Abstract
Ant–fungus associations are well known from attine ants, whose nutrition is based on a symbiosis with basidiomycete fungi. Otherwise, only a few non-nutritional ant–fungus associations have been recorded to date. Here we focus on one of these associations involving Allomerus plant-ants that build galleried structures on their myrmecophytic hosts in order to ambush prey. We show that this association is not opportunistic because the ants select from a monophyletic group of closely related fungal haplotypes of an ascomycete species from the order Chaetothyriales that consistently grows on and has been isolated from the galleries. Both the ants' behaviour and an analysis of the genetic population structure of the ants and the fungus argue for host specificity in this interaction. The ants' behaviour reveals a major investment in manipulating, growing and cleaning the fungus. A molecular analysis of the fungus demonstrates the widespread occurrence of one haplotype and many other haplotypes with a lower occurrence, as well as significant variation in the presence of these fungal haplotypes between areas and ant species. Altogether, these results suggest that such an interaction might represent an as-yet undescribed type of specific association between ants and fungus in which the ants cultivate fungal mycelia to strengthen their hunting galleries.
Keywords: ant–fungus association, Cordia nodosa, Chaetothyriales, Hirtella physophora, myrmecophyte, population structure
1. Introduction
Insect fungiculture has evolved independently in four orders of insects; i.e. ants, termites, ambrosia beetles and gall midges [1–3]. In ants, species of the Attini tribe are engaged in a highly coevolved nutritional symbiosis with basidiomycetes in which both partners are codependent, and that shows their reciprocal specializations and codispersal [4–6]. Besides such cultivation of fungal crops, few non-food associations between ants and fungi have been reported to date in which fungi occur in ant constructions or inside the domatia of ant-plants [7–12]. These occurrences of fungi in the constructions or inside the domatia are not the result of the growth of opportunistic species, but of specific interactions [9,12]. These studies also suggest that the active maintenance and management of fungi by ants for non-nutritional purposes may be more widespread than currently assumed [13].
Here we address this issue in Neotropical plant-ants of the genus Allomerus, associated with the myrmecophytes Hirtella physophora Martius & Zuccharini and Cordia nodosa Lamarck. To ambush prey, these ants build galleries under the stems of their host plants using trichomes that they assemble into a frame on which then grows a fungal mycelium that reinforces the structure [8]. It has not yet been demonstrated, however, whether the ants cultivate the fungi or if the fungi are opportunistic species. We studied unknown aspects of the biology of this ant–fungus association, and both characterized and analysed the genetic population structure of the fungi from the galleries and of their associated ants (Allomerus decemarticulatus Mayr and Allomerus octoarticulatus Mayr), sampled from geographically distant areas within French Guiana.
2. Material and methods
(a). Sampling ants and traps
Workers from 197 A. decemarticulatus and 43 A. octoarticulatus colonies were sampled along with parts of their corresponding galleries from four areas in French Guiana between 2008 and 2009: Petit Saut, Sinnamary (05°04′27″ N, 53°03′21″ W; n = 133/19 A. decemarticulatus/A. octoarticulatus colonies, respectively), Montagne des Singes (05°04′20″ N, 52°41′43″ W; n = 26/6), Montagne de Kaw (04°32′34″ N, 52°09′14″ W; n = 29/18) and Nouragues (04°05′16″ N, 52°40′49″ W; n = 9/0). In addition, 18 domatia recently occupied by founding queens were dissected. Such domatia were recognizable from the outside because the queen's wings remained at the entrance and because the queen had built a stockade to close the entrance.
Fungal samples were precultured in wet cotton and 15–30 single hyphae from the new mycelium were then cultured in solid yeast and malt extract with glucose medium for 6–20 days (see details in the electronic supplementary material).
