Abstract
Siblings within the same family often differ dramatically in phenotype. Some differences are attributable to initial maternal handicaps (birth or hatching asynchrony, differences in egg or neonate size and hormonal or antioxidant titre); but differences among siblings may also arise from differences in the brood-rearing environment that offspring experience. Here, I use a model system—a long-term study of nestlings in an altricial bird—to study how an initial maternal handicap, hatching asynchrony, regulates the effective social environment of siblings in the same family as measured by offspring survival. The interaction of family size and structure generated wide differences in the effective environments of siblings living in the same physical space (a nest), and reared by the same parents, in the same family structure. Social rank was the key component of the unshared environment of contemporary siblings, and was alone sufficient to generate near-maximal differences in offspring performance. Nestlings sitting side-by-side effectively lived in different worlds.
Keywords: social rank, birth rank, hatching asynchrony, maternal effect, shared and non-shared environment, effective and objective environment
1. Introduction
Phenotypic variation among siblings is widespread in taxa ranging from parasitoid wasps to birds and mammals [1]. The obvious question is why? Some variations stem from initial manipulations of offspring phenotype that often arise from maternal effects—e.g. differences in egg or neonate size, birth or hatching asynchrony and hormone or antioxidant titre (reviews in [2,3]). But these maternal handicaps also alter the developmental environment experienced by contemporary siblings. Recent work in birds shows that environmental influences during brood-rearing may induce phenotypic variation in the progeny [4,5]. A large and parallel body of work on human families examines the environmental origins of phenotypic diversity among children—e.g. in personality, intelligence, behaviour and growth [6–11]. Here the non-shared environment is posited to explain differences among siblings [12]: these are the environmental influences unique to each child, such as birth or social rank and peer relations, and not those general to the entire family (the shared environment).
Identifying the features of the non-shared environment contributing to within-family diversity requires measurement of the relationship between the objective and effective environments that offspring experience [12]. The objective environment is defined by characteristics that can be measured directly such as family size, birth rank or the number of parents. It provides a quantitative measure of the offspring environment. The effective environment provides an estimate of the consequences of the objective environment, and is not directly measurable. Rather, it is inferred from the outcome of offspring performance. In short, the objective environment helps us to explain why offspring differ; the effective environment tells us how much.
Nestling birds are a useful model to study the objective and effective environments of siblings within a family. They share the same physical space (a nest), parents and family characteristics (all properties of the shared environment), but often differ in social rank owing to asynchronous hatching [13,14], an element of the unshared environment. Here, in an altricial songbird, I measure the relationship between the objective and effective environments experienced by siblings within the same family. I use a simple metric—whether an offspring lives or dies over the nestling period—to show how the effective environment is derived from properties of the initial objective environment: the size and the structure of the family in which a nestling lives, and most importantly, offspring social rank.
2. Material and methods
I studied red-winged blackbirds (Agelaius phoeniceus) nesting in near Winnipeg, Manitoba over 17 field seasons (1993–2009). Daily censuses were conducted at an average of 278 nests each year with the assistance of a field crew. Detailed methods have been described elsewhere [15,16] and I present only a précis here. Nests were visited from egg-laying until nestlings reached at least 8 days of age (hatching = day 1). Nestlings were marked for individual identification and generally not handled after day 10 to prevent premature fledging.
I divide the brood into core and marginal elements [17,18] based upon the hatching pattern of individual offspring. All nest-mates hatching on the first day of the nestling period are core offspring; nestlings hatching one or more days later are marginal offspring (see [16] for detailed methods of classifying core versus marginal progeny). I further subdivide the marginal brood into three levels: m1, m2 and m3. Because of the incubation pattern of red-winged blackbirds, these subscript designations correspond both to the number of days that a marginal offspring hatches after its core counterparts, and its rank within the marginal brood. If, for example, three marginal offspring are present alongside one or more core offspring, the m1, m2 and m3 progeny will usually have hatched 1, 2 and 3 days after the core offspring. I denote family size and structure as cj where c is the number of core hatchlings and j the number of marginal hatchlings [15]. Rank within the brood is denoted as either core or mi where m denotes marginal status and the subscript i denotes rank within the marginal brood); m1 progeny are found in all broods with at ≥1 marginal offspring, m3 progeny are only found in broods with ≥3 marginal progeny. Table 1 shows the mean survival to day 8 of core, m1, m2 and m3 progeny in the 13 most common brood structures. Although survival to leave the nest is an obviously incomplete measure of fitness, it is still a useful proxy, as survival among specific classes of offspring showed near-maximal variation and fledging success is the robust predictor of recruitment to breeding [19]. I computed bootstrap confidence intervals because data for core broods had a polytomous distribution.
