Abstract
In many species, females display brightly coloured and elaborate traits similar to those that males use in intra- and inter-sexual selection processes. These female characters are sometimes related to fitness, and might function as secondary sexual characteristics that have evolved through sexual selection. Here, we used descriptive data from 674 females in 10 populations and an experimental removal of Trichostrongylus tenuis parasites in four populations, to examine the effects of season, age, condition, and parasites on the size of supra-orbital combs displayed by female red grouse Lagopus lagopus scoticus. We found that comb size (i) was greater during the breeding than the non-breeding season, (ii) was greater in adult than in young females, (iii) was positively correlated with body condition, and (iv) negatively correlated with parasite abundance. Experimentally, we showed that comb size increased proportionally to the number of worms removed after parasite dosing. Our findings provide a better understanding of proximate mechanisms behind the expression of a male-like trait in females, and we discuss its possible function as a female ornament.
Keywords: female, comb size, Trichostrongylus tenuis, red grouse, body condition
1. Introduction
Studies examining why animals display conspicuous or elaborate traits have traditionally focused on males [1]. By contrast, female traits have often been considered to be non-functional by-products of sexual selection processes acting on males, with females not gaining any selective benefit from displaying these characters [2]. However, several alternative functional hypotheses have also been put forward to explain elaborate traits in females [3,4]. Among them, the sexual selection hypothesis [3] is receiving growing attention. It predicts that the expression of elaborate traits in females helps mates or competitors assess individual quality [3,4]. Yet the link between trait expression and individual quality (e.g. condition, parasite abundance) in females has received limited empirical support, and has not been tested in natural conditions. This link is crucial for the validity of this hypothesis.
Parasites deplete resources from their hosts, thus creating trade-offs when hosts invest limited resources among different fitness-related traits [5]. For instance, parasites may reduce body condition [6], survival [7–9], reproductive output [9] and, ultimately, fitness [10]. In females, previous studies have suggested that parasites influence body condition and reproduction [11], which have also been linked to the expression of conspicuous traits [12–15]. Therefore, parasites might impose a physiological cost on females that could ultimately limit the expression of their conspicuous traits [13,16]. However, in the absence of experimental validation, previous studies provide inconclusive evidence that parasites mediate the expression and condition-dependence of conspicuous traits in females (but see [17,18]).
Here, we first describe the temporal (breeding versus non-breeding season), age- and condition-dependent variation of comb size in female red grouse. We also analyse correlatively and experimentally the relationship between comb size and infection by the intestinal nematode Trichostrongylus tenuis, a parasite known to reduce reproductive output and survival in red grouse [9]. According to the sexual selection hypothesis, traits should be indexes of individual quality in sexual contexts [3], so we expect that comb size should be greater during the breeding season, and in adult females compared with first-year females. Also, to provide support to the suggestion that sexual selection drives the evolution of elaborate female traits, it should be expected that: (i) comb size would correlate positively with body condition and negatively with parasite abundance; and (ii) experimental parasite reductions would increase comb size and body condition.
2. Material and methods
(a). Correlative data
In 2001–2005 and 2009–2010, we captured 674 females from 10 populations in spring (n = 544) and autumn (n = 130) in the UK (see electronic supplementary material). Each was individually ringed and aged as adult or first-year females [19]. We measured body mass (nearest value in grams), wing length (nearest millimetre) and comb size (comb length × width, square millimetre—see electronic supplementary material). Females were kept overnight in individual boxes in order to collect faecal samples to estimate T. tenuis abundance (see below).
(b). Parasite abundance
We estimated T. tenuis abundance by determining parasite egg concentration in red grouse faecal samples, using a standard method previously validated for this species [20,21].
(c). Experiment
In spring 2009, we caught 47 females from four populations. Each was fitted with a radio collar (TW3-necklace tag, Biotrack) and randomly assigned to one of two treatments: dosed with anthelmintic (n = 27) or control (n = 20). Control and dosed females were orally given 1 ml of water or 1 ml of anthelmintic (Levamisole hydrochloride, Nilverm Gold), respectively (see electronic supplementary material). We re-captured 43 females (dosed, n = 26; control, n = 17) 21 ± 3 days after treatment. Upon each capture, we measured comb size, body mass and wing length, and collected faecal samples for estimating parasite abundance.
(d). Statistical analyses
We used SAS v. 9.1. For correlative data, female comb size and body mass were the dependent variables, with T. tenuis abundance and body mass as covariates and with age and season as fixed factors. Body condition was also analysed by including body mass with wing length as a covariate.
Date of capture was included as a covariate and the interactions between age or season and the studied variables were tested; these variables were removed from the models if they were not significant. We collected data for more than one year per site, so we included the year nested within site as a random variable in all models. If females were captured more than once, we only included the data from the first capture. Sample size differs between analyses because some variables were not measured or age was not determined for some females.
