Abstract
Long embryonic periods are assumed to reflect slower intrinsic development that are thought to trade off to allow enhanced physiological systems, such as immune function. Yet, the relatively rare studies of this trade-off in avian offspring have not found the expected trade-off. Theory and tests have not taken into account the strong extrinsic effects of temperature on embryonic periods of birds. Here, we show that length of the embryonic period did not explain variation in two measures of immune function when temperature was ignored, based on studies of 34 Passerine species in tropical Venezuela (23 species) and north temperate Arizona (11 species). Variation in immune function was explained when embryonic periods were corrected for average embryonic temperature, in order to better estimate intrinsic rates of development. Immune function of offspring trades off with intrinsic rates of embryonic development once the extrinsic effects of embryonic temperatures are taken into account.
Keywords: embryonic temperature, embryonic period, immune function, trade-offs
1. Introduction
Slower embryonic development, reflected by longer embryonic periods, is thought to allow enhanced development of physiological systems, like the immune system, that increase survival and longevity [1–3]. Such trade-offs are thought to underlie the well-known difference between tropical and north temperate birds in length of embryonic periods and adult survival [2–4]. Yet, the only two tests of a trade-off between length of the incubation period and immune function of nestling birds found no support for a trade-off [5,6] and no study has included offspring in tropical and temperate sites. Thus, a trade-off between embryonic development rate and offspring immune function remains unclear, especially across latitudes, but is critical to furthering our understanding of trade-offs in the evolution of offspring quality and life-history strategies.
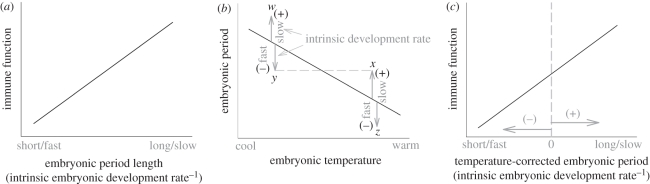
Previous tests relied on the assumption that embryonic periods reflect the inverse of intrinsic embryonic development rate, yielding a predicted increase in strength of immune defence with length of the embryonic period (figure 1a). This assumption may be problematic for ectothermic embryos, like birds, because of temperature effects. Temperatures experienced by ectothermic embryos influence both embryonic development rate and the amount of energy converted into tissue [7,8]. Variation in parental incubation behaviour determines temperatures experienced by avian embryos, and experimental swapping of eggs showed that temperature affects length of the embryonic period [9]. Differences in embryonic period owing to such extrinsic effects of temperature do not reflect differences in intrinsic rates of development [10]. Instead, intrinsic rates of embryonic development should be better estimated by deviations from period lengths predicted by embryonic temperature while controlling for any allometric effects of size (figure 1b). In particular, species with embryonic periods that are long for their temperature (points above the line in figure 1b) reflect slower intrinsic development, controlling for extrinsic effects of temperature. Conversely, species with embryonic periods that are short for their temperature (points below the line in figure 1b) reflect faster intrinsic development, controlling for temperature. If this is true, species differences in immune function should be best predicted by the temperature-corrected embryonic period as an estimate of intrinsic development rates (figure 1c). Here, we test these alternatives.
Figure 1.
Immune function is predicted to trade off with intrinsic embryonic development rate. (a) Length of the embryonic period is assumed to reflect the inverse of intrinsic development rate and, so immune defence is predicted to increase with embryonic period length. (b) Embryonic temperature is an extrinsic effect on embryonic period, where cooler temperatures cause longer embryonic periods. Points above (w,x) and below (y,z) the line reflect species with relatively longer versus shorter periods and slower versus faster intrinsic development (shown in grey), respectively, corrected for temperature. Species at points x and y have the same absolute embryonic period but differ in their intrinsic development rates. (c) Immune function is expected to increase with embryonic period corrected for possible extrinsic temperature effects to provide a more accurate index of intrinsic embryonic development rate.