Table 1.
Mean proportion of offspring surviving to 8 days of age (a measure of the effective environment) in relation to social rank (core, m1, m2, m3) and family structure in red-winged blackbirds nesting near Winnipeg, Manitoba, 1993–2009; n is the number of broods; 95% bootstrap confidence intervals are shown.
| family structure | social rank |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| core | 95% CI | n | m1 | 95% CI | n | m2 | 95% CI | n | m3 | 95% CI | n | |
| 10 | 1.000 | 22 | ||||||||||
| 11 | 0.959 | 1.000–0.868 | 49 | 0.898 | 0.924–0.717 | 49 | ||||||
| 12 | 0.904 | 0.963–0.842 | 73 | 0.868 | 0.945–0.795 | 68 | 0.750 | 0.863–0.658 | 68 | |||
| 13 | 0.988 | 1.000–0.965 | 84 | 0.952 | 0.988–0.905 | 83 | 0.843 | 0.917–0.762 | 83 | 0.366 | 0.482–0.265 | 82 |
| 20 | 0.949 | 0.955–0.836 | 39 | |||||||||
| 21 | 0.888 | 0.928–0.845 | 129 | 0.722 | 0.775–0.627 | 126 | ||||||
| 22 | 0.912 | 0.939–0.877 | 181 | 0.717 | 0.788–0.658 | 180 | 0.400 | 0.473–0.337 | 180 | |||
| 23 | 0.904 | 0.981–0.815 | 26 | 0.920 | 1.000–0.800 | 25 | 0.480 | 0.680–0.280 | 25 | 0.160 | 0.320–0.040 | 25 |
| 30 | 0.874 | 0.944–0.840 | 45 | |||||||||
| 31 | 0.825 | 0.879–0.800 | 156 | 0.382 | 0.500–0.359 | 152 | ||||||
| 32 | 0.917 | 0.967–0.789 | 28 | 0.481 | 0.667–0.296 | 27 | 0.111 | 0.259–0 | 27 | |||
| 40 | 0.800 | 0.850–0.613 | 15 | |||||||||
| 41 | 0.875 | 1.000–0.800 | 8 | 0.375 | 0.600–0 | 8 | ||||||
3. Results
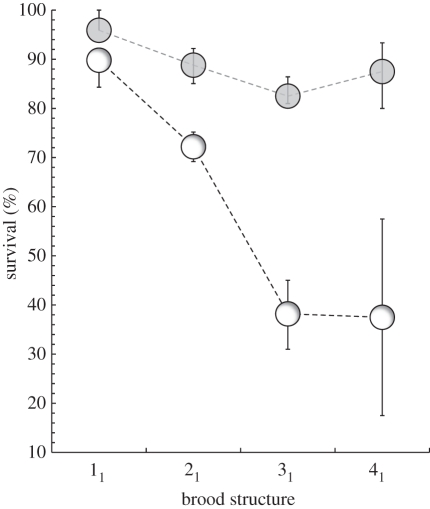
Table 1 shows point estimates of mean survival of focal offspring by family size and structure for the entire 17 year dataset. Survival to day 8 showed a near-maximal range of variation from the virtually assured survival of both core and marginal progeny in small broods to the near-certain death of low-ranking marginal nestlings in large broods. Overall, there was a greater than eightfold range of variation in mean nestling survival, with core progeny generally enjoying higher survival than marginal siblings in the same family: as brood size grew, particularly those broods with more core progeny, the gap between the survival of core and marginal siblings also grew (table 1). An easily measured property of the objective environment, hatching asynchrony, had very different effects on progeny survival that were conditional on family size and structure: m1 progeny (1 day asynchrony) enjoyed near-certain survival in 11 broods but survival fell sharply as core brood size grew (figure 1). Similarly, m2 progeny (2 day asynchrony) enjoyed high (75%) survival in 12 broods, but just 11 per cent survival in 32 broods (table 1), a sevenfold difference in mean nestling survival.
Figure 1.
Example of how the effective environment is conditional upon social rank and family size, and not the initial maternal handicap (a 1 day hatching asynchrony) per se. The mean survival of core (shaded circles) and m1 marginal nestlings (open circles) to 8 days of age in relation to brood structure is shown. Whiskered lines represent 95% bootstrap confidence intervals.