(b). Molecular and phylogenetic analysis of the fungi and the ants
The total DNA from all of the fungal and ant samples was extracted using a 10 per cent Chelex (BioRad) solution. A phylogenetic analysis was conducted on the entire internal transcribed spacer (ITS) region and the EF1α segment of the fungi, as well as the cytochrome c-oxydase (COI) and a segment of the cytochrome b gene of the ants. In total, 114 fungal samples out of the 240 collected were successfully sequenced for both the ITS and EF1α segment (fungal isolation success of about 50%), and 91 ant colonies (69 A. decemarticulatus and 22 A. octoarticulatus) were successfully sequenced for both the COI and cytochrome b. Capronia pilosella and Exophiala pisciphila were used as the fungal outgroup, and Monomorium subopacum, Diplorhoptrum sp., Solenopsis saevissima and Wasmannia auropunctata were used as the ant outgroup. Phylogenetic inferences were made using different criteria: maximum parsimony (MP), neighbour-joining (NJ), maximum-likelihood (ML) and Bayes. The genetic structure of the fungal variants detected in the cladograms was analysed using Arlequin v. 3.1 [14] and the AMOVA values for the FST were calculated after 10 000 permutations (see the electronic supplementary material).
3. Results
Eleven out of the 18 founding queens recorded in the dissected domatia had only been installed for a very short time, as they had laid few or no eggs. In all of these 11 cases, a black pellet was clearly visible in the domatium wall or already pasted to the trichomes that the queen had cut and piled at the entrance to the domatium to close it (figure 1a). The pellet is made from plant material and the first hyphae grow in the suberous crust. It remains, however, unknown whether the queen brings the pellet and the fungus from its mother colony or if it is already present in the new host plant (figure 1b–d). The seven remaining queens had already bred some larvae and pupae and the hyphae had entirely covered the domatia entrances. The first workers produced must excavate a tunnel through this barrier to leave the domatia (figure 1d). They then cut the trichomes along the stem, creating a path to new domatia and use the cut trichomes to build the frame of a vault above the path (figure 1e). The workers scarify the epidermis and mesophyll of the inner walls of the domatia to prepare pellets of vegetal dough. These pellets are carried out of the domatia and pasted along the trichomes that form the future gallery (figure 1f–h).
Figure 1.
Culture of the fungus and construction of the galleries. (a) Trichomes inside and outside a recently colonized domatium; (b) pellet made from plant material from the domatium close to the first eggs laid in the newly colonized domatium (arrow); (c) the founding queen scarifies the domatia wall (vertical arrow) where the first hyphae will grow in the suberous crust (horizontal arrow); (d) the growing fungus seals the entrance of the domatium until the first workers make an exit hole (arrow); (e) scaffold of trichomes; (f) highly scarified domatium; (g) the workers paste pellets made from the suberous crust of the domatia (arrows) on the frame of trichomes; and (h) the fungus grows from each of the pellets strengthening the galleries that the ants use as a trap (white arrow, trichomes; black arrow, pellets). Scale bar, 1 mm.
Forty-four fungal species present as spores were isolated directly from the galleries (table 1), but none of them grow on these galleries unless the ants have been removed. Moreover, an examination of the infrabuccal pellets of the workers demonstrated the active removal of fungal spores and contaminant hyphae (M. X. Ruiz-Gonzalez 2009, unpublished results). By contrast, the mycelium of only one fungal species, a sooty mould, was repeatedly noted in both the galleries covering the plant stems and the domatia occupied by the founding queens. This melanized fungus, with elongated hyphae of 4.5–9 µm in diameter, consistently grew from the multi-replicated cultures (108 and 31 different colonies of A. decemarticulatus and A. octoarticulatus, respectively). Also, separate cultures sampled from the same colonies were genetically identical, highlighting the monoculture of one fungal strain per colony.
Table 1.