2. Material and methods
Study sites were high-elevation (2300 m elevation) mixed forest in Arizona (34° N, 111° W) and cloud forest (1350–2000 m) in the Andes of Venezuela (9° N, 69° W). We intensively measured embryonic temperatures, embryonic periods and immune function for 11 Passerine species in north temperate Arizona, and 23 species in tropical Venezuela (appendix A), which comprise the set of species used for analyses here. Study sites were searched for nests during breeding seasons of 1988–2009 in Arizona and 2002–2008 in Venezuela. Embryonic periods were quantified as the difference in days between last egg laid and last egg hatched [9] for nests found prior to laying of the last egg and for which hatch day was observed.
Embryonic temperatures were measured by placing a thermister in the centre of one egg and measuring temperature experienced by the embryo every 12 or 24 s in three to nine nests per species over 5 days per nest, on average [9]. We used two common assays of immune function [5,11,12] that have been related to offspring survival and disease resistance (e.g. [13]). In particular, we measured the inflammatory response to injection of phytohaemagglutinin (PHA, Sigma L-8754) at a standardized developmental stage (the day nestling primary pin-feathers broke their sheaths) and averaged the response for each nest. Nestlings were injected in the patagium with 0.04 ml of a solution of 5 mg ml−1 of PHA in phosphate-buffered saline (PBS). The inflammatory response at the point of injection was measured after 24 h with a pressure-sensitive micrometer to the nearest 0.01 mm [5]. This inflammatory response reflects an induced innate and adaptive immune response [11,14]. We also measured the activity of natural antibodies that circulate in normal individuals in the absence of exogenous antigenic stimulation as a measure of constitutive innate immune defences [14]. We followed the protocol by Matson et al. [12] scaled to our sample sizes of plasma (between 15 and 25 µl). Plasma samples were serially diluted twofold with 0.01 M PBS (Sigma P3813) and incubated at 37°C for 90 min with a 1 per cent rabbit blood cell suspension (Colorado Serum, CO, USA). We collected blood samples from nestlings when the primary feathers broke their sheaths in order to standardize stage of development. Blood samples were kept on ice in the field and centrifuged within 8 h to separate the plasma. Plasma samples were stored frozen at −20°C until analyses were performed.
General linear models were used to examine relationships within and between sites. When interactions between sites were significant or nearly so (p < 0.10), we analysed each site separately. We tested the models without (standard in previous approaches—figure 1a) and with (figure 1c) embryonic temperature averaged over 24 h sampling periods. We calculated temperature-corrected embryonic periods as the residuals from analysis of covariance (ANCOVA) models of the log-transformed embryonic period with site as a factor and 24 h embryonic temperature and body mass as covariates. We controlled for possible phylogenetic effects using independent contrasts [15] based on the CRUNCH option of program CAIC [16].
3. Results
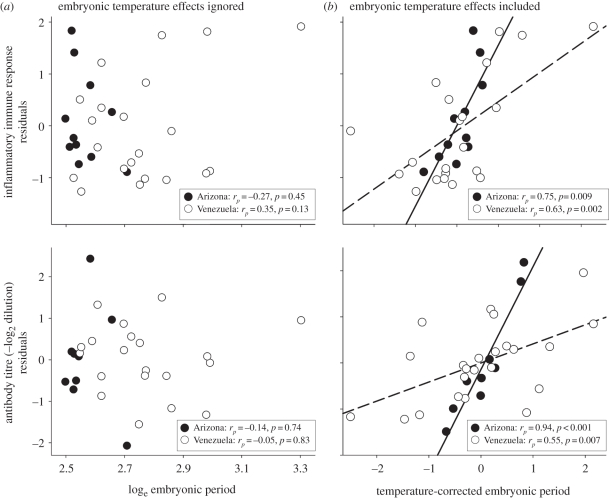
The expected positive relationship between length of the embryonic period and immune function (i.e. figure 1a) was not observed for either inflammatory or natural antibody measures of immune function (figure 2a and table 1). Indeed, if anything, the relationships tended towards negative patterns (figure 2a), rather than the expected positive (i.e. figure 1a) patterns.