For the experiment, we used general linear mixed models. We completed two sets of analyses with treatment (dosed versus control), age (young versus adult) and their interactions included as fixed effects. Site was included as a random variable. When testing for treatment effects, we included ‘female’ as a random effect to account for repeated measures and re-capture (before versus after treatment) as a factor. In a first model, comb size or body mass were the dependent variables. In a second set of models, we calculated individual changes in comb size and body mass between first and second captures, and included them as response variables. We used changes in T. tenuis before and after treatment as an explanatory variable with initial parasite levels as a fixed effect to correct for initial values. In these models, body condition was calculated as the residuals of the regression between body mass and wing length. The difference of the residuals before and after treatment was included as the change in body condition. Sample size may vary because some individuals did not produce the faecal samples necessary to assess parasite abundance.
3. Results
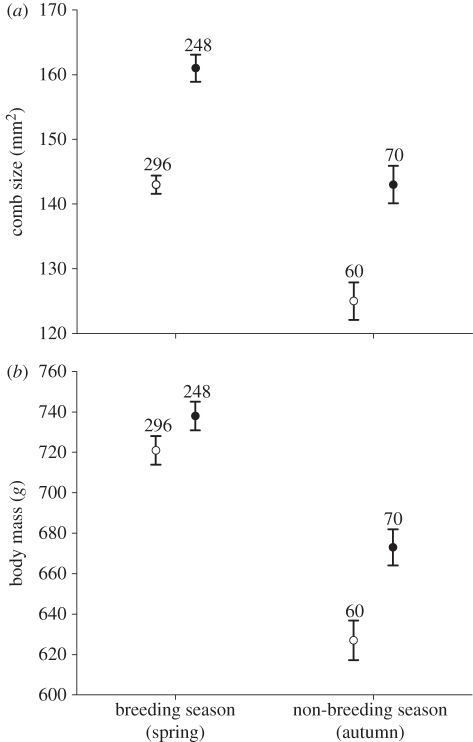
Females had bigger combs in spring than in autumn (F1,656 = 15.39, p < 0.001) and adult females had larger combs than young females (F1,656 = 39.03, p < 0.001; figure 1) independent of season (F1,328 = 0.03, p = 0.629). Comb size was negatively associated with T. tenuis worm abundance (estimate = −0.002 ± 0.0007, F1,329 = 7.63, p = 0.006) and positively with body mass (estimate = 0.060, F1,329 = 5.19, p = 0.023), once correcting by season and age (both p < 0.001). Any other interactions between the variables included in the initial models were not significant (all p > 0.245).
Figure 1.
Comparison of (a) comb size and (b) body mass of female red grouse according to season and age. Filled circles, adult females; open circles, young females.
Body mass variation was explained by age (F1,329 = 21.78, p < 0.001; figure 1), season (F1,329 = 72.86, p < 0.001), and T. tenuis abundance (estimate = −0.003 ± 0.001, F1,329 = 6.13, p = 0.014). Including wing length as a covariate did not change the significance of the results (all p < 0.041). Adult females were heavier than young females in spring and in autumn, although the difference was higher in autumn (F1,329 = 10.76, p = 0.001).
Prior to treatment, comb size, body mass, parasite abundance and capture date did not differ between control and dosed females (all p > 0.290). Dosing was effective at purging parasites (F1,108 = 28.36, p < 0.001) and this effect was independent of site and age (both p > 0.236).
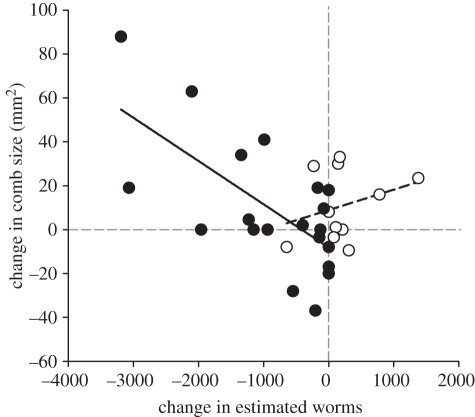
The difference in comb size before and after treatment was not affected by dosing (F1,39 = 0.04, p = 0.851) regardless of female age (F1,28 = 0.70, p = 0.410). However, at the individual level, dosing resulted in a relationship between individual changes in comb size and changes in parasite abundance (F1,30 = 6.25, p = 0.019; figure 2): a greater reduction in parasite abundance was associated with a greater increase in comb size in dosed females (estimate = −0.019 ± 0.005, F1,19 = 14.41, p = 0.003), but not in control females (estimate = 0.009 ± 0.009, F1,10 = 0.90, p = 0.367). Including body mass as a covariate did not change this result (F1,30 = 5.39, p = 0.028; body mass F1,30 = 1.17, p = 0.224).
Figure 2.
