Abstract
An intriguing new annelid, Teuthidodrilus samae (Annelida, Cirratuliformia) gen. and sp. nov., was observed and collected during deep-water column exploration of the western Celebes Sea. The Celebes Sea is a deep pocket basin, effectively isolated from surrounding deep water, and is part of the Coral Triangle, a focal area for conservation because of its high diversity and unique geological history. Collected specimens reached 94 mm in length and possessed 10 anterior appendages that were as long or longer than the body. Two characters distinguish T. samae from other polychaetes: notochaetae forming broad, concavo-convex paddles and six pairs of free-standing, oppositely branched nuchal organs. Phylogenetic analysis of five genes and a 29-character morphological matrix showed that T. samae is an acrocirrid (primarily benthic polychaetes) belonging to the morphologically diverse swimming clade. Pelagic animals within primarily benthic clades are of particular interest in evolutionary biology, because their adaptations to life in the water column inform us of the evolutionary possibilities and constraints within the clade and indirectly of the selective pressures at work in this unfamiliar habitat. This new genus illustrates how much we have to learn about even the large, abundant inhabitants of deep-pelagic communities.
Keywords: Acrocirridae, Celebes Sea, pelagic, Polychaeta, Teuthidodrilus samae
1. Introduction
The Celebes Sea (figure 1a) is a deep basin (maximum depth approx. 6200 m) located between the Philippines and Indonesia, which is thought to have formed in the Eocene (44–42 Myr ago) far from any major land mass [1]. During the Pleistocene, the basin was isolated by significantly lower sea level that exposed surrounding islands. The deep-water habitat of the Celebes basin continues to be isolated from surrounding deep water by relatively shallow sills (approx. 500 m to a maximum depth of 1350 m). Density differences inhibit water flowing over the sills from mixing with basin water, resulting in long residence times for water below 1500 m [2]. The basin is at the centre of the Coral Triangle, an area now considered a conservation hotspot owing to the high diversity and endemism of shallow-water corals and fishes [3]. This area is also the centre of geographical distributions and diversity of lanternfish, hatchetfish, dragonfish and anglerfish [4]. Based on the unique geology and history of the basin and the extreme diversity of the shallow-water fauna, the hypothesis that the deep fauna may be equivalently diverse and unique was a motivation for the exploratory expedition to the Celebes Sea.
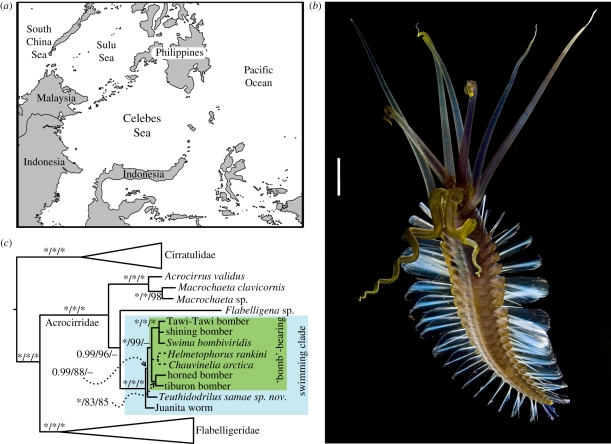
Figure 1.
(a) Map of Celebes Sea and surroundings. (b) Ventro-lateral view of live paratype 3 or 4. Photograph, Michael Aw 2007. Scale bar, 15 mm. (c) Simplified version of the tree from Osborn & Rouse [12] showing the phylogenetic position of T. samae gen. and sp. nov. (Online version in colour.)
