Abstract
The tendency of females to mate with multiple males is often explained by direct and indirect benefits that could outweigh the many potential costs of multiple mating. However, behaviour can only evolve in response to costs and benefits if there is sufficient genetic variation on which selection can act. We followed 108 mating chases of 85 North American red squirrels (Tamiasciurus hudsonicus) during 4 years, to measure each female's degree of multiple male mating (MMM), and used an animal model analysis of our multi-generational pedigree to provide what we believe is the first estimate of the heritability of MMM in the wild. Female red squirrels were highly polyandrous, mating with an average of 7.0 ± 0.2 males on their day of oestrus. Although we found evidence for moderate levels of additive genetic variation (CVA = 5.1), environmental variation was very high (CVE = 32.3), which resulted in a very low heritability estimate (h2 < 0.01). So, while there is genetic variation in this trait, the large environmental variation suggests that any costs or benefits associated with differences among females in MMM are primarily owing to environmental and not genetic differences, which could constrain the evolutionary response to natural selection on this trait.
Keywords: animal model, heritability, genetic variation, multiple mating, polyandry, promiscuity
1. Introduction
Male reproductive success is typically not limited by numbers of gametes, time or energy, but by the number of successful copulations [1]. In contrast, the advantages of multiple mating for females are less immediately obvious, particularly in mammals, because of constraints related to gamete number and maternal care [2]. Females who copulate with multiple males may also experience immune suppression, physical damage and harassment by males, increased risk of sexually transmitted disease, decreased foraging efficiency and increased predation risk [3]. Nevertheless, there are many species in which females engage in multiple male mating (MMM; [4]).
The potential costs of MMM have led to many hypothesized direct or indirect benefits that might explain the persistence of an otherwise costly trait [4–6]. MMM by females could also be affected by selection for increased numbers of copulations in males, either because MMM by females is functionally required for increased male copulation rates [7], or because MMM by females and copulation rates in males are genetically correlated [8]. However, MMM might also persist in natural populations despite being costly, because of insufficient genetic variation for the reduction of MMM to evolve. Previous studies of captive insects have found evidence for large broad-sense heritabilities, but in the few cases in which maternal effects were controlled the narrow-sense heritability of MMM was found to be quite small (reviewed in [9]). Heritability estimates can also depend on the particular environment in which they are measured [10] and estimates from the laboratory are typically greater than those estimated in the wild (e.g. [11]). As a result, estimates are needed of the genetic basis to phenotypic variation in MMM expressed in the natural environment, in which selection acts on MMM. We, therefore, calculated the heritability of MMM for female North American red squirrels (Tamiasciurus hudsonicus) in the southwest Yukon of Canada. Red squirrels are highly promiscuous, with females mating with an average of 6.9 males during a single day of oestrus [12]. We used behavioural observations of a free-ranging squirrel population to document the number of males with whom females copulated while in oestrus, and estimated the heritability of MMM using an ‘animal model’ analysis [13] of a multi-generational pedigree for this same population.
2. Material and methods
A wild population of red squirrels (Tamiasciurus hudsonicus) was monitored in southwestern Yukon (61° N, 138° W) from 1989 to 2009 [14] to establish a complete pedigree for 6512 individuals [12]. In 2003, 2004, 2005 and 2008, individual female squirrels were monitored during their mating chase, and attending males were identified through unique coloured markings attached to their ear tags [12]. Copulations were recorded when seen, heard or when a male and female spent at least 60 s underground. Indices were used for the number of mates and the number of males who attended the mating chase in order to account for the time when a female was out of sight. The index was calculated as the number of mates (or number of attending males), divided by the product of the total time observed and the proportion of intervals the female was in sight [12].
We estimated the heritability of MMM using an animal model based on a Markov chain Monte Carlo for generalized linear mixed models (MCMCglmm) analysis in the R statistical package [15,16]. This approach allowed us to estimate the additive genetic variance, maternal effects, and permanent and residual environmental variance of MMM using data from 108 mating chases by 85 squirrels for whom the relationship matrix was determined from our entire multi-generational pedigree (see electronic supplementary material, table S1). The number of attending males and year were included as fixed effects in the model, although our results were unaffected by their exclusion (see electronic supplementary material, table S2). We had repeated observations for a relatively small number of females, but we retained the additional random effect for female identity to avoid overestimating the additive genetic variance [17]. Maternal effects were estimated to be small and did not substantially improve the model so they were excluded, but this did not affect our overall conclusions (see electronic supplementary material, table S3). MCMCglmm follows a Bayesian framework [15] so the requisite priors were assigned to be twice the regression coefficient from a mother–daughter regression [18], in which we controlled for the number of attendant males and the year in which the data were collected. However, using priors based on a heritability as high as 0.85, which was found for the remating interval of Pieris napi [19], did not affect our conclusions (see electronic supplementary material for details on prior specification and model assessment). We report 95% CI as an indication of our uncertainty around our estimates of variance components and heritabilities even though these are constrained to be positive in MCMCglmm. We assessed the significance of variance components by comparing the deviance information criteria (DIC) of our nested models.