I used multiple linear regression of the point estimates of focal offspring survival (weighted by sample size) to determine how much variation in the effective environment was attributable to two properties of the objective environment, family size and structure. Data were arcsine √x transformed for normality. Overall social rank, core brood size at hatching, and their interaction explained approximately 90 per cent of variation in survival (r2adj = 0.898, F3,25 = 82.98, p ≪ 0.001). Lower social rank (β = −0.093, t = −1.930, p = 0.065) and particularly a larger core brood at hatching (β = −0.133, t = −4.591, p ≪ 0.001), and the interaction of rank and core brood size (β = −0.105, t = −3.938, p = 0.001) was strongly associated with reduced nestling survival.
4. Discussion
Family size and offspring social rank, two elements of the objective environment, together explained striking differences in the effective environments for siblings within families. Both within and across families, there was a greater than eightfold difference in mean nestling survival and most of this variation was accounted for by the social rank of the focal offspring, the number of core hatchlings in the family, and its interaction. Mothers constructed different social niches for their progeny with their initial choices of family size and structure, and even identical phenotypic handicaps could lead to dramatically different outcomes for offspring—e.g. a 1 day hatching interval. Survival costs for high-ranking marginal progeny in small broods compared with their core counterparts were negligible. Conversely, the costs were great for low-ranking marginal progeny in large broods. Although key properties of the initial objective environment were shared among siblings—parents, the same nest, family size and structure—a key unshared component, social rank created different effective environments.
The result is important, not just because social rank can generate diversity in offspring performance within the family. The different social environments they create also present very different challenges for contemporary siblings during brood-rearing. Elegant work by Mainwaring et al. [5] shows that social rank, and not maternal effects, is the chief determinant of phenotypic variation in nestling blue tits (Cyanistes caeruleus) in a suite of traits linked to sibling competition—e.g. nestlings prioritized growth of tarsi over feather development in order to maintain standing within the nest. Similarly, Gil et al. [4] showed that gape area of nestling spotless starlings (Sturnus unicolor) increases under the influence of sibling competition. Offspring phenotypes, evidently, are plastic and respond to different effective environments. As shown here, such environments can be very different indeed.
It is not hard to draw parallels between nestling birds that differ in hatching rank, and human children that differ in birth rank. There is vigorous debate among behavioural geneticists, developmental psychologists, sociologists and demographers over the origins of differences among siblings in personality, behaviour, intelligence, growth or health status [7,8,10,20–22]. Much of the controversy centres on the explanatory features of the non-shared environment: is the intra-family (e.g. birth rank, [8,11]) or extra-family environment (e.g. peer relations, [6,21]) more important? My work on a non-human animal model eliminates the extra-family environment as a potential source of variation, as contemporary offspring share the same confined physical space, the nest. I show that differences in social rank are alone sufficient to generate near-maximal differences in offspring performance, supporting the view that the non-shared environment within the family is important. Offspring sitting side-by-side can live in different worlds.
Acknowledgements
This study meets the terms of the ethics committee at the institution where the experiment was carried out.
I am grateful for the assistance of a large team of field workers and funding from NSERC. I thank Douglas Mock, Mark Hauber and anonymous reviewers for helpful comments.