Animal density typically decreases with depth in the open ocean but then increases again when approaching the deep-sea floor [5–7]. The concentration of mobile animals within a few hundred metres of the seafloor forms a diverse demersal community that we know little about. Many organisms found in this broad demersal or bentho-pelagic zone (the bottom several hundred metres that is influenced by the seafloor) have long been inaccessible for two main reasons. First, traditional sampling gear was designed to collect benthic (living on the seafloor) or pelagic (living in the water column) animals. Swimming organisms can escape collection devices towed on the seafloor and midwater nets are not often towed near the seafloor at great depth because of the risk of damaging the relatively fragile gear [8]. Second, many of the community's animals are delicate and thus damaged beyond usefulness by indiscriminate sampling gear and the long trip back to the surface [9]. Development of vehicles capable of free operation in the deep-water column has allowed direct access to deep-pelagic habitats. These vehicles have the unique ability to observe animals undisturbed in their natural habitat and to collect them without damage, and thus are providing a more complete picture of the inhabitants of the largest habitat on the Earth [10–11].
Here, we introduce Teuthidodrilus samae (figure 1b), an unusual new genus and species of swimming polychaete from the deep bentho-pelagic zone of the Celebes Sea discovered by direct observation with a remotely operated vehicle. We also present notes on this species' behaviour and ecology in the electronic supplementary material. Based on combined molecular and morphological analyses, T. samae gen. and sp. nov. belongs to Acrocirridae, is a member of the swimming clade (figure 1c) and is sister to the ‘bomb’-bearing clade [12]. Teuthidodrilus samae gen. and sp. nov. is an example of the type of discoveries we can anticipate with continued exploration of the least known and largest habitat on the Earth, the deep-water column.
2. Material and methods
Seven specimens of T. samae were collected from the deep-water column of the Celebes Sea in October of 2007 (table 1). All in situ observations were made with the remotely operated vehicle Max Rover Global Explorer operated from Philippines research vessel BRP Hydrographer Presbitero. In situ video was taken with a Panasonic high-definition camera. Specimens were captured in 6.5 l detritus samplers or with a high-flow suction sampler [13]. Live specimens were photographed immediately, then relaxed in magnesium chloride and fixed in formalin. Tissue removed from three animals was placed in chilled 95 per cent ethanol for genetic analysis.
Table 1.
Teuthidodrilus samae gen. and sp. nov. collection information.
| number | accession number | depth (m) | length (mm) live, fixed | date | locality | number of chaetigers | nuchal shape |
|---|---|---|---|---|---|---|---|
| holotype | NMA 04342 | 2830 | 90, 65 | 10 Oct 2007 | 4°57′43″ N, 120°09′42″ E | 25 | smooth |
| paratype 1 | SIO-BIC A2250 | approx. 2500–2800 | —, 38 | 6 Oct 2007 | 4°58′00″ N, 120°14′36″ E | 25 | smooth |
| paratype 2 | SIO-BIC A2251 | 2912 | 20, 10+ | 10 Oct 2007 | 4°57′43″ N, 120°09′42″ E | 17+ | unknown |
| paratype 3 | SIO-BIC A2252 | 2259 | 65, 60 | 12 Oct 2007 | 4°42′37″ N, 120°07′30″ E | 25 | smooth |
| paratype 4 | SIO-BIC A2253 | 2028 | 94, 84 | 12 Oct 2007 | 4°42′37″ N, 120°07′30″ E | 32 | frilly |
| paratype 5 | MCZ IZ 99582 | approx. 2500–2800 | — | 6 Oct 2007 | 4°58′00″ N, 120°14′36″ E | — | frilly |
| paratype 6 | MCZ IZ 99583 | 2039 | —, 85 | 10 Oct 2007 | 4°57′43″ N, 120°09′42″ E | — | unknown |
Specimens were examined in the laboratory with light and scanning electron microscopy. Dissected branchiae and chaetae were embedded in Spurr's resin, sectioned and stained with toluidine blue for histological examination. Five genes (18S, 28S, 16S, COI and CytB) were sequenced and a 29-character morphological matrix generated and analysed both individually and in combination in order to determine phylogenetic relationships of this and six other recently discovered swimming species to other cirratuliform polychaetes [12].
3. Systematics
Teuthidodrilus samae, new genus and species (figures 1b and 2).