3. Results
The mean MMM index was 1.02 ± 0.04 males per hour of observation with a mean attendant male index of 1.63 ± 0.04 males per hour. This MMM index corresponded to an average of 7.0 ± 0.2 males mated by each female on her single day of oestrus (following [12]). The fixed effects (consisting of attendant males and year as a factor) explained 40.2 per cent of the variation in the MMM index (F4,103 = 17.3, p < 0.0001). MMM was positively associated with the number of attending males (b = 0.29 ± 0.04, t103 = 7.5, p < 0.0001) and mean MMM differed depending on the year in which the data were collected (F3,103 = 4.1, p = 0.009).
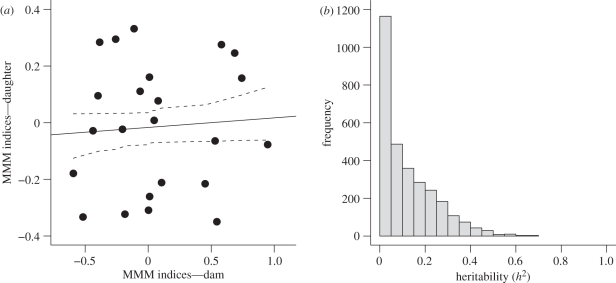
The heritability from the parent–offspring regression was estimated to be 0.06 ± 0.22, which was not significantly different from zero (t21 = 0.30, p = 0.77; figure 1a). The animal model provided evidence for moderate levels of additive genetic variation (CVA = 5.1; 95% CI = 0.03–22.6; change in DIC = 7.4; table 1), but very high levels of environmental (residual) variation (CVE = 32.3; 95% CI = 24.7–38.5), which resulted in a very low heritability estimate for MMM (h2 = 0.002; table 1 and figure 1b).
Figure 1.
(a) The heritability of MMM by female North American red squirrels was not significantly different from zero based on a mother–daughter regression. (b) The MCMC posterior distribution from an ‘animal model’ based on a multi-generational pedigree indicated a very low heritability.
Table 1.
Sources of variation in MMM by female North American red squirrels. Variance components are presented for each of three candidate models as coefficients of variation (CV = 100 × s.d./mean), where the mean MMM was 1.02 males per hour of observation. Fixed effects (FE) were the number of attending males and year. Individual effects (Vi) represent all sources of individual variation (genetic and non-genetic) in the model where additive genetic effects (VA) are excluded, but represent permanent environmental effects in models that included additive genetic effects. Heritability (h2), and individual effects (Vi/Vp) were calculated relative to total phenotypic variation. Model comparison was based on the DIC; 95% CI for each parameter are in parentheses.
| model | DIC | CVe | CVi | CVa | Vi/Vp | h2 |
|---|---|---|---|---|---|---|
| 1. FE | 90.2 | 35.8 (30.5–40.3) | — | — | — | — |
| 2. FE and individual effects (Vi) | 84.7 | 32.8 (24.8–39.3) | 15.0 (0.06–26.3) | — | 0.002 (7.2e−8–0.49) | — |
| 3. FE + Vi + genetic effects (Va) | 82.8 | 32.3 (24.7–38.5) | 3.7 (0.003–23.5) | 5.1 (0.03–22.6) | 0.002 (8.0e−9–0.42) | 0.002 (4.5e−7–0.36) |
4. Discussion
While the costs and benefits of MMM have attracted much research attention (e.g. [4,5]), the prerequisite of sufficient genetic variation in MMM for rates of multiple mating to evolve in response to these fitness costs and benefits has been largely ignored outside of the laboratory. Here we provide what we believe is the first estimate of the heritability of MMM from a wild population. While we found moderate levels of additive genetic variation (in the 50th percentile of published estimates based on data in [20]), levels of residual environmental variation (i.e. variation not attributed to additive genetic or permanent environmental effects) were very high relative to previously reported values (92nd percentile based on data in [20]), which resulted in a heritability of MMM by red squirrels that was very low (h2 = 0.002).
