Abstract
While studies of sexual selection focus primarily on female choice and male–male competition, males should also exert mate choice in order to maximize their reproductive success. We examined male mate choice in mosquitofish, Gambusia holbrooki, with respect to female size and female dominance. We found that the number of mating attempts made by a male was predicted by the dominance rank of females in a group, with dominant females attracting more mating attempts than subordinates. The number of mating attempts made by males was independent of the female size. The observed bias in the number of mating attempts towards dominant females may be driven either by straightforward male mate choice, since dominance and female fecundity are often closely related, or via the dominant females mediating male mating behaviour by restricting their access to subordinate females.
Keywords: mate choice, fitness, dominance, hierarchy, fecundity
1. Introduction
While studies of mate selection have historically focused on female choice, there are many scenarios where males may also benefit from being choosy with respect to their mates [1]. Theory predicts that male mate choice should be most likely to occur where females vary in quality and where males have either to make a sizeable investment in reproduction or where the encounter rate with potential mates is high [2]. Where there is a large variation in characteristics that correlate with female fecundity, males may potentially benefit by biasing their reproductive effort according to their appraisal of these characteristics [3,4]. In many species, female fecundity is positively correlated with size, and males exhibit preferences for larger females [5–7].
Males may also benefit from selecting females on the basis of social rank. Female dominance hierarchies are found in species representing a wide range of taxa [8–11]. The fitness benefits of occupying higher ranks in a dominance hierarchy are often considerable, both for males [12] and for females. For example, dominant females benefit from increased foraging efficiency and can displace subordinates from feeding patches [13] or steal their food [14]. Additionally, stress experienced by subordinates can impair reproductive function [15]. Overall, dominant females may provide offspring better and produce larger and healthier broods than subordinates [16], potentially making them more attractive as mates.
The mosquitofish, Gambusia holbrooki, is a livebearing Poecilid with a promiscuous mating system. Adult males are typically smaller than adult females and do not court, but rely on coercion. Both sexes are organized into dominance hierarchies [17] where the dominant individual is often the largest. In this species, the hierarchies are often ‘monarchistic’ in nature, with a single individual dominating a small group of relatively equal subordinates [17]. While prior studies have investigated male preference for larger females [18], it is not known whether and how this relates to a female's position in the dominance hierarchy. While dominance often covaries with size and growth, this is by no means always the case [19,20]; hence it is insufficient to take size as a proxy for dominance. If male mosquitofish gain fitness benefits from mating with dominant females, then they may be expected to be choosy in this respect. Alternatively, females may mediate male mating preferences through their behaviour, particularly through dominant females restricting male access to subordinate females. Here, we examine whether male mosquitofish allocate their mating efforts according to female dominance rank, in the absence of major size differences.
2. Material and methods
We collected mosquitofish during late 2009 from Manley Dam, Australia (33°46′35.45″ S, 151°14′50.38″ E). Captured mosquitofish were held in 180 l vats at the University of Sydney at 23.1 ± 1°C with 12 : 12 h light regime. The fish were stocked at high densities (approx. 200 fish with an even sex ratio per 180 l vat) to prevent the possible development of familiarity preferences [21]. Each female used in the experiment was given a unique individual identification marker using elastomer (NorthWest Marine Technology, Washington, USA) at least one week prior to beginning experiments.
Two experimental tanks measuring 40 × 19.5 × 23 cm (l × w × d) were filled with aged, conditioned water to a depth of 17 cm. Both tanks had aquarium gravel added to a depth of 1 cm with two artificial plants to provide refuge for the fish and an airstone to circulate the water. Each tank was lit from above and enclosed within a black plastic shelter to minimize external disturbance.
For each experimental replicate, five adult females measuring 29.22 ± 1.52 mm (mean ± s.d.), range 26–33.4 mm, were placed in the tank and left for a period of 20–24 h, following which we conducted a 20 min observational period. During this period, we recorded agonistic interactions between females; individuals chase their subordinates and are chased by those dominant to them, hence rank is simple to determine [22]. Following this, a single male measuring 19.54 ± 0.85 mm (mean ± s.d.) was placed in the tank and allowed to acclimate for 20 min. This was followed by a further 20 min observation period where the number of mating attempts the male made towards each female was recorded. As with other Poecilid fishes, a mating attempt is clear and unambiguous, which involves the male rotating his intromittent organ (the gonopodium) towards the female's genital pore. We conducted 12 replicates, each time with new individuals.
(a). Data analysis
The data were analysed using a linear multiple regression technique, with the number of mating attempts made by each male towards each of the five females as the outcome and both female size and female dominance as predictors. We used a Durbin–Watson procedure to determine residual independence in the model, assessed collinearity by examining tolerance and the variance inflation factor and tested assumptions of homoscedacity and normality of distribution using residual plots and probability–probability plots, respectively [23].