Relationship between the change in T. tenuis abundance and the change in comb size according to treatment. Open circles, control females; filled circles, dosed females.
Parasite dosing did not influence changes in body mass (F1,39 = 0.32, p = 0.573) or condition (F1,37 = 0.06, p = 0.808) between captures. Individual changes in body mass were not explained by changes in worm burdens between groups (F1,31 = 0.47, p = 0.501). However, changes in body mass over time were related to initial parasite levels (F1,33 = 3.80, p = 0.048): the increase in body mass between captures was smaller when initial levels of parasites were high in control birds (estimate = −0.142 ± 0.057, F1,12 = 5.99, p = 0.034), but not in dosed ones (F1,20 = 0.29, p = 0.598). This was not the case for body condition (F1,31 = 0.43, p = 0.515).
4. Discussion
Our results demonstrate that female comb size is condition-dependent and limited by intestinal parasites in red grouse. We found that comb size positively covaried with different indexes of individual quality, such as age and body mass. Adult and heavier females had bigger combs throughout the year. The cause of this age-dependent variation remains unclear because it is difficult to determine whether ageing affects trait expression or whether individuals with enhanced traits are more likely to survive [22]. The positive relationship between comb size and body mass is consistent with previous findings in female birds (reviewed in [3]), and supports the hypothesis that expression of the studied trait is condition-dependent. Female combs are also bigger during the breeding period, as predicted for traits functioning as indicators of quality in sexual contexts [23]. The mechanism underlying this pattern remains unknown, but sexual hormones whose concentrations increase during the breeding period could have mediating effects on the expression of this trait. Altogether, our descriptive results indicate that comb size could be used as a cue to indicate female quality: larger combs could signal an older age, better condition and fewer parasites.
These results were in part confirmed by our experiment. Although we did not find a significant difference in comb size between females in the control and dosed groups, we did find that comb size at the individual level increased in proportion to the number of parasites removed by treatment. This suggests that parasites constrain comb size in females, and that they may do so above a given threshold of parasite abundance. This highlights the possibility that the relationship between parasites and comb size may be complex and nonlinear. We also did not find any treatment-level effects on body mass, but changes in body mass were mediated by parasites in control females: their mass changed proportionately to the number of parasites that they had at the beginning of the experiment, with greater increases observed when initial parasite levels were low. However, changes in body mass in dosed females were not associated with the number of T. tenuis. Therefore, parasites might limit the expected increase in body mass before breeding, potentially compromising female fecundity [9]. Overall, our results are consistent with the hypothesis that parasites mediate the condition-dependency of female comb size.
In conclusion, our correlative and experimental results are consistent with the hypothesis that female quality can be inferred from comb size. Our results do not allow us to rule out the effect of genetic correlation between sexes. Instead, our results support the suggestion that sexual selection deserves to be further considered as a driver of the evolution of elaborate traits in females. If comb size is a reliable and honest signal in females, it might be used within intra- or inter-sexual selection contexts. This possibility should now be tested, as there is growing evidence suggesting that elaborate female traits can evolve by sexual selection, rather than exclusively being a by-product of selection acting on males because of genetic correlations.
Acknowledgements
We held all the necessary UK Home Office licenses for conducting these procedures (PPL 60/3824).
We are grateful to the owners and gamekeepers, the British Army, Royal Society for Protection of Birds and English Natural Heritage for allowing us to conduct the work on their grouse moors. This study was funded by a Natural Environment Research Council grant (NE/D014352/1).
