification of skin alkaloids from Neotropical poison frogs (Dendrobatidae) with a general survey of toxic/noxious substances in the Amphibia. Toxicon 25, 1023–1095 10.1016/0041-0101(87)90265-0 (doi:10.1016/0041-0101(87)90265-0) [DOI] [PubMed] [Google Scholar]
- 4.Daly J. W. 1995. The chemistry of poisons in amphibian skin. Proc. Natl Acad. Sci. USA 92, 9–13 10.1073/pnas.92.1.9 (doi:10.1073/pnas.92.1.9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habermehl G., Haaf A. 1968. Cholesterin als Vorstufe in der Biosynthese der Salamanderalkaloide. Chemische Berichte 101, 198–200 10.1002/cber.19681010126 (doi:10.1002/cber.19681010126) [DOI] [PubMed] [Google Scholar]
- 6.Daly J. W. 1998. Thirty years of discovering arthropod alkaloids in amphibian skin. J. Nat. Prod. 61, 162–172 10.1021/np970460e (doi:10.1021/np970460e) [DOI] [PubMed] [Google Scholar]
- 7.Daly J. W., Spande T. F., Garraffo H. M. 2005. Alkaloids from amphibian skin: a tabulation of over eight-hundred compounds. J. Nat. Prod. 68, 1556–1575 10.1021/np0580560 (doi:10.1021/np0580560) [DOI] [PubMed] [Google Scholar]
- 8.Daly J. W., et al. 2004. Biologically active substances from amphibians: preliminary studies on anurans from twenty-one genera of Thailand. Toxicon 44, 805–812 10.1016/j.toxicon.2004.08.016 (doi:10.1016/j.toxicon.2004.08.016) [DOI] [PubMed] [Google Scholar]
- 9.Hedges S. B., Duellman W. E., Heinicke M. P. 2008. New World direct-developing frogs (Anura: Terrarana): molecular phylogeny, classification, biogeography, and conservation. Zootaxa 1737, 1–182 [Google Scholar]
- 10.AmphibiaWeb 2010. Information on amphibian biology and conservation (Web application). Berkeley, California: AmphibiaWeb; (available: http://amphibiaweb.org/ (accessed: Sep 5, 2010)). [Google Scholar]
- 11.Díaz L. M., Cádiz A. 2008. Guia taxonómica de los anfibios de Cuba. ABC Taxa 4, 1–294 [Google Scholar]
- 12.Alonso R., Rodríguez A., Márquez R. 2007. Sound guide of the amphibians from Cuba. ALOSA sons de la natura. Audio CD & booklet, p. 46 [Google Scholar]
- 13.Estrada A. R., Hedges S. B. 1996. At the lower size limit in tetrapods: a new diminutive frog from Cuba (Leptodactylidae: Eleutherodactylus). Copeia 1996, 852–859 10.2307/1447647 (doi:10.2307/1447647) [DOI] [Google Scholar]
- 14.Saporito R. A., Donnelly M. A., Norton R., Garraffo H. M., Spande T. F., Daly J. W. 2007. Oribatid mites as a major dietary source for alkaloids in poison frogs. Proc. Natl Acad. Sci. USA 104, 8885–8890 10.1073/pnas.0702851104 (doi:10.1073/pnas.0702851104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saporito R. A., Garraffo H. M., Donnelly M. A., Edwards A. L., Longino J. T., Daly J. W. 2004. Formicine ants: an arthropod source for the pumiliotoxin alkaloids of dendrobatid frogs. Proc. Natl Acad. Sci. USA 101, 8045–8050 10.1073/pnas.0402365101 (doi:10.1073/pnas.0402365101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly J. W., Garraffo H. M., Spande T. F., Yeh H. J. C., Peltzer P. M., Cacivio P. M., Baldo J. D., Faivovich J. 2008. Indolizidine 239Q and quinolizidine 275I. Major alkaloids in two Argentinian bufonid toads (Melanophryniscus). Toxicon 52, 858–870 10.1016/j.toxicon.2008.08.016 (doi:10.1016/j.toxicon.2008.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldwell J. P. 1996. The evolution of myrmecophagy and its correlates in poison frogs (family Dendrobatidae). J. Zool. 40, 75–101 [Google Scholar]
- 18.Vences M., Glaw F., Böhme W. 1998. Evolutionary correlates of microphagy in alkaloid-containing frogs (Amphibia: Anura). Zool. Anz. 236, 217–230 [Google Scholar]
- 19.Darst C. R., Menéndez-Guerrero P. A., Coloma L. A., Cannatella D. C. 2005. Evolution of dietary specialization and chemical defense in poison frogs (Dendrobatidae): a comparative analysis. Am. Nat. 165, 56–69 10.1086/426599 (doi:10.1086/426599) [DOI] [PubMed] [Google Scholar]
- 20.Simon M. P., Toft C. A. 1991. Diet specialization in small vertebrates: mite-eating in frogs. Oikos 61, 263–278 10.2307/3545344 (doi:10.2307/3545344) [DOI] [Google Scholar]
- 21.Glaw F., Vences M. 2006. Phylogeny and genus-level classification of mantellid frogs. Org. Divers. Evol. 6, 236–253 10.1016/j.ode.2005.12.001 (doi:10.1016/j.ode.2005.12.001) [DOI] [Google Scholar]