Figure 2.
Measures of immune function based on the inflammatory response to phytohemagglutinin (PHA) injection and natural antibody activity relative to lengths of embryonic periods. Plots reflect the relationships of residuals from analyses in table 1. (a) Inflammatory response and natural antibody activity residuals correct for body mass and site and are not related to log-transformed length of embryonic periods for Passerine species in tropical Venezuela and north temperate Arizona (table 1). (b) The immune measure residuals correct for embryonic temperature, body mass, and site and are related to temperature-corrected embryonic periods that correct for embryonic temperature, body mass and site (table 1). Note that shorter temperature-corrected periods (left side of x-axis) reflect faster embryonic development for a given temperature and longer temperature-corrected periods (right side) reflect slower development for a given temperature (from figure 1c). Solid lines reflect the regression line for Arizona and the dashed line is Venezuela.
Table 1.
Results of analyses of covariance to explain interspecific variation in immune function for raw data and phylogenetically independent contrasts. Interactions were dropped from the models when p > 0.10, but main effects were always retained.
| variables | raw data |
independent contrasts |
||||
|---|---|---|---|---|---|---|
| d.f. | F-value | p-value | d.f. | F-value | p-value | |
| (a) ignoring embryonic temperature effects (figure 2a) | ||||||
| inflammatory immune response (error d.f. = 28; R2 = 0.33) | ||||||
| site | 1 | 1.60 | 0.22 | 1 | 2.99 | 0.10 |
| loge body mass | 1 | 18.50 | <0.001 | 1 | 1.72 | 0.20 |
| loge embryonic period | 1 | 2.38 | 0.13 | 1 | 1.59 | 0.22 |
| natural antibody activity (error d.f. = 28; R2 = 0.20) | ||||||
| site | 1 | 0.42 | 0.52 | 1 | 0.75 | 0.39 |
| loge body mass | 1 | 4.63 | 0.04 | 1 | 0.68 | 0.42 |
| loge embryonic period | 1 | 0.18 | 0.67 | 1 | 0.03 | 0.88 |
| (b) accounting for embryonic temperature effects (figure 2b) | ||||||
| inflammatory immune response (error d.f. = 27; R2 = 0.59) | ||||||
| site | 1 | 1.29 | 0.27 | 1 | 1.42 | 0.24 |
| loge body mass | 1 | 10.43 | 0.003 | 1 | 1.23 | 0.28 |
| embryonic temperature | 1 | 11.64 | 0.002 | 1 | 5.95 | 0.022 |
| loge embryonic period | 1 | 14.81 | 0.001 | 1 | 7.78 | 0.010 |
| natural antibody activity (error d.f. = 25; R2 = 0.72) | ||||||
| site | 1 | 22.32 | <0.001 | 1 | 2.99 | 0.096 |
| loge body mass | 1 | 0.97 | 0.34 | 1 | 1.08 | 0.31 |
| embryonic temperature | 1 | 45.98 | <0.001 | 1 | 12.21 | 0.002 |
| loge embryonic period | 1 | 33.28 | <0.001 | 1 | 9.35 | 0.005 |
| site × embryonic temperature | 1 | 22.32 | <0.001 | 1 | 3.54 | 0.071 |
| site × loge embryonic period | 1 | 15.59 | 0.001 | 1 | 4.18 | 0.052 |
When embryonic temperature was included in models, the embryonic period became highly significant and positive in direction (table 1). However, interactions with site were also highly significant (table 1), so we analysed each site separately. Tests of both measures of immune function against the temperature-corrected embryonic period (i.e. figure 1c) were positive and significant in both sites (figure 2b).