Our heritability of MMM was substantially smaller than most previous broad-sense heritabilities for polyandry in captive insects [9] and for behavioural traits in general (h2 = 0.30 ± 0.03 reviewed by [21]). It is not uncommon for laboratory measures to overestimate wild heritabilities (e.g. [11]), in part because sources of environmental variation are controlled in captivity. For example, over 40 per cent of the variation in MMM in our study was explained by the number of males that attended a female's mating chase, whereas the number of potential sires presented to a female is typically controlled in captivity. Our low heritability estimate for MMM, however, did not depend on the inclusion or exclusion of the number of attending males as a covariate in the analysis (see electronic supplementary material, table S2).
In the two previous studies that were able to separate direct genetic effects from dam effects on MMM, the narrow-sense heritability was also low (h2 < 0.1) and dam effects (including maternal, epistasis and dominance effects) were found to be large [22,23]. We estimated maternal effects on MMM to be small and their inclusion did not affect our heritability estimate (see electronic supplementary material). Given limitations in the available data, our estimates of additive genetic effects, permanent environmental effects and maternal effects were likely confounded to some degree [17]. The combined contribution of all of these sources of variation to total phenotypic variation, however, remained very small.
Despite a relatively large multi-generational pedigree and four years of field data on MMM, confidence intervals around our estimates of CVA and h2 remained broad. The 95% CI for CVA spanned much of the range of, but did not exceed, previous estimates of CVA (3rd–94th percentile of values reviewed by [20]). So, while genetic variation for this trait exists, the exact level remains uncertain. What was certain, however, is that environmental (residual) variation was large (95% CI for CVE ranged from the 87th percentile to 94th percentile of values reviewed by [20]) and far exceeded additive genetic variation, such that the heritability of this trait was extremely low. These findings indicate, first, that there is genetic variation in MMM in red squirrels, and hence the trait is evolvable (sensu [20]). However, the evolution of MMM ultimately depends on an association between genetic variation in MMM and variation in fitness [24]. Furthermore, genetic variation affects fitness only through a functioning phenotype within a particular environment. In our case, the very large environmental effects mean that phenotypic differences in MMM among females that might affect fitness were largely attributable to non-genetic differences. As such, any phenotypic selection on MMM, resulting from the costs and benefits of mating multiply is likely to act on environmental deviations (e.g. [25]) and not genetically based differences among females, and hence will not contribute to an evolutionary response to selection in this trait. Whether high levels of environmental variation generally constrain the contemporary adaptation of rates of MMM, however, awaits further estimates of the sources of variation in MMM in other natural populations.
Acknowledgements
We thank all squirrelers for their fieldwork, and the McAdam Laboratory and three anonymous reviewers for many helpful comments. Jarrod Hadfield provided valuable advice on MCMCglmm and prior specification. This project was funded by the Natural Sciences and Engineering Research Council (D.W.C., M.M.H., S.B., A.G.Mc.), the National Science Foundation (A.G.Mc.), the Natural Environment Research Council (D.W.C.) and the Ontario Ministry of Research and Innovation (A.G.Mc.). This is contribution number 57 of the Kluane Red Squirrel Project.
References
- 1.Bateman A. J. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349–368 10.1038/hdy.1948.21 (doi:10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 2.Queller D. C. 1997. Why do females care more than males? Proc. R. Soc. Lond. B 264, 1555–1557 10.1098/rspb.1997.0216 (doi:10.1098/rspb.1997.0216) [DOI] [Google Scholar]
- 3.Daly M. 1978. The cost of mating. Am. Nat. 112, 771–776 10.1086/283319 (doi:10.1086/283319) [DOI] [Google Scholar]
- 4.Jennions M. D., Petrie M. 2000. Why do females mate multiply? A review of genetic benefits. Biol. Rev. 75, 21–64 10.1017/S0006323199005423 (doi:10.1017/S0006323199005423) [DOI] [PubMed] [Google Scholar]