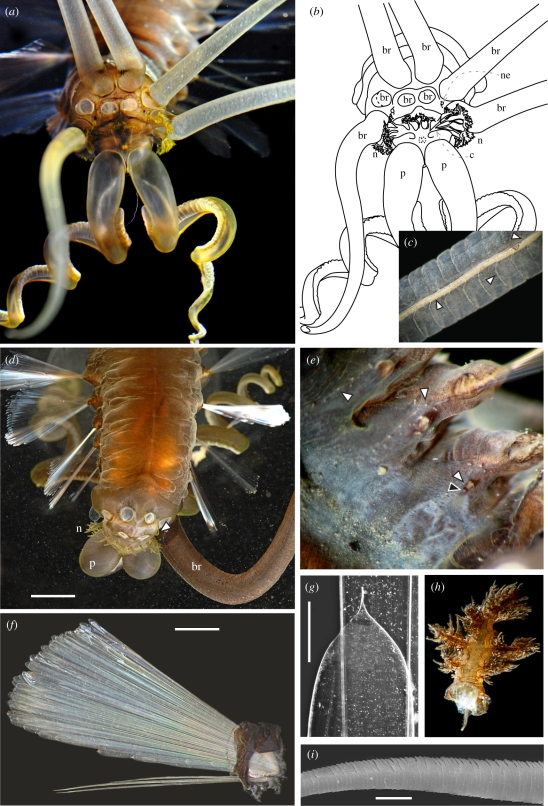
Figure 2.
Teuthidodrilus samae, gen. and sp. nov. (a and b) Anterior view of paratype 5 showing five attached branchiae (br), nephridiopores (black papillae, ne), branched nuchal organs (n) and the ciliary ridge joining them (c), and palps (p). (c) Shaft of elongate branchia from holotype showing longitudinal vessel (lower arrow), circular vessels (centre arrow) and nerve (upper arrow). (d) Dorsal view of holotype showing attached palps, branchial scars, branched nuchal organs, bubbled remains of the gelatinous sheath and nephridiopore (arrow). (e) Ventral view of paratype 3 showing notopodial (right) and neuropodial lobes (that of third chaetiger, lower white arrow), gonopore (black arrow), row of papillae (top right arrow) between noto- and neuropodial lobes and row of papillae (top left arrow) lateral across ventrum of segment. (f) Parapodium from holotype showing notochaetal fan (top) and neurochaetae (bottom). (g) Tip of notochaeta from holotype; 10× magnification is not sufficient to resolve the spinous nature of the distal tip. (h) Dissected free-standing, nuchal structure from paratype 4, frilly. (i) Neurochaeta distal shaft from holotype seen with scanning electron microscopy. Scale bars, d = 4 mm, f = 3 mm, g = 0.2 mm and i = 10 µm. (Online version in colour.)
(a). Diagnosis
A member of Acrocirridae having a pair of grooved palps longer than body. Branchial membrane dorsal, with four pairs of elongate, tapered branchiae equal in length to the body. Nuchal organs as ciliated ridge continuous on six pairs of free-standing, oppositely branched structures. Neuropodia contain two to four simple chaetae, which are flattened in the distal three-quarters. Prominent notopodial lobes contain 50 or more simple, flattened, concavo-convex chaetae that taper abruptly at the tip forming a fine point. All chaetae with fine rings of tiny spines, most obvious at distal tips. First chaetiger with reduced number of chaetae, and no obvious achaetous anterior segments. Adults with 25 or more chaetigers. Papillae small, clavate, reduced to a single lateral row on the ventrum of each segment and a small longitudinal row between noto- and neuropodial lobes. Gonopores as broad papillae immediately ventral to neuropodia on the second and third chaetigers. Body wall darkly pigmented and gelatinous sheath thin.
(b). Holotype and paratypes
The holotype female was collected off Tawi-Tawi, Philippines, October 2007, at 2830 m in 2950 m deep water and is deposited at the National Museum of the Philippines (NMA 04342; table 1). Four paratypes are deposited at the Scripps Institution of Oceanography Benthic Invertebrate Collection (SIO-BIC A2250–A2253) and two are deposited at the Museum of Comparative Zoology, Harvard University (MCZ IZ 99582–99583).