3. Results
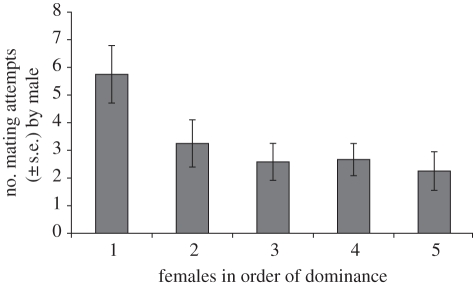
The model successfully predicted the number of matings attempted by the male mosquitofish (regression: r = 0.413, F2,57 = 5.88, p = 0.005). Of the two predictor variables, female rank (standardized β = −0.37, t = 3.02, p = 0.004; figure 1), but not the female size (standardized β = 0.15, t = 1.22, p = 0.23), was a significant predictor of the number of mating attempts made by the male—higher ranked females attracted more mating attempts from the males than the lower ranked females. All regression analysis assumptions were met.
Figure 1.
Mean mating attempts directed towards each female according to the female's dominance rank.
4. Discussion
Male mosquitofish allocate their mating attempts according to the female's position in the dominance hierarchy, but the experiments failed to detect an effect of the female size. This bias in male mating effort may be driven by their preference for dominant females, or as a result of the aggressive interactions between females increasing their probability of encountering dominant females, or an interaction of these two. Male preference for dominant females has previously been documented in animals with particular social systems, especially in hierarchical social mammals [24–26] and where sex roles are reversed [27]. To our knowledge, this is the first demonstration of male preference for dominant females in a promiscuous mating system with regular (non-reversed) sex roles.
Why should males choose to mate with dominant females? Dominant animals may be better provisioned [13,28], and more fecund than subordinates [29], hence mating with a dominant female may confer a direct fitness benefit to a male since the quantity and quality of food the female consumes affects her reproductive success. Food availability and offspring number are positively correlated in egg-laying fish [30], while in livebearers, like G. holbrooki, females may reabsorb oocytes [31] or produce smaller offspring when food is scarce [32]. Furthermore, dominant females can sometimes directly suppress reproduction in subordinates through chemical means [33], including in Gambusia [34].
Despite the coercive nature of the mosquitofish mating system, females are not passive participants. For example, the fact that males showed a strong preference for the dominant female but appeared to show little discrimination between the other ranks may be indicative of the monarchic nature of mosquitofish hierarchies and the agonistic and chemical suppression of subordinates by the most dominant fish [17,34]. Furthermore, the structure of the female dominance hierarchy and the aggression between its members are likely to feed back to affect male behaviour. The behaviour of dominant females probably affects the encounter rate of males with potential mates, since dominant individuals aggressively drive-off their subordinates and thus occupy a greater proportion of available space.
In many species, size may be a proxy for dominance. Here, female size did not play a part in male choice, although given a wider size range, males may choose larger females, on the basis of size-related fecundity [35,36], or, in light of current findings, on the basis of dominance-related fecundity.
Even where females vary in quality, male choosiness should only occur when there is a high male investment in courtship or mating, or when there is a low cost to choosiness [2,37]. In most other cases, males might be best served to take any mating opportunity. For most of their breeding season in Australia, mosquitofish live at extremely high densities, allowing males a high encounter rate with their mates and, importantly, imposing low costs for choosiness. Under these circumstances, males may potentially maximize fitness by being selective. However, if all males were to target dominant females, the payoff for selecting dominant females would drop and a less choosy male strategy could be favoured. Intriguingly, therefore, male strategy may be dependent on female density, switching from choosiness to an indiscriminate strategy when the ratio of females to males drops below some threshold value.
Acknowledgements
All experiments were approved in advance by the University of Sydney Animal Ethics Committee.
The authors would like to thank the editor and two anonymous referees for their comments, which greatly improved the manuscript.
