Abstract
Although many studies have examined social learning capabilities in apes and monkeys, experiments involving prosimians remain largely absent. We investigated the potential for social learning in black-and-white ruffed lemurs using a two-action foraging task. Eight individuals were divided into two experimental groups and exposed to conspecifics using one of two techniques to access food. Subjects were then given access to the apparatus and their retrieval techniques were recorded and compared. All subjects made their first retrieval using the technique they observed being demonstrated, and there were significant differences between the two groups in their overall response patterns. These results suggest that prosimians are capable of social learning and that additional long-term field studies may reveal the presence of behavioural traditions similar to those found in other primates.
Keywords: behavioural traditions, culture, two-action method
1. Introduction
Social learning is an important cognitive skill that allows an animal to acquire information about its environment through the observation of conspecifics rather than through trial-and-error learning. As such, social learning has been investigated in several animal taxa, but has been an area of particular interest in the study of non-human primates [1]. One reason for such extensive interest in this topic among primatologists is that social learning is thought to play a central role in the spread and maintenance of group-specific traditions, or ‘cultures’, a characteristic once thought to be uniquely human [2]. Perhaps not surprisingly, some of the most compelling evidence for non-human culture can be found among the apes, with both chimpanzees and orangutans exhibiting an impressive number of behavioural traditions [3,4]. Although relatively few monkey species have been studied extensively enough to allow for cross-community comparisons, in-depth studies of both Japanese macaques and white-faced capuchins have revealed a more modest number of traditions [5].
Despite the attention that such findings have generated, the field of cultural primatology remains very much in its infancy. Indeed, there is much debate in the literature over how ‘culture’ ought to be defined, making it difficult to reach a consensus regarding which animals exhibit it [2,5]. Less contentious is the idea that social learning is a necessary precursor to culture; therefore, a critical investigation of social learning across phylogenetically diverse species can provide important information on the evolutionary origin of culture, as well as the cognitive and socioecological factors that may precede it.
Prosimians, the most primitive evolutionary branch of primates, have been largely ignored in studies of social learning. This lack of attention is probably, in part, a result of early reports suggesting that lemurs do not possess many of the cognitive abilities present in other primates [6]. Nevertheless, some recent studies suggest that lemur cognitive abilities are more developed than previously thought [7–10] and so they may be capable of social learning. Although there have been some observational studies of the social influences on learning in lemurs, experiments focusing specifically on the social transmission of information remain limited [11–15].
In this study, our goal was to experimentally examine social learning in black-and-white ruffed lemurs (Varecia variegata) using a two-action foraging task [16]. The methodology involves first training a demonstrator to retrieve food from an apparatus using one of two possible techniques, in this case either by lifting or swinging a hinged door. After observing a demonstrator using one technique, subjects are given access to the apparatus with both techniques available for use. If there is a reliable bias in favour of the observed action rather than the alternative method, the observer is said to have acquired the behaviour socially [16]. It is important to note that our focus here was not to determine the specific type of social learning taking place (imitation, emulation, etc.) but rather to investigate lemurs' abilities to engage in social learning in general.
2. Material and methods
(a). Subjects
A family group of nine black-and-white ruffed lemurs housed at Zoo Atlanta served as subjects. Animals were divided into two test groups: Lift group (IA, KA, PO and PH) and Swing group (ML, MN, LU and UM). Both groups contained adults and juveniles. An additional individual (MV) acted only as a demonstrator. Testing occurred in their indoor enclosure, which was divided into multiple rooms and thus enabled individual animals to be isolated from one another during testing. All subjects had access to their usual daily diet throughout testing.