(c). Brief description of holotype
Body brown in live specimen, black when preserved. Thin gelatinous sheath. Total preserved body length 65 mm (90 mm before preservation), and body width 10 mm, with 25 chaetigers. Body papillae few, small, clavate.
Prostomium reduced to tissue supporting nuchal organs. Nuchal organs begin anteriorly as a laterally opening, horseshoe-shaped ciliate ridge that continues posteriorly to loop up each major branch of the lateral-most branched free-standing structures, then back down towards the medial line of the head and back, and up each successively more medial pair of free-standing nuchal structures, finally meeting between the medial-most pair of free-standing structures (figure 2d, shown in paratype figure 2a,b; orange ridge visible in figure 2h). Grooved palps frontal, coiled. Mouth antero-ventrally located, pharnynx unarmed. Four pairs of elongate, tapered branchiae attached in two rows posterior to the nuchal organs, longest at least 68 mm in length. Anterior nephridiopores as pair of broad-based, darkly pigmented papillae lateral to antero-medial-most branchiae (black dots seen in paratype figure 2a).
Chaetigers with prominent notopodia, distinct from neuropodia, supporting greater than 50 chaetae, except the first chaetiger with less than 10. Notochaetae external length up to 15 mm, simple, broad, flattened, concavo-convex paddles tapering abruptly to a fine point at the distal tip; distal-most margins with fine spines in tightly spaced rows. Neuropodial lobes small, rounded projections, supporting two to four simple chaetae with a round cross section at the base that flattens to a fine-tipped, tapered blade distally, with tiny, closely spaced rows of spines along the entire length (figure 2i).
Internal anatomy somewhat visible through the partially transparent body of live specimens. Ventrally located double nerve cord with two pairs of fused ganglia per segment. Gut forms three loops within chaetigers 2–6. Heart body present. Large efferent, afferent and circular vessels supply elongate branchiae, innervation with identical arrangement (figure 2c). Single pair anterior nephridia extend back to the fifth chaetiger. Gonads in chaetigers 2–4 consisting of grape-like clusters of variously sized, beige ova, maximum size nearly 1 mm. Septa complete. Detailed description, etymology, ecological information, and discussion of variation between specimens and taxonomic remarks available in the electronic supplementary material.
4. Discussion
The relative inaccessibility of the deep sea has left most of its vast spaces unexplored, so discovery of new species is seldom surprising. The unusual morphology, large size, numerous observations (16 within seven dives), behaviour and phylogenetic position of T. samae are however a surprise. How could such an animal evade collection until now? We believe that the immense volume of deep, pelagic habitat, the difficulty of sampling deep demersal communities and T. samae's ability to swim away from towed observational or sampling gear probably all contributed to its long seclusion.
Although currently monotypic, we do not expect the genus to remain so as exploration with submersibles continues in other areas. A similar animal was observed off western India (10°11′80″ N, 75°30′80″ E) by the submersible Hercules 7 in October of 2004. That single observation (specimen not collected) was made at 1005 m depth and was recorded by the SERPENT project (http://archive.serpentproject.com/231/). Observable differences in swimming behaviour, posture (see video included in the electronic supplementary material), and observation depth (2000–3000) suggest that the Indian Ocean animal may represent a second species of Teuthidodrilus.
The numerous T. samae observations collected within just a few dives and all within 100 m of the seafloor suggest that this animal is a common member of the deep bentho-pelagic community of the Celebes Sea basin. The video obtained allows speculation on the ecology (a suspension feeder using large aggregates of marine snow) and insight into the mechanics of swimming. This discovery illustrates how much we have to learn about even the large, common inhabitants of deep-pelagic communities.