References
- 1.Jones K. M., Monaghan P., Nager R. G. 2001. Male mate choice and female fecundity in zebra finches. Anim. Behav. 62, 1021–1026 10.1006/anbe.2001.1843 (doi:10.1006/anbe.2001.1843) [DOI] [Google Scholar]
- 2.Kokko H., Johnstone R. A. 2002. Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Phil. Trans. R. Soc. Lond. B 357, 319–330 10.1098/rstb.2001.0926 (doi:10.1098/rstb.2001.0926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonduriansky R. 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. 76, 305–339 10.1017/S1464793101005693 (doi:10.1017/S1464793101005693) [DOI] [PubMed] [Google Scholar]
- 4.Sargent R. C., Gross M. R., Vandenberghe E. P. 1986. Male mate choice in fishes. Anim. Behav. 34, 545–550 10.1016/S0003-3472(86)80123-3 (doi:10.1016/S0003-3472(86)80123-3) [DOI] [Google Scholar]
- 5.Rowland W. J. 1989. The ethological basis of mate choice in male threespine sticklebacks, Gasterosteus aculeatus. Anim. Behav. 38, 112–120 10.1016/S0003-3472(89)80070-3 (doi:10.1016/S0003-3472(89)80070-3) [DOI] [Google Scholar]
- 6.Wong B. B. M., Jennions M. D. 2003. Costs influence male mate choice in a freshwater fish. Proc. R. Soc. Lond. B 270, S36–S38 10.1098/rsbl.2003.0003 (doi:10.1098/rsbl.2003.0003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shine R., Mason R. T. 2001. Courting male garter snakes (Thamnophis sirtalis parietalis) use multiple cues to identify potential mates. Behav. Ecol. Sociobiol. 49, 465–473 10.1007/s002650100334 (doi:10.1007/s002650100334) [DOI] [Google Scholar]
- 8.Appleby M. C. 1980. Social rank and food access in red deer stags. Behaviour 74, 294–309 10.1163/156853980X00519 (doi:10.1163/156853980X00519) [DOI] [Google Scholar]
- 9.Whitten P. L. 1983. Diet and dominance among female vervet monkeys (Cercopithecus aethiops). Am. J. Primatol. 5, 139–159 10.1002/ajp.1350050205 (doi:10.1002/ajp.1350050205) [DOI] [PubMed] [Google Scholar]
- 10.Whiteman E. A., Cote I. M. 2004. Dominance hierarchies in group-living cleaning gobies: causes and foraging consequences. Anim. Behav. 67, 239–247 10.1016/j.anbehav.2003.04.006 (doi:10.1016/j.anbehav.2003.04.006) [DOI] [Google Scholar]
- 11.Skog M. 2009. Intersexual differences in European lobster (Homarus gammarus): recognition mechanisms and agonistic behaviours. Behaviour 146, 1071–1091 10.1163/156853909X406437 (doi:10.1163/156853909X406437) [DOI] [Google Scholar]
- 12.Qvarnström A., Forsgren E. 1998. Should females prefer dominant males? Trends. Ecol. Evol. 13, 498–501 10.1016/S0169-5347(98)01513-4 (doi:10.1016/S0169-5347(98)01513-4) [DOI] [PubMed] [Google Scholar]
- 13.Barrette C., Vandal D. 1986. Social rank, dominance, antler size, and access to food in snow-bound wild woodland caribou. Behaviour 97, 118–146 10.1163/156853986X00342 (doi:10.1163/156853986X00342) [DOI] [Google Scholar]
- 14.Huck U. W., Lisk R. D., McKay M. V. 1988. Social-dominance and reproductive success in pregnant and lactating golden hamsters (Mesocricetus auratus) under seminatural conditions. Physiol. Behav. 44, 313–319 10.1016/0031-9384(88)90031-5 (doi:10.1016/0031-9384(88)90031-5) [DOI] [PubMed] [Google Scholar]
- 15.Carragher J. F., Sumpter J. P., Pottinger T. G., Pickering A. D. 1989. The deleterious effects of cortisol implantation on reproductive function in 2 species of trout, Salmo trutta and Salmo gairdneri. Gen. Comp. Endocrinol. 76, 310–321 10.1016/0016-6480(89)90163-9 (doi:10.1016/0016-6480(89)90163-9) [DOI] [PubMed] [Google Scholar]
- 16.Hoy S., Bauer J., Borberg C., Chonsch L., Weirich C. 2009. Impact of rank position on fertility of sows. Livest. Sci. 126, 69–72 10.1016/j.livsci.2009.05.018 (doi:10.1016/j.livsci.2009.05.018) [DOI] [Google Scholar]
- 17.Caldwell M. C., Caldwell D. K. 1962. Monarchistic dominance in small groups of captive mosquitofish, Gambusia affinis patruelis. Bull. S. Calif. Acad. Sci. 61, 37–43 [Google Scholar]
- 18.Bisazza A., Marconato A., Marin G. 1989. Male mate preferences in the mosquitofish Gambusia holbrooki. Ethology 83, 335–343 10.1111/j.1439-0310.1989.tb00541.x (doi:10.1111/j.1439-0310.1989.tb00541.x) [DOI] [Google Scholar]