(b). Apparatus
The experimental apparatus consisted of a 30 cm tube of 3 inch diameter PVC (polyvinyl chloride) capped at both ends, with a 3.5 cm opening cut in the centre through which subjects could retrieve food (figure 1). There were two methods through which subjects could access the opening—either by lifting a lightweight aluminum hinge (Lift) or by sliding a circular piece of aluminum to the left (Swing). The Swing was mounted on top of the Lift so that both covered the same opening, but could not be used simultaneously. Both actions could be closed and locked to restrict usage to a single action during demonstrator training or observation sessions. The apparatus was loaded with food and mounted in the subjects' enclosure at the start of each session and removed immediately following each session. All sessions were a minimum of 10 min in length.
Figure 1.
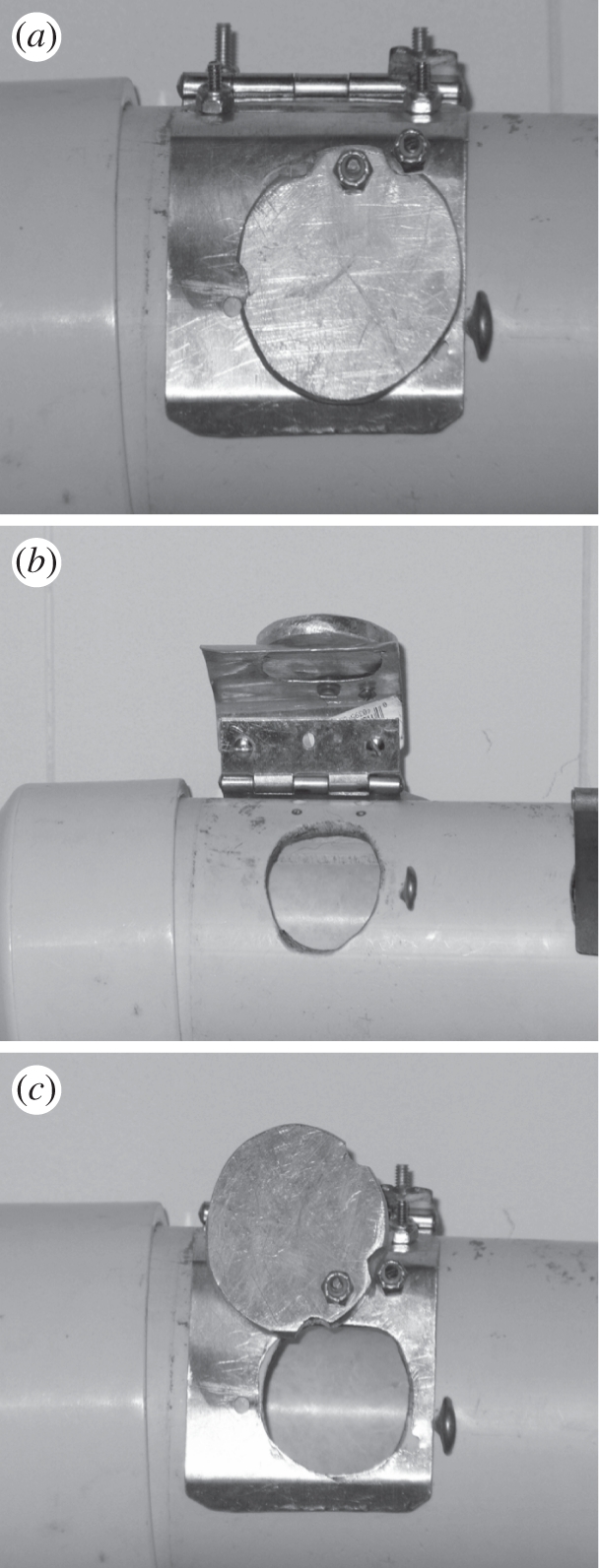
(a) The apparatus with both actions closed. (b) The apparatus with the Lift open. (c) The apparatus with the Swing open.