4. Discussion
A trade-off between intrinsic embryonic development rate and offspring quality traits, like immune function, is fundamental to life-history theory [17]. Yet, two broad measures of immune function showed no relationship with the absolute length of the embryonic period in bird species from both tropical and north temperate sites (i.e. figure 2a). Once embryonic temperature effects on length of embryonic periods are incorporated, the trade-off is clear (figure 2b).
These interspecific results mesh well with a recent intraspecific experimental study where nestlings from eggs that were cooled during the embryonic period had lower immune function than control nestlings [7]. Cooler embryonic temperatures can cause longer embryonic periods and decreased developmental efficiency from increased metabolic costs, and thereby compromise postnatal offspring quality [8,18]. The assumption that the absolute length of the embryonic period is an estimate of intrinsic embryonic development rate may be misplaced when embryonic temperature varies extensively, as commonly seen among passerines [9]. Embryonic development period became a significant positive predictor of nestling immune function once the extrinsic effects of temperature on embryonic development were considered.
Acknowledgements
All research was carried out under permission of University of Montana's IACUC committee.
We are grateful to C. Breuner, M. G. Palacios and two anonymous reviewers for helpful comments on the article. This work was supported by National Science Foundation, U.S.G.S. Climate Change Research Programme and USDA CSREES. E.A. was supported by a postdoctoral fellowship from the Spanish Ministry of Science and Education. Any use of trade names is for descriptive purposes only and does not imply endorsement by the US Government.
Appendix A
Species included in the sampling and analyses by location
| Cardellina rubrifrons | Arizona |
| Catharus guttatus | Arizona |
| Empidonax occidentalis | Arizona |
| Junco hyemalis | Arizona |
| Pipilo chlorurus | Arizona |
| Poecile gambeli | Arizona |
| Sialia mexicana | Arizona |
| Troglodytes aedon | Arizona |
| Turdus migratorius | Arizona |
| Vermivora celata | Arizona |
| Vermivora virginiae | Arizona |
| Atlapetes semirufus | Venezuela |
| Basileuterus tristriatus | Venezuela |
| Buarremon brunneinucha | Venezuela |
| Catharus aurantiirostris | Venezuela |
| Catharus fuscater | Venezuela |
| Dysithamnus mentalis | Venezuela |
| Grallaricula ferrugineipectus | Venezuela |
| Henicorhina leucophrys | Venezuela |
| Masius chrysopterus | Venezuela |
| Mionectes olivaceus | Venezuela |
| Myadestes ralloides | Venezuela |
| Myioborus miniatus | Venezuela |
| Myrmotherula schisticolor | Venezuela |
| Platycichla flavipes | Venezuela |
| Platycichla leucops | Venezuela |
| Premnoplex brunnescens | Venezuela |
| Saltator maximus | Venezuela |
| Tachyphonus rufus | Venezuela |
| Thraupis episcopus | Venezuela |
| Thraupis palmarum | Venezuela |
| Troglodytes aedon | Venezuela |
| Turdus olivater | Venezuela |
| Zimmerius chrysops | Venezuela |
References
- 1.Lee K. A., Wikelski M., Robinson W. D., Robinson T. R., Klasing K. C. 2008. Constitutive immune defences correlate with life-history variables in tropical birds. J. Anim. Ecol. 77, 356–363 10.1111/j.1365-2656.2007.01347.x (doi:10.1111/j.1365-2656.2007.01347.x) [DOI] [PubMed] [Google Scholar]
- 2.Ricklefs R. E. 1992. Embryonic development period and the prevalence of avian blood parasites. Proc. Natl Acad. Sci. USA 89, 4722–4725 10.1073/pnas.89.10.4722 (doi:10.1073/pnas.89.10.4722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricklefs R. E. 1993. Sibling competition, hatching asynchrony, incubation period, and life span in altricial birds. Curr. Ornithol. 11, 199–275 [Google Scholar]