- 5.Simmons L. W. 2005. The evolution of polyandry: sperm competition, sperm selection, and offspring viability. Annu. Rev. Ecol. Evol. Syst. 36, 125–146 10.1146/annurev.ecolsys.36.102403.112501 (doi:10.1146/annurev.ecolsys.36.102403.112501) [DOI] [Google Scholar]
- 6.Wolff J. O., Macdonald D. W. 2004. Promiscuous females protect their offspring. Trends Ecol. Evol. 19, 127–134 10.1016/j.tree.2003.12.009 (doi:10.1016/j.tree.2003.12.009) [DOI] [PubMed] [Google Scholar]
- 7.Schwagmeyer P. L., Parker G. A. 1990. Male mate choice as predicted by sperm competition in thirteen-lined ground squirrels. Nature 348, 62–64 10.1038/348062a0 (doi:10.1038/348062a0) [DOI] [Google Scholar]
- 8.House C. M., Walling C. A., Stamper C. E., Moore A. J. 2009. Females benefit from multiple mating but not multiple mates in the burying beetle Nicrophorus vespilloides. J. Evol. Biol. 22, 1961–1966 10.1111/j.1420-9101.2009.01800.x (doi:10.1111/j.1420-9101.2009.01800.x) [DOI] [PubMed] [Google Scholar]
- 9.Evans J. P., Simmons L. W. 2008. The genetic basis of traits regulating sperm competition and polyandry: can selection favour the evolution of good- and sexy-sperm? Genetica 134, 5–19 10.1007/s10709-007-9162-5 (doi:10.1007/s10709-007-9162-5) [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann A. A., Merilä J. 1999. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 14, 96–101 10.1016/S0169-5347(99)01595-5 (doi:10.1016/S0169-5347(99)01595-5) [DOI] [PubMed] [Google Scholar]
- 11.Conner J. K., Franks R., Stewart C. 2003. Expression of additive genetic variances and covariances for wild radish floral traits: comparison between field and greenhouse environments. Evolution 57, 487–495 [DOI] [PubMed] [Google Scholar]
- 12.Lane J. E., Boutin S., Gunn M. R., Slate J., Coltman D. W. 2008. Female multiple mating and paternity in free-ranging North American red squirrels. Anim. Behav. 75, 1927–1937 10.1016/j.anbehav.2007.10.038 (doi:10.1016/j.anbehav.2007.10.038) [DOI] [Google Scholar]
- 13.Kruuk L. E. B. 2004. Estimating genetic parameters in natural populations using the ‘animal model’. Phil. Trans. R. Soc. Lond. B 359, 873–890 10.1098/rstb.2003.1437 (doi:10.1098/rstb.2003.1437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAdam A. G., Boutin S., Sykes A. K., Humphries M. M. 2007. Life histories of female red squirrels and their contributions to population growth and lifetime fitness. Ecoscience 14, 362–369 10.2980/1195-6860(2007)14[362:LHOFRS]2.0.CO;2 (doi:10.2980/1195-6860(2007)14[362:LHOFRS]2.0.CO;2) [DOI] [Google Scholar]
- 15.Hadfield J. D. 2010. MCMC methods for multi-response generalized linear models: the MCMCglmm R Package. J. Stat. Softw. 33, 1–2220808728 [Google Scholar]
- 16.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3-900051-07-0. See http://www.R-project.org [Google Scholar]
- 17.Kruuk L. E. B., Hadfield J. D. 2007. How to separate genetic and environmental causes of similarity between relatives. J. Evol. Biol. 20, 1890–1903 10.1111/j.1420-9101.2007.01377.x (doi:10.1111/j.1420-9101.2007.01377.x) [DOI] [PubMed] [Google Scholar]
- 18.Lynch M., Walsh B. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates [Google Scholar]
- 19.Wedell N. 2001. Female remating in butterflies: interaction between female genotype and nonfertile sperm. J. Evol. Biol. 14, 746–754 10.1046/j.1420-9101.2001.00327.x (doi:10.1046/j.1420-9101.2001.00327.x) [DOI] [Google Scholar]
- 20.Houle D. 1992. Comparing evolvability and variability of quantitative traits. Genetics 130, 195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mousseau T. A., Roff D. A. 1987. Natural selection and the heritability of fitness components. Heredity 59, 181–197 10.1038/hdy.1987.113 (doi:10.1038/hdy.1987.113) [DOI] [PubMed] [Google Scholar]
- 22.Shuker D. M., Phillimore A. J., Burton-Chellew M. N., Hodge S. E., West S. A. 2007. The quantitative genetics basis of polyandry in the parasitoid wasp, Nasonia vitripennis. Heredity 98, 69–73 10.1038/sj.hdy.6800897 (doi:10.1038/sj.hdy.6800897) [DOI] [PubMed] [Google Scholar]
- 23.Simmons L. W. 2003. The evolution of polyandry: patterns of genotypic variation in female mating frequency, male fertilization success and a test of the sexy-sperm hypothesis. J. Evol. Biol. 16, 623–634 10.1046/j.1420-9101.2003.00572.x (doi:10.1046/j.1420-9101.2003.00572.x) [DOI] [PubMed] [Google Scholar]
- 24.Price G. 1970. Selection and covariance. Nature 227, 520–521 10.1038/227520a0 (doi:10.1038/227520a0) [DOI] [PubMed] [Google Scholar]
- 25.Kruuk L. E. B., Slate J., Pemberton J. M., Brotherstone S., Guinness F., Clutton-Brock T. 2002. Antler size in red deer: heritability and selection but no evolution. Evolution 56, 1683–1695 [DOI] [PubMed] [Google Scholar]