(c). Procedure
(i). Lift group
All subjects were shown only the Lift method by a single demonstrator (MV). The demonstrator was trained to use the Lift by locking the Swing action during training sessions. After nine training sessions, we unlocked the Swing action, and she continued to use the Lift 100 per cent of the time. We then gave all four subjects visual access to the demonstrator using the apparatus through a 4 × 2.5 m wall of wire mesh for 13 observation sessions. The demonstrator made an average of 25 successful retrievals per session. Both the demonstrator and four observers were then given access to the apparatus with both actions available (testing sessions); however, it was quickly clear that the demonstrator was monopolizing the apparatus, and we then began testing individuals in physical and visual isolation (average = 15.5 sessions per individual).
(ii). Swing group
All subjects were shown only the Swing method by the demonstrators. Because of housing changes (i.e. the need to subgroup while inside) and an illness in MV, multiple individuals (IA, KA, ML and UM) acted as demonstrators. Individuals only served as demonstrators after completing all of their own testing sessions. Because several of the demonstrators had previously been exposed to the Lift technique, only the Swing method was available during all observation sessions. Subjects were given visual access to a demonstrator as described above for a minimum of 10 (up to 15) observation sessions. Demonstrators made an average of 12 successful retrievals per session. Each individual was then visually and physically isolated from the group and given access to the apparatus, with both actions available, for three testing sessions. Fewer testing sessions were used under this condition because individuals needed to be available to move to another institution.
(d). Data collection
For all observation sessions, we collected data on the amount of time each individual spent observing the demonstrator(s), defined as being within two body lengths of the apparatus and directing visual attention towards it while the demonstrator made retrievals. Although subjects may have observed retrievals from a greater distance, time spent observing from within two body lengths could be determined with the highest degree of accuracy. During testing sessions, we recorded the first retrieval attempt and first successful retrieval. We also recorded the total number of successful retrievals using the Swing and Lift. We tested for differences between the experimental groups based on each subject's first 42 retrievals, which was the minimum number of retrievals made by a subject; however, results remain significant when all retrievals are included.
3. Results
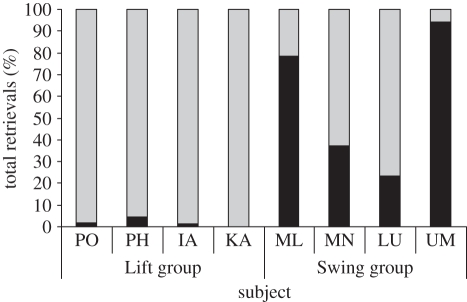
IA, KA, PO and PH (Lift group) observed the demonstrator manipulate the apparatus for a total of 12 (6.2%), 37 (19.0%), 44 (22.5%) and 38 (19.3%) min, respectively. ML, MN, LU and UM (Swing group) observed the demonstrator(s) manipulate the apparatus for a total of 16 (25.5%), 3 (4.6%), 4 (5.3%) and 9 (20.6%) min, respectively. We found a significant positive correlation between the amount of time spent observing a model and copying fidelity (e.g. the per cent of responses that matched the model; Spearman's ρ = 0.719, p = 0.045). All subjects in both experimental groups made their first retrieval attempts (which were all successful) using the demonstrated method. There were significant differences between the two experimental groups in the percentage of lift/swing + lift (Wilcoxon–Mann–Whitney test, z = −2.309, p = 0.029). Figure 2 shows the per cent of all retrievals using the Swing and Lift for each individual. Although all four Lift subjects maintained a high degree of copying fidelity across testing sessions, only two of the Swing subjects were observed to do so (table 1).
Figure 2.
Per cent of total retrievals for each subject using the Swing (black bars) versus the Lift (grey bars).
Table 1.