- 4.Martin T. E. 2002. A new view for avian life history evolution tested on an incubation paradox. Proc. R. Soc. Lond. B 269, 309–316 10.1098/rspb.2001.1879 (doi:10.1098/rspb.2001.1879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palacios M. G., Martin T. E. 2006. Incubation period and immune function: a comparative field study among coexisting birds. Oecologia 146, 505–512 10.1007/s00442-005-0220-3 (doi:10.1007/s00442-005-0220-3) [DOI] [PubMed] [Google Scholar]
- 6.Tella J. L., Scheuerlein A., Ricklefs R. E. 2002. Is cell-mediated immunity related to the evolution of life-history strategies in birds? Proc. R. Soc. Lond. B 269, 1059–1066 10.1098/rspb.2001.1951 (doi:10.1098/rspb.2001.1951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardia D. R., Pérez J., Clotfelter E. D. 2010. Experimental cooling during incubation leads to reduced innate immunity and body condition in nestling tree swallows. Proc. R. Soc. B 277, 1881–1888 10.1098/rspb.2009.2138 (doi:10.1098/rspb.2009.2138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson C. R., Vleck C. M., Vleck D. 2006. Periodic cooling of bird eggs reduces embryonic growth efficiency. Phys. Biochem. Zool. 79, 927–936 10.1086/506003 (doi:10.1086/506003) [DOI] [PubMed] [Google Scholar]
- 9.Martin T. E., Auer S. K., Bassar R. D., Niklison A. M., Lloyd P. 2007. Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution 61, 2558–2569 10.1111/j.1558-5646.2007.00204.x (doi:10.1111/j.1558-5646.2007.00204.x) [DOI] [PubMed] [Google Scholar]
- 10.Martin T. E., Schwabl H. 2008. Variation in maternal effects and embryonic development rates among passerine species. Phil. Trans. R. Soc. B 363, 1663–1674 10.1098/rstb.2007.0009 (doi:10.1098/rstb.2007.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin L. B., Han P., Lewittes J., Kuhlman J. R., Klasing K. C., Wikelski M. 2006. Phytohemagglutinin (PHA) induced skin swelling in birds: histological support for a classic immunoecological technique. Funct. Ecol. 20, 290–299 10.1111/j.1365-2435.2006.01094.x (doi:10.1111/j.1365-2435.2006.01094.x) [DOI] [Google Scholar]
- 12.Matson K. D., Ricklefs R. E., Klasing K. C. 2004. A hemolysis–hemagglutination assay for characterizing constitutive innate humoral immunity in wild and domestic birds. Devel. Comp. Immunol. 29, 275–286 10.1016/j.dci.2004.07.006 (doi:10.1016/j.dci.2004.07.006) [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez G., Sorci G., Møller A. P., Ninni P., Haussy C., De Lope F. 1999. Immunocompetence and condition-dependent sexual advertisement in male house sparrows (Passer domesticus). J. Anim. Ecol. 68, 1225–1234 10.1046/j.1365-2656.1999.00364.x (doi:10.1046/j.1365-2656.1999.00364.x) [DOI] [Google Scholar]
- 14.Palacios M. G., Cunnick J. E., Vleck D., Vleck C. M. 2009. Ontogeny of innate and adaptive immune defense components in free-living tree swallows, Tachycineta bicolor. Devel. Comp. Immunol. 33, 456–463 10.1016/j.dci.2008.09.006 (doi:10.1016/j.dci.2008.09.006) [DOI] [PubMed] [Google Scholar]
- 15.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15 10.1086/284325 (doi:10.1086/284325) [DOI] [Google Scholar]
- 16.Purvis A., Rambaut A. 1995. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. Comput. Appl. Biosci. 11, 247–251 [DOI] [PubMed] [Google Scholar]
- 17.Roff D. A. 1992. The evolution of life histories. London, UK: Chapman and Hall Publishers [Google Scholar]
- 18.Qualls C. P., Andrews R. M. 1999. Cold climates and the evolution of viviparity in reptiles: cold incubation temperatures produce poor-quality offspring in the lizard, Sceloporus virgatus. Biol. J. Linn. Soc. 67, 353–376 [Google Scholar]