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 10.2307/2407393 (doi:10.2307/2407393) [DOI] [PubMed] [Google Scholar]
- 3.Amundsen T., Pärn H. 2006. Bird Coloration, vol. II (eds Hill G. E., McGraw K. J.), pp. 280–345 Cambridge, MA: Harvard University Press [Google Scholar]
- 4.Kraaijeveld K., Kraaijeveld-Smit F. J. L., Komdeur J. 2007. The evolution of mutual ornamentation. Anim. Behav. 74, 657–677 10.1016/j.anbehav.2006.12.027 (doi:10.1016/j.anbehav.2006.12.027) [DOI] [Google Scholar]
- 5.Clayton D., Moore J. 1997. Host-parasite evolution: general principles and avian models. Oxford, UK: Oxford University Press [Google Scholar]
- 6.Booth D., Clayton D. H., Block B. A. 1993. Experimental demonstration of the energetic cost of parasitism in free-ranging hosts. Proc. R. Soc. Lond. B 253, 125–129 10.1098/rspb.1993.0091 (doi:10.1098/rspb.1993.0091) [DOI] [Google Scholar]
- 7.Hudson P. J., Dobson A. P., Newborn D. 1998. Prevention of population cycles by parasite removal. Science 282, 2256–2258 10.1126/science.282.5397.2256 (doi:10.1126/science.282.5397.2256) [DOI] [PubMed] [Google Scholar]
- 8.Hanssen S. A., Hasselquist D., Folstad I., Erikstad K. E. 2004. Costs of immunity: immune responsiveness reduces survival in a vertebrate. Proc. R. Soc. Lond. B 271, 925–930 10.1098/rspb.2004.2678 (doi:10.1098/rspb.2004.2678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson P. J. 1986. The effect of a parasitic nematode on the breeding production of red grouse. J. Anim. Ecol. 55, 85–92 10.2307/4694 (doi:10.2307/4694) [DOI] [Google Scholar]
- 10.Møller A. P., Allander K., Dufva R. 1990. Population biology of passerine birds: an integrated approach (eds Blondel J., Gosler A. G., Lebreton J. D., McCleery R. H.), pp. 269–280 Berlin, Germany: Springer [Google Scholar]
- 11.Ilmonen P., Taarna T., Hasselquist D. 2000. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc. R. Soc. Lond. B 267, 665–670 10.1098/rspb.2000.1053 (doi:10.1098/rspb.2000.1053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velando A., Lessells C. M., Márquez J. C. 2001. The function of female and male ornaments in the Inca Tern: evidence for links between ornament expression and both adult condition and reproductive performance. J. Avian Biol. 32, 311–318 10.1111/j.0908-8857.2001.320404.x (doi:10.1111/j.0908-8857.2001.320404.x) [DOI] [Google Scholar]
- 13.Potti J., Merino S. 1996. Decreased levels of blood trypanosome infection correlate with female expression of a male secondary sexual trait: implications for sexual selection. Proc. R. Soc. Lond. B 263, 1199–1204 10.1098/rspb.1996.0176 (doi:10.1098/rspb.1996.0176) [DOI] [Google Scholar]
- 14.Morales J., Moreno J., Merino S., Sanz J. J., Tomá G., Arriero E., Lobato E., Martínez-De La Puente J. 2007. Female ornaments in the pied flycatcher Ficedula hypoleuca: associations with age, health and reproductive success. Ibis 149, 245–254 10.1111/j.1474-919X.2006.00635.x (doi:10.1111/j.1474-919X.2006.00635.x) [DOI] [Google Scholar]
- 15.Doutrelant C., Grégoire A., Grnac N., Gomez D., Lambrechts M. M., Perret P. 2008. Female coloration indicates female reproductive capacity in blue tits. J. Evol. Biol. 21, 226–233(doi:10.1111/j.1420-9101.2007.014511.x) [DOI] [PubMed] [Google Scholar]
- 16.Ezenwa V. O., Jolles A. E. 2008. Horns honestly advertise parasite infection in male and female African buffalo. Anim. Behav. 75, 2013–2021 10.1016/j.anbehav.2007.12.013 (doi:10.1016/j.anbehav.2007.12.013) [DOI] [Google Scholar]
- 17.Fitze P. S., Richner H. 2002. Differential effects of a parasite on ornamental structures based on melanins and carotenoids. Behav. Ecol. 13, 401–407 10.1093/beheco/13.3.401 (doi:10.1093/beheco/13.3.401) [DOI] [Google Scholar]
- 18.Hill G. E. 2002. A red bird in a brown bag. The function and evolution of colorful plumage in the house finch. New York, NY: Oxford University Press [Google Scholar]
- 19.Cramp S., Simmon K. E. L. 1980. The birds of the Western Paleartic. Oxford, UK: Oxford University Press [Google Scholar]
- 20.Seivwright L., Redpath S. M., Mougeot F., Watt L., Hudson P. J. 2004. Faecal egg counts provide a reliable measure of Trichostrongylus tenuis intensities in free-living red grouse Lagopus lagopus scoticus. J. Helmintol. 78, 69–76 10.1079/JOH2003220 (doi:10.1079/JOH2003220) [DOI] [PubMed] [Google Scholar]
- 21.Seivwright L. J., Redpath S. M., Mougeot F., Leckie F., Hudson P. J. 2005. Interactions between intrinsic and extrinsic mechanisms in a cyclic species: testosterone increases parasite infection in red grouse. Proc. R. Soc. B 272, 2299–2304 10.1098/rspb.2005.3233 (doi:10.1098/rspb.2005.3233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gil D., Cobb J. L. S., Slater P. J. B. 2001. Song characteristics are age dependent in the willow warbler, Phylloscopus trochilus. Anim. Behav. 62, 689–694 10.1006/anbe.2001.1812 (doi:10.1006/anbe.2001.1812) [DOI] [Google Scholar]
- 23.Pérez-Rodríguez L. 2008. Carotenoid-based ornamentation as a dynamic but consistent individual trait. Behav. Ecol. Sociobiol. 62, 995–1005 10.1007/s00265-007-0527-7 (doi:10.1007/s00265-007-0527-7) [DOI] [Google Scholar]