Number of retrievals by subject using the demonstrated method over time. Swing subjects in italics.
| subject | retrievals (1–10) | retrievals (11–20) | retrievals (21–30) | … final 10 retrievals |
|---|---|---|---|---|
| PO (Potter) | 10 | 10 | 6 | 10 |
| PH (Phinneaus) | 10 | 6 | 7 | 10 |
| IA (Ian) | 10 | 10 | 10 | 10 |
| KA (Kahloh) | 10 | 10 | 10 | 10 |
| ML (Malaky) | 8 | 7 | 9 | 9 |
| MN (Menabe) | 8 | 4 | 2 | 1 |
| LU (Luna) | 5 | 4 | 2 | 0 |
| UM (unnamed male) | 8 | 10 | 9 | 10 |
4. Discussion
Using a two-action paradigm, the current study found evidence of social learning in black-and-white ruffed lemurs. All of the experimental subjects used the demonstrated technique on their very first attempt, which is considered an important indication of social learning [17]. Furthermore, the two experimental groups showed significant differences in the percentage of retrievals made using the two different methods. Although a recent open diffusion study by Kendal et al. [15] found evidence of social learning in ring-tailed lemurs using a similar two-action foraging task, it only pertained to which of three variations on the Lift technique was used. Thus, this is the first lemur study to find evidence of social learning on a two-action task that is similar to what has been observed in other primate species [16].
Despite clear evidence of social learning, the low level of copying fidelity shown by two of the Swing subjects makes it unlikely that a pattern of distinct behavioural traditions, as has been observed in other primates [16,18], would have been maintained. This could be a result of the two individuals' limited observation time, as suggested by the positive relationship between time spent observing and copying fidelity. Alternatively, this task was also easy to solve (pilot data found response frequencies on the Swing and Lift in naive animals were roughly equivalent), and Kendal et al. [15] reported a potential underlying bias towards the Lift method among ring-tailed lemurs. In a more complex task where spontaneous discovery of a preferred method was less likely, we might expect to see greater fidelity. Finally, these findings could reflect a qualitative difference between lemurs and other primates in the degree to which social learning influences behaviour. These questions are ones that future research should address.
The results presented here indicate that neglecting to include prosimians in studies of cognition results in an incomplete picture of social learning processes and the origin of behavioural traditions. Because prosimians represent a link between anthropoids and other mammals, cognitive studies of lemurs can uniquely contribute to the debate regarding whether instances of social learning within the Primate order, as well as the wider mammalian taxon, are the result of convergent evolution or common ancestry. In addition, our results suggest that interpopulation behavioural traditions, like those observed in other wild primates [2,5], may be present in wild populations of lemurs. Hopefully, additional long-term field studies can answer this question and thus shed further light on the evolution of culture.
Acknowledgements
All experiments comply with animal care and safety laws and were approved by Zoo Atlanta's scientific review board.
Special thanks to the Zoo Atlanta primate staff, Becky Antworth, Angela Legg, Marietta Dindo and Hannah Jaicks.
References
- 1.Caldwell C. A., Whiten A. 2002. Evolutionary perspectives on imitation: is a comparative psychology of social learning possible? Anim. Cogn. 5, 193–208 10.1007/s10071-002-0151-x (doi:10.1007/s10071-002-0151-x) [DOI] [PubMed] [Google Scholar]
- 2.McGrew W. C. 1998. Culture in nonhuman primates. Annu. Rev. Anthropol. 27, 301–328 10.1146/annurev.anthro.27.1.301 (doi:10.1146/annurev.anthro.27.1.301) [DOI] [Google Scholar]