Acknowledgements
Special thanks to the science party of Exploring the Inner Space of the Celebes Sea 2007 who were involved in the ROV operations and collection of polychaete specimens, Russ Hopcroft, Erich Horgan, Bill Hamner and Emory Kristof. Thank you to the captain and crew of the BRP Hydrographer Presbitero and the pilots of Max Rover Global Explorer, particularly Joe Caba for his superb collection skills. Thanks to Michael Aw for use of his photographs and to Drs Filemon and Mely Romero for liaison with authorities in Tawi-Tawi. Funding was provided to L.P.M. by grants from NOAA's Office of Ocean Exploration and WHOI Ocean Life Institute, with additional support from the National Geographical Society. The University of California President's Postdoctoral Fellowship provided funding to K.J.O. Thanks to Bruce Robison, Fredrik Pleijel and Adrian Glover for comments that improved this manuscript and to Linda Kuhnz for assistance with figure 1a.
References
- 1.Nichols G., Hall R. 1999. History of the Celebes Sea based on its stratigraphic and sedimentological record. J. Asian Earth Sci. 17, 47–59 10.1016/S0743-9547(98)00034-8 (doi:10.1016/S0743-9547(98)00034-8) [DOI] [Google Scholar]
- 2.Gordon A., Giulivi C. F., Gani Ilahude A. 2003. Deep topographic barriers within the Indonesian seas. Deep Sea Res. 50, 2205–2228 10.1016/S0967-0645(03)00053-5 (doi:10.1016/S0967-0645(03)00053-5) [DOI] [Google Scholar]
- 3.Allen G. R. 2008. Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 18, 541–556 10.1002/aqc.880 (doi:10.1002/aqc.880) [DOI] [Google Scholar]
- 4.Robison B. H., Hamner W. M. 2009. Pocket basins and deep-sea speciation. In Encyclopedia of islands (eds Gillespie R., Clague D. A.), pp. 755–757 Berkeley, CA: University of California Press [Google Scholar]
- 5.Wishner K. F. 1980. The biomass of the deep-sea benthopelagic plankton. Deep Sea Res. 27, 203–216 10.1016/0198-0149(80)90012-6 (doi:10.1016/0198-0149(80)90012-6) [DOI] [Google Scholar]
- 6.Smith K. L., Kaufmann R. S., Edelman J. L., Baldwin R. J. 1992. Abyssoplagic fauna in the central North Pacific: comparison of acoustic detection and trawl and bated trap collections to 5800 m. Deep Sea Res. 39, 659–685 10.1016/0198-0149(92)90094-A (doi:10.1016/0198-0149(92)90094-A) [DOI] [Google Scholar]
- 7.Robison B. R., Sherlock R. E., Reisenbichler K. R. 2010. The bathypelagic community of Monterey Canyon. Deep Sea Res. 57, 1551–1556 10.1016/j.dsr2.2010.02.021 (doi:10.1016/j.dsr2.2010.02.021) [DOI] [Google Scholar]
- 8.Robison B. H. 2004. Deep pelagic biology. J. Exp. Mar. Biol. Ecol. 300, 253–272 10.1016/j.jembe.2004.01.012 (doi:10.1016/j.jembe.2004.01.012) [DOI] [Google Scholar]
- 9.Haddock S. H. D. 2004. A golden age of gelata: past and future research on planktonic ctenophores and cnidarians. Hydrobiologia 530/531, 549–556 10.1007/s10750-004-2653-9 (doi:10.1007/s10750-004-2653-9) [DOI] [Google Scholar]
- 10.Vecchione M., et al. 2001. Worldwide observations of remarkable deep-sea squids. Science 294, 2505. 10.1126/science.294.5551.2505 (doi:10.1126/science.294.5551.2505) [DOI] [PubMed] [Google Scholar]
- 11.Eschmeyer W. N., Fricke R., Fong J. D., Polack D. A. 2010. Marine fish diversity: history of knowledge and discovery (Pisces). Zootaxa 2525, 19–50 [Google Scholar]
- 12.Osborn K. J., Rouse G. W. In press Phylogenetics of Acrocirridae and Flabelligeridae (Cirratuliformia, Annelida). Zool. Scr. 10.1111/j.1463-6409.2010.00460.x (doi:10.1111/j.1463-6409.2010.00460.x) [DOI] [Google Scholar]
- 13.Robison B. H. 1992. Midwater research methods with MBARI's ROV. Mar. Technol. Soc. J. 26, 32–39 [Google Scholar]