Fungi present in the galleries as spores and their closest TAXID from GenBank.
| phylum | class | order | species | closest TAXID from GenBank |
|---|---|---|---|---|
| Ascomycota | Dothideomycetes | Botryosphaeriales | 5 spp, 4 genera | AJ938005, FJ799942, EU687005, FJ904913, FJ904840 |
| Capnodiales | 5 spp, 5 genera | AF502837, EU019265, EU167591, AJ582964, EU707900 | ||
| Pleosporales | 5 spp, at least 3 genera | DQ914713, EU686970, EU489931, GQ179976, FJ904919 | ||
| Chaetothyriales | 4 spp, at least 3 genera | FJ475797, FJ475797, EU520597, AJ244263 | ||
| Eurotiomycetes | Eurotiales | Penicillium sp. | AF510496 | |
| Sordariomycetes | Hypocreales | 11 spp., 7 genera | EU552110, FJ037741, AM410612, FJ612897, FJ612897, FJ605099, AJ301990, FJ487919, FJ808013, AY746002, AB067714 | |
| Microascales | Scopulariopsis sp. | AY625066 | ||
| Xylariales | 6 spp, at least 1 genus | FJ884143, EF451799, EF423541, EF451799, AF377296, FJ613106 | ||
| Basidiomycota | Agaricomycetes | Agaricales | 1 Coprinellus sp. | AY461838 |
| Auriculariales | 1 Exidiosis sp. | AF395309 | ||
| Tremellomycetes | Tremellales | 2 spp., 2 genera | AF314985, FJ882009 | |
| Ustilaginomycetes | 1 Pseudozyma sp. | DQ008954 | ||
| Incertae sedis | Mucorales | 1 Rhizomucor sp. | EF583637 |
BLAST search and the phylogenetic analyses of the ITS region in 124 samples revealed that a clade with seven monophyletic taxa was the closest (85–91% identity) to our sequences. Three out of the seven taxa are uncultured, environmental samples (GenBank accession nos: FJ820738, AJ582964, AY969659); three others are fungi found on the carton galleries of Azteca brevis (FJ538958, FJ538959, FJ538960) and the seventh is an ascomycete species described as Trimmatostroma cordae Sharma & Singh (GenBank accession nos: AJ244263). All of the sequences are grouped within the order Chaetothyriales.
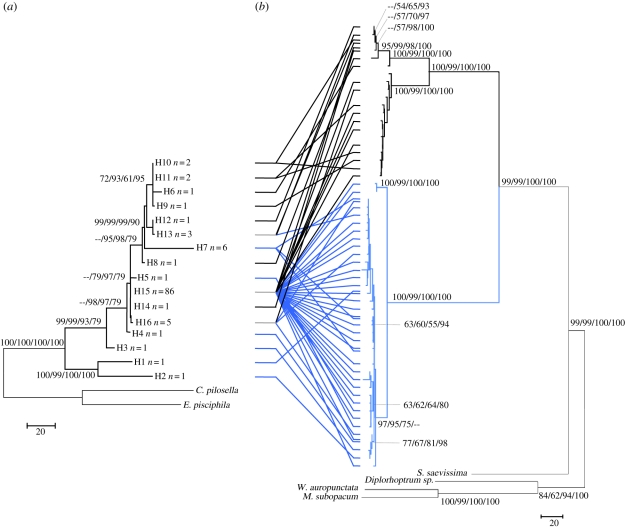
An analysis of the concatenated fungal ITS and EF1α regions revealed the presence of 16 haplotypes belonging to a monophyletic clade (figure 2). More than 75 per cent of the samples had the same haplotype. Haplotypes 1–5 and 7 were exclusive to A. decemarticulatus and haplotypes 6, 8–12 and 14 were exclusive to A. octoarticulatus. Finally, haplotypes 13, 15 and 16 were present in the constructions of both ant species. The haplotype composition varied significantly between the different areas (FST = 0.131, p-value = 0.0004) and between host ant species (FST = 0.108, p-value = 0.0016).
Figure 2.
Phylograms of the ITS–EF1α fungal and COI-cytochrome b ant haplotypes. Bootstrap values above 50% for each branch are shown as NJ/MP/ML/BY. (a) The 16 haplotypes of fungal cultivars isolated from Allomerus decemarticulatus (grey) or A. octoarticulatus (black) colonies. (b) The 57 ant haplotypes.