- 3.Whiten A., Goodall J., McGrew W. C., Nishida T., Reynolds V. 1999. Cultures in chimpanzees. Nature 399, 682–685 10.1038/21415 (doi:10.1038/21415) [DOI] [PubMed] [Google Scholar]
- 4.van Schaik C. P., Ancrenaz M., Borgen G., Galdikas B., Knott C. D., Singleton I., Suzuki A., Utami S. S., Merrill M. 2003. Orangutan cultures and the evolution of material culture. Science 299, 102–105 10.1126/science.1078004 (doi:10.1126/science.1078004) [DOI] [PubMed] [Google Scholar]
- 5.Perry S., Manson J. H. 2003. Traditions in monkeys. Evol. Anthropol. 12, 71–81 10.1002/evan.10105 (doi:10.1002/evan.10105) [DOI] [Google Scholar]
- 6.Jolly A. 1966. Lemur social behavior and primate intelligence. Science 153, 501–506 10.1126/science.153.3735.501 (doi:10.1126/science.153.3735.501) [DOI] [PubMed] [Google Scholar]
- 7.Santos L. R., Mahajan N., Barnes J. L. 2005. How prosimian primates represent tools: experiments with two lemur species (Eulemur fulvus and Lemur catta). J. Comp. Psychol. 119, 394–403 10.1037/0735-7036.119.4.394 (doi:10.1037/0735-7036.119.4.394) [DOI] [PubMed] [Google Scholar]
- 8.Lewis K. P., Jaffe S., Brannon E. M. 2005. Analog number representations in mongoose lemurs (Eulemur mongoz): evidence from a search task. Anim. Cogn. 8, 247–252 10.1007/s10071-004-0251-x (doi:10.1007/s10071-004-0251-x) [DOI] [PubMed] [Google Scholar]
- 9.MacLean E. L., Merritt D. J., Brannon E. M. 2008. Social complexity predicts transitive reasoning in prosimian primates. Anim. Behav. 76, 479–486 10.1016/j.anbehav.2008.01.025 (doi:10.1016/j.anbehav.2008.01.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruiz A., Gomez J. C., Roeder J. J., Byrne R. W. 2009. Gaze following and gaze priming in lemurs. Anim. Cogn. 12, 427–434 10.1007/s10071-008-0202-z (doi:10.1007/s10071-008-0202-z) [DOI] [PubMed] [Google Scholar]
- 11.Hosey G. R., Jacques M., Pitts A. 1997. Drinking from tails: social learning of a novel behaviour in a group of ring-tailed lemurs (Lemur catta). Primates 38, 415–422 10.1007/BF02381881 (doi:10.1007/BF02381881) [DOI] [Google Scholar]
- 12.Kappeler P. M. 1987. The acquisition process of a novel behavior pattern in a group of ring-tailed lemurs (Lemur catta). Primates 28, 225–228 10.1007/BF02382571 (doi:10.1007/BF02382571) [DOI] [Google Scholar]
- 13.Fornasiere I., Anderson J. R., Roeder J. J. 1990. Responses to a novel food acquisition task in three species of lemurs. Behav. Process. 21, 143–156 10.1016/0376-6357(90)90021-7 (doi:10.1016/0376-6357(90)90021-7) [DOI] [PubMed] [Google Scholar]
- 14.Anderson J. R., Fornasieri I., Ludes E., Roeder J. J. 1992. Social processes and innovative behaviour in changing groups of Lemur fulvus. Behav. Process. 27, 101–112 10.1016/0376-6357(92)90020-E (doi:10.1016/0376-6357(92)90020-E) [DOI] [PubMed] [Google Scholar]
- 15.Kendal R. L., Custance D., Kendal J. R., Vale G., Stoinski T., Rakotomalala N. I., Rasaminanana H. 2010. Evidence for social learning in wild lemurs (Lemur catta). Learn. Behav. 38, 220–234 10.3758/LB.38.3.220 (doi:10.3758/LB.38.3.220) [DOI] [PubMed] [Google Scholar]
- 16.Caldwell C. A., Whiten A. 2007. Social learning in apes and monkeys: cultural animals? In Primates in perspective (eds Campbell C. J., Fuentes A., MacKinnon K. C., Panger A., Bearder S. K.), pp. 652–663 New York, NY: Oxford University Press [Google Scholar]
- 17.Whiten A. 2002. The imitator's representation of the imitated. In The imitative mind: development, evolution, and brain bases (eds Meltzoff A. N., Prinz W.), pp. 98–121 Cambridge, UK: Cambridge University Press [Google Scholar]
- 18.Whiten A., Horner V., de Waal F. B. M. 2005. Conformity to cultural norms of tool use in chimpanzees. Nature 437, 737–740 10.1038/nature04047 (doi:10.1038/nature04047) [DOI] [PubMed] [Google Scholar]