- 19.Harwood A. J., Armstrong J. D., Metcalfe N. B., Griffiths S. W. 2003. Does dominance status correlate with growth in wild stream-dwelling Atlantic salmon (Salmo salar)? Behav. Ecol. 14, 902–908 10.1093/beheco/arg080 (doi:10.1093/beheco/arg080) [DOI] [Google Scholar]
- 20.Huntingford F. A., Metcalfe N. B., Thorpe J. E., Graham W. D., Adams C. E. 1990. Social-dominance and body size in Atlantic salmon parr, Salmo salar. J. Fish Biol. 36, 877–881 10.1111/j.1095-8649.1990.tb05635.x (doi:10.1111/j.1095-8649.1990.tb05635.x) [DOI] [Google Scholar]
- 21.Ward A. J. W., Hart P. J. B. 2003. The effects of kin and familiarity on interactions between fish. Fish Fish. 4, 348–358 10.1046/j.1467-2979.2003.00135.x (doi:10.1046/j.1467-2979.2003.00135.x) [DOI] [Google Scholar]
- 22.Gorlick D. L. 1976. Dominance hierarchies and factors influencing dominance in guppy Poecilia reticulata. Anim. Behav. 24, 336–346 10.1016/S0003-3472(76)80041-3 (doi:10.1016/S0003-3472(76)80041-3) [DOI] [Google Scholar]
- 23.Field A. 2000. Discovering statistics using SPSS. London, UK: Sage Publications [Google Scholar]
- 24.Powell D. M. 2008. Female-female competition or male mate choice? Patterns of courtship and breeding behavior among feral horses (Equus caballus) on Assateague Island. J. Ethol. 26, 137–144 10.1007/s10164-007-0043-2 (doi:10.1007/s10164-007-0043-2) [DOI] [Google Scholar]
- 25.Rasa O. A. E. 1977. Ethology and sociology of dwarf mongoose (Helogale undulate rufula). J. Comp. Ethol. 43, 337–406 [Google Scholar]
- 26.Kuester J., Paul A. 1996. Female-female competition and male mate choice in Barbary macaques (Macaca sylvanus). Behaviour 133, 763–790 10.1163/156853996X00468 (doi:10.1163/156853996X00468) [DOI] [Google Scholar]
- 27.Berglund A., Rosenqvist G. 2001. Male pipefish prefer dominant over attractive females. Behav. Ecol. 12, 402–406 10.1093/beheco/12.4.402 (doi:10.1093/beheco/12.4.402) [DOI] [Google Scholar]
- 28.Gilmour K. M., DiBattista J. D., Thomas J. B. 2005. Physiological causes and consequences of social status in salmonid fish. Int. Comp. Biol. 45, 263–273 10.1093/icb/45.2.263 (doi:10.1093/icb/45.2.263) [DOI] [PubMed] [Google Scholar]
- 29.King W. J., Allaine D. 2002. Social, maternal, and environmental influences on reproductive success in female Alpine marmots (Marmota marmota). Can. J. Zool. 80, 2137–2143 10.1139/z02-205 (doi:10.1139/z02-205) [DOI] [Google Scholar]
- 30.Wootton R. J. 1977. Effect of food limitation during breeding season on size, body components and egg-production of female sticklebacks (Gasterosteus aculeatus). J. Anim. Ecol. 46, 823–834 10.2307/3643 (doi:10.2307/3643) [DOI] [Google Scholar]
- 31.Hester F. J. 1964. Effects of food supply on fecundity in the female guppy, Lebistes reticulatus. J. Fish. Res. Board Can. 21, 757–764 [Google Scholar]
- 32.Reznick D., Callahan H., Llauredo R. 1996. Maternal effects on offspring quality in poeciliid fishes. Am. Zool. 36, 147–156 [Google Scholar]
- 33.Gerlach G. 2006. Pheromonal regulation of reproductive success in female zebrafish: female suppression and male enhancement. Anim. Behav. 72, 1119–1124 10.1016/j.anbehav.2006.03.009 (doi:10.1016/j.anbehav.2006.03.009) [DOI] [Google Scholar]
- 34.Lutnesky M. M. F., Adkins J. W. 2003. Putative chemical inhibition of development by conspecifics in mosquitofish, Gambusia affinis. Environ. Biol. Fish. 66, 181–186 10.1023/A:1023696609963 (doi:10.1023/A:1023696609963) [DOI] [Google Scholar]
- 35.Herdman E. J. E., Kelly C. D., Godin J. G. J. 2004. Male mate choice in the guppy (Poecilia reticulata): do males prefer larger females as mates? Ethology 110, 97–111 10.1111/j.1439-0310.2003.00960.x (doi:10.1111/j.1439-0310.2003.00960.x) [DOI] [Google Scholar]
- 36.Bisazza A., Manfredi S., Pilastro A. 2000. Sexual competition, coercive mating and mate assessment in the one-sided livebearer, Jenynsia multidentata: are they predictive of sexual dimorphism? Ethology 106, 961–978 10.1046/j.1439-0310.2000.00620.x (doi:10.1046/j.1439-0310.2000.00620.x) [DOI] [Google Scholar]
- 37.Barry K. L., Kokko H. 2010. Male mate choice: why sequential choice can make its evolution difficult. Anim. Behav. 80, 163–169 10.1016/j.anbehav.2010.04.020 (doi:10.1016/j.anbehav.2010.04.020) [DOI] [Google Scholar]