Abstract
Animal contests often involve the use of repeated signals, which are assumed to advertise stamina, and hence fighting ability. While an individual may be predicted to give up once it has crossed an energetic threshold, costs inflicted by its opponent may also contribute to the giving-up decision. Therefore, physical strength should be of key importance in contests, allowing high signal magnitude as well as potentially inflicting costs. We investigated this using hermit crab shell fights, which employ a ‘hybrid signal’ of shell rapping, which advertises stamina but also imposes potentially deleterious consequences for the receiver. We examined the links between contest outcomes and two proxies for strength; the protein content and relative mass of hermit crab abdominal muscles, the main muscle group used in shell rapping. Our results indicate that there was no difference in muscle protein between winners and losers, whereas winners had significantly greater muscle mass : body mass ratios. Thus, while stamina has been assumed by theory to be an important determinant of agonistic success, the present results demonstrate the importance of muscle size and thereby strength.
Keywords: assessment, game theory, hermit crab, fight, muscle, strength
1. Introduction
Theoretical models of contest behaviour vary in their assumptions about which traits should most strongly influence resource-holding potential (RHP) [1]. The Energetic War of Attrition [2] assumes that repeated agonistic signals demonstrate the sender's stamina, which may be quantified through analysis of post-fight energetic status [3,4], whole body endurance capacities [5,6] or aerobic capacity [7]. Models such as the Sequential Assessment Model [8] and the Cumulative Assessment Model [9] assume that the ability to harm the opponent may be important. In these cases an individual's strength, as well as its stamina, should influence RHP, because this will determine the ability to inflict injuries or disorienting blows. Indeed, lizards with high bite forces [10], and crabs with high claw strength [11], have been shown to defeat weaker opponents. If strength (the force generated by muscle contraction) is a key component of RHP, we would expect to see greater investment in traits such as muscle mass or quality in individuals that win fights compared with the opponents they defeat.
In some contests, both opponents may perform displays that advertise their strength [11,12]. In many examples, however, there are asymmetric roles, in which the animals fight in different ways, and the benefits of being strong may vary between each opponent. In dung beetles, an intruder male will attempt to pull a resident out of its tunnel and the resident will attempt to resist [13]. When hermit crabs, Pagurus bernhardus, fight over gastropod shells, the attacker performs shell-rapping signals [14] by repeatedly hitting its shell against the defender's shell, while the defender remains tightly withdrawn into the shell for most of the encounter (figure 1). The fight concludes when either the defender decides to give up, allowing itself to be evicted, or the attacker decides to give up first without evicting the defender. Attackers who rap more vigorously are more likely to win [14] and analyses of post-fight metabolites [4,15] and endurance capacities [5] indicate that temporal vigour is related to stamina. Successful attackers also hit harder [15,16] than those that give up, indicating that strength as well as stamina may be important. Indeed, overall body size has already been shown to be an important factor influencing agonistic success in hermit crab fights [17], but the relative strength of opponents has yet to be explored.
Figure 1.
A shell fight in Pagurus bernhardus. (a) The attacker contacts the shell of the defender, (b) inserts its chelipeds into the aperture of the defender's shell and (c) performs bouts of shell rapping, (d) which may result in the eviction of the defender.
If the ability to inflict direct costs on defenders, as well as advertising stamina, is a significant feature of these encounters, we would expect to see greater investment in muscle quality in victorious attackers compared with attackers who give up, such that successful attackers should have greater muscle mass and protein content than attackers who give up. Similarly, abdominal muscle quality might influence the ability of defenders to resist eviction such that defenders who retain their shells should have greater muscle mass and protein concentration [18] than those that are evicted. Furthermore, we would expect to see differences in muscle quality between opponents within fights, such that successful attackers should have greater muscle quality compared with the defenders they evict, and defenders who retain their shells should have superior muscle quality to the attackers that fail to evict them.
2. Material and methods
Crabs were collected between May and July 2007 from Hannafore Point, UK (50°20′ N, 4°27′ W). They were maintained in tanks of aerated sea water at 15°C and fed a diet of catfish pellets. Crabs were removed from their shells by cracking in a bench vice, and only undamaged male crabs, free from obvious parasites and intermoult were used in the experiment. Unused crabs were provided with new shells and returned to the sea.
Crabs were assigned to pairs consisting of a small and large crab (mean weights + s.e.; small, 0.802 + 0.016 g; large, 1.053 + 0.023 g). The larger crab of the pair was provided with a Littorina littorea shell that was 50 per cent of its preferred shell weight, while the smaller crab was provided with a shell that was 100 per cent adequate for the larger crab.