An analysis of the concatenated ant COI and cytrochrome b regions revealed the presence of 57 haplotypes. Two main clades corresponding to A. octoarticulatus and A. decemarticulatus, respectively, were strongly supported in all cases (figure 2). Allomerus octoarticulatus (20 haplotypes) comprises two subpopulations: one from the east of French Guiana (Montagne de Kaw), and the other from the west (Petit Saut and Montagne des Singes). For A. decemarticulatus (37 haplotypes), five clades were statistically supported; two of them were noted both at Nouragues and the Montagne de Kaw, the three others at Petit Saut and the Montagne des Singes.
4. Discussion
Although the spores of many fungal species were present in the galleries, the Allomerus workers' behaviour favours the growth of only one species, corresponding to a monophyletic group of closely related haplotypes from the order Chaetothyriales. The occurrence of a single fungal species associated with the Allomerus galleries and of a single haplotype per colony argue for a specific association between Allomerus ants and this fungus, and thus towards host specificity. Furthermore, the ants provide the fungus with a substrate, control for potential invasions by alien fungi and the association with the ants is obligate for the survival of the fungus [8].
Such host-specificity is common in nutritional associations between ants and fungi in which fungal cultivars are mainly transmitted vertically across generations [5,6]. On the contrary, in fungus-growing termites, the fungus can reproduce and the interaction is transmitted horizontally resulting in a low specificity [15]. Note that nutritional associations involve basidiomycetes, while the non-nutritional ant–fungi interactions identified so far, including the present study, are associated with ascomycetes. Moreover, a striking parallel can be drawn between the Allomerus species studied here and Az. brevis [11]. Both build galleries pierced with holes and three of the fungi associated with Az. brevis are also relatives of T. cordae, suggesting the possible specialization of this group of fungi with arboreal ants.
In contrast to the specificity in the Allomerus-fungus association, the fungal community associated with the building material from Az. brevis galleries is composed of at least six distantly related species, while that associated with four European Lasius species is composed of five fungal species (three related species occur invariably; the two others, distantly related, only occasionally) [11,12]. These examples might represent different degrees of coevolutionary interdependence between ants and the fungi they use as building material. According to the geographical mosaic theory of coevolution [16], the higher the strictness of the interaction, the lower the number of species involved. The presence of one widespread haplotype and many haplotypes with a lower occurrence suggests the emergence of generalist and specialist strains that might be the product of the evolutionary dynamics of the fungus with each Allomerus species.
Acknowledgements
We are grateful to D. Reynolds for his help with the determination of the fungi; to R. Boulay for providing us with the Monomorium subopacum sample; and to A. Yockey-Dejean, K. G. Dexter and M. A. Fares for proofreading the manuscript. We would also like to thank the Laboratoire Environnement de Petit Saut for furnishing logistical help. Financial support for this study was provided by a research programme of the French Agence Nationale de la Recherche (research agreement no. ANR-06-JCJC-0109-01), by the ESF-EUROCORES/TECT/BIOCONTRACT programme, by the Programme Amazonie II of the French Center National de la Recherche Scientifique (CNRS), by the Programme interdisciplinaire ‘Interface Physique Chimie Biologie: soutien à la prise de risque’ of the CNRS, and by a Nouragues research grant from the CNRS.