References
- 1.Mock D. W., Parker G. A. 1997. The evolution of sibling rivalry. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Groothuis T. G. G., Muller W., von Engelhardt N., Carere C., Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352 10.1016/j.neubiorev.2004.12.002 (doi:10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 3.Sockmann K. W., Sharp P. J., Schwabl H. 2006. Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility in clutch size, incubation behavior, and yolk androgen deposition. Biol. Rev. 81, 629–666 10.1017/S1464793106007147 (doi:10.1017/S1464793106007147) [DOI] [PubMed] [Google Scholar]
- 4.Gil D., Bulmer E., Celis P., López I. 2008. Adaptive developmental plasticity in growing nestlings: sibling competition induces differential gape growth. Proc. R. Soc. B 275, 549–554 10.1098/rspb.2007.1360 (doi:10.1098/rspb.2007.1360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mainwaring M. C., Dickens M., Hartley I. R. 2010. Environmental and not maternal effects determine variation in offspring phenotypes in a passerine bird. J. Evol. Biol. 23, 1302–1311 10.1111/j.1420-9101.2010.01997.x (doi:10.1111/j.1420-9101.2010.01997.x) [DOI] [PubMed] [Google Scholar]
- 6.Harris J. R. 1995. Where is the child's environment? A group socialization theory of development. Psychol. Rev. 102, 458–489 10.1037/0033-295X.102.3.458 (doi:10.1037/0033-295X.102.3.458) [DOI] [Google Scholar]
- 7.Plomin R., Daniels D. 1987. Why are children in the same family so different from one another? Behav. Brain Sci. 10, 1–60 10.1017/S0140525X00055941 (doi:10.1017/S0140525X00055941) [DOI] [Google Scholar]
- 8.Sulloway F. J. 1996. Born to rebel: birth order, family dynamics, and creative lives. New York, NY: Pantheon Books [Google Scholar]
- 9.Kristensen P., Bjerkedal T. 2007. Explaining the relation between birth order and intelligence. Science 316, 1717. 10.1126/science.1141493 (doi:10.1126/science.1141493) [DOI] [PubMed] [Google Scholar]
- 10.Lawson D. W., Mace R. 2008. Sibling configuration and childhood growth in contemporary British families. Int. J. Epidem. 37, 1408–1421 10.1093/ije/dyn116 (doi:10.1093/ije/dyn116) [DOI] [PubMed] [Google Scholar]
- 11.Sulloway F. J. 2007. Birth order and sibling competition. In Handbook of evolutionary psychology (eds Dunbar R., Barrett L.), pp. 297–311 Oxford, UK: Oxford University Press [Google Scholar]
- 12.Turkheimer E., Waldron M. 2000. Nonshared environment: a theoretical methological, and quantitative review. Psychol. Bull. 126, 78–108 10.1037/0033-2909.126.1.78 (doi:10.1037/0033-2909.126.1.78) [DOI] [PubMed] [Google Scholar]
- 13.Lack D. 1947. The significance of clutch-size. Ibis 89, 302–352 10.1111/j.1474-919X.1947.tb04155.x (doi:10.1111/j.1474-919X.1947.tb04155.x) [DOI] [Google Scholar]
- 14.Magrath R. D. 1990. Hatching asynchrony in altricial birds. Biol. Rev. 65, 587–622 10.1111/j.1469-185X.1990.tb01239.x (doi:10.1111/j.1469-185X.1990.tb01239.x) [DOI] [Google Scholar]
- 15.Forbes S. 2009. Portfolio theory and how parent birds manage investment risk. Oikos 118, 161–169 10.1111/j.1600-0706.2009.17702.x (doi:10.1111/j.1600-0706.2009.17702.x) [DOI] [Google Scholar]
- 16.Forbes S. 2010. Family structure and variation in reproductive success in blackbirds. Behav. Ecol. Sociobiol. 64, 475–483 10.1007/s00265-009-0863-x (doi:10.1007/s00265-009-0863-x) [DOI] [Google Scholar]
- 17.Forbes L. S., Thornton S., Glassey B., Forbes M., Buckley N. J. 1997. Why parent birds play favourites. Nature 390, 351–352 10.1038/37025 (doi:10.1038/37025) [DOI] [Google Scholar]
- 18.Mock D. W., Forbes L. S. 1995. The evolution of parental optimism. Trends Ecol. Evol. 10, 130–134 10.1016/S0169-5347(00)89014-X (doi:10.1016/S0169-5347(00)89014-X) [DOI] [PubMed] [Google Scholar]
- 19.Weatherhead P. J., Dufour L. W. 2000. Fledging success as an index of recruitment in red-winged blackbirds. Auk 117, 627–633 10.1642/0004-8038(2000)117[0627:FSAAIO]2.0.CO;2 (doi:10.1642/0004-8038(2000)117[0627:FSAAIO]2.0.CO;2) [DOI] [Google Scholar]
- 20.Freese J., Powell B., Steelman L. C. 1999. Rebel without a cause or effect: birth order and social attitudes. Am. Sociol. Rev. 64, 207–231 10.2307/2657528 (doi:10.2307/2657528) [DOI] [Google Scholar]
- 21.Harris J. R. 1998. The nurture assumption. New York, NY: Free Press [Google Scholar]
- 22.Jenkins J., Simpson A., Dunn J., Rasbash J., O'Connor T. 2005. Mutual influence of marital conflict and children's behavior problems: shared and nonshared family risks. Child Dev. 76, 24–39 10.1111/j.1467-8624.2005.00827.x (doi:10.1111/j.1467-8624.2005.00827.x) [DOI] [PubMed] [Google Scholar]