References
- 1.Farrell B. D., Sequeira A. S., O'Meara B. C., Normark B. B., Chung J. H., Jordal B. H. 2001. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 55, 2011–2027 [DOI] [PubMed] [Google Scholar]
- 2.Mueller U. G., Gerardo N. M., Aanen D. K., Six D. L., Schultz T. R. 2005. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 36, 563–595 10.1146/annurev.ecolsys.36.102003.152626 (doi:10.1146/annurev.ecolsys.36.102003.152626) [DOI] [Google Scholar]
- 3.Rohfritsch O. 2008. Plants, gall midges, and fungi: a three component system. Entomol. Exp. Appl. 128, 208–216 10.1111/j.1570-7458.2008.00726.x (doi:10.1111/j.1570-7458.2008.00726.x) [DOI] [Google Scholar]
- 4.Mikheyev A. S., Mueller U. G., Abbot P. 2010. Comparative dating of Attine ant and Lepiotaceous cultivar phylogenies reveals coevolutionary synchrony and discord. Am. Nat. 175, E126–E133 10.1086/652472 (doi:10.1086/652472) [DOI] [PubMed] [Google Scholar]
- 5.Mueller U. G., Rehner S. A., Schultz T. R. 1998. The evolution of agriculture in ants. Science 281, 2034–2038 10.1126/science.281.5385.2034 (doi:10.1126/science.281.5385.2034) [DOI] [PubMed] [Google Scholar]
- 6.Weber N. A. 1966. Fungus-growing ants. Science 153, 587–604 10.1126/science.153.3736.587 (doi:10.1126/science.153.3736.587) [DOI] [PubMed] [Google Scholar]
- 7.Bailey I. W. 1920. Relations between ants and fungi. Ecology 1, 174–189 10.2307/1929134 (doi:10.2307/1929134) [DOI] [Google Scholar]
- 8.Dejean A., Solano P. J., Ayroles J., Corbara B., Orivel J. 2005. Arboreal ants build traps to capture prey. Nature 434, 973. 10.1038/434973a (doi:10.1038/434973a) [DOI] [PubMed] [Google Scholar]
- 9.Defossez E., Selosse M. A., Dubois M. P., Mondolot L., Faccio A., Djieto-Lordon C., McKey D., Blatrix R. 2009. Ants-plants and fungi: a new threeway symbiosis. New Phytol. 182, 942–949 10.1111/j.1469-8137.2009.02793.x (doi:10.1111/j.1469-8137.2009.02793.x) [DOI] [PubMed] [Google Scholar]
- 10.Maschwitz U., Hölldobler B. 1970. Der kartonnestbau bei Lasius filiginosus Latr. (Hym. Formicidae). Z. vergl. Physiol. 66, 176–189 10.1007/BF00297777 (doi:10.1007/BF00297777) [DOI] [Google Scholar]
- 11.Mayer V. E., Voglmayr H. 2009. Mycelial carton galleries of Azteca brevis (Formicidae) as a multi-species network. Proc. R. Soc. B 276, 3265–3273 10.1098/rspb.2009.0768 (doi:10.1098/rspb.2009.0768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlick-Steiner B. C., Steiner F. M., Konrad H., Seifert B., Christian E., Moder K., Stauffer C., Crozier R. H. 2008. Specificity and transmission mosaic of ant nest-wall fungi. Proc. Natl Acad. Sci. USA 105, 940–943 10.1073/pnas.0708320105 (doi:10.1073/pnas.0708320105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulsen M., Currie C. R. 2009. On ants, plants and fungi. New Phytol. 182, 785–788 10.1111/j.1469-8137.2009.02863.x (doi:10.1111/j.1469-8137.2009.02863.x) [DOI] [PubMed] [Google Scholar]
- 14.Excoffier L., Laval G., Schneider S. 2005. Arlequin v. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1, 47–50 [PMC free article] [PubMed] [Google Scholar]
- 15.Aanen D. K., Ross V. I. D., de Fine Licht H. H., Mitchell J., de Beer Z. W., Slippers B., Rouland-Lefèvre C., Boomsma J. J. 2007. Patterns of interaction specificity of fungus-growing termites and Termitomyces symbionts in South Africa. BMC Evol. Biol. 7, 115. 10.1186/1471-2148-7-115 (doi:10.1186/1471-2148-7-115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson J. N. 2005. The geographic mosaic of coevolution. Chicago, IL: The University of Chicago Press [Google Scholar]