Crabs were then housed individually and allowed to acclimate for 16 h prior to the staged encounters. Of these 67 encounters, 46 resulted in an eviction and 21 ended with a non-eviction. It is unlikely that muscle protein concentration would change as a result of engaging in a fight, but to test this we also staged 11 encounters in a control group where fights were terminated when the attacker made initial contact with the shell of the defender. This confirmed that protein concentration did not change during a fight (see electronic supplementary material), and the control group was excluded from subsequent analyses.
Following the fights, each crab was humanely killed by immersion in liquid nitrogen and stored at −20°C. While still frozen, the abdominal musculature was removed by dissection. The muscle tissue of each crab was weighed, then analysed for protein concentration following the Bradford method [19]. The muscle : body weight ratio was calculated for each individual by dividing the abdominal muscle weight by the overall crab weight.
We used a two-way (‘one-within and one-between’) repeated-measures ANOVA to analyse muscle quality and relative size. The within-subjects factor was ‘role’ (‘attacker’ or ‘defender’ from the same fight) and the between-subjects factor was ‘outcome’ (‘eviction’ or ‘non-eviction’). This paired approach was required because a fight is an experimental unit, whereas measures taken from attackers and defenders within fights are non-independent experimental sub-units [20].
3. Results
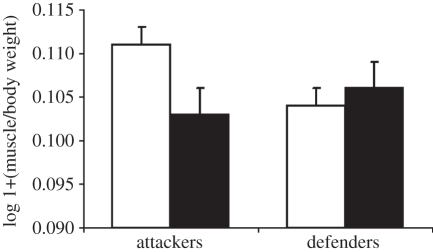
There was no difference in muscle protein concentration between outcomes (F1,65 = 0.024, p = 0.8768), or between roles (F1,65 = 3.356, p = 0.0715) and there was no significant interaction effect (F1,65 = 0.007, p = 0.9353). There was no difference in the muscle : body weight ratio between outcomes (F1,65 = 0.973, p = 0.3276) or roles (F1,65 = 0.895, p = 0.3476). However, a significant interaction between role and outcome (F1,65 = 5.155, p = 0.0265; figure 2) indicates that winners (attackers who effected evictions and defenders who resisted eviction) had greater muscle : body weight ratios than losers (attackers who failed to evict defenders and defenders who were evicted).
Figure 2.
Effects of fight outcome on muscle : body weight ratios in attackers and defenders. Error bars show standard errors. Unfilled bars, eviction; filled bars, non-eviction.
4. Discussion
Many studies on fighting have focused on the role of overall body size, but here we investigated the influence of investment in muscles, potentially a key component of body size driving RHP. Muscle quality (protein concentration) did not vary between the fight outcomes (evictions or non-evictions) but the interaction term indicates that victorious crabs (attackers who evicted defenders, and defenders who resisted eviction; figure 2) had bigger abdominal muscles as a proportion of total body mass than losers. For attackers, the ability to hit the opponent's shell hard enhances the chance of victory [15,16] and here we show that investment in large muscles also improves the chance of evicting the defender. Rapping with high temporal vigour could advertise the attacker's stamina but the need for large muscles and powerful impacts is less clear. Attackers give up on the basis of energetic costs but the decision of defenders is linked to the raps they receive [15]. While rapping may provide information about the attacker, it may also directly influence the defender's ability to maintain a grip on their shells [15,16] and shell rapping may be a ‘hybrid’ signalling activity [21] that performs both functions. While high stamina would enhance the signal, large muscles might enhance the ability to inflict direct effects, as assumed by the Cumulative Assessment Model. Indeed, if in addition to gathering information by monitoring the temporal pattern, defenders must resist direct effects imposed by shell rapping, we would expect defenders as well as attackers to benefit from investing in larger abdominal musculature.
In contrast to the muscle : body weight ratios, the lack of interaction effect indicates that muscle quality in terms of protein concentration does not differ between the winners and losers. Thus, muscle size appears to be more important than muscle quality. It is likely that the relative size of the muscles is important, as larger muscles will contain more fibres devoted to contraction, and longer fibres, which would increase contraction force [22]. Protein concentration may be less important than other properties of muscles such as size and glycogen reserves [23].
Previous studies on diverse systems have shown that stamina is a key correlate of RHP [5]. Here we show that a morphological correlate of strength also influences RHP. Investment in large muscles improves the prospect of victory for both roles, but the effect appears to be greater for attackers. The decision of attackers seems to be based on the Energetic War of Attrition, giving up when the costs cross a threshold [4]. The decision of defenders appears to have elements in common with the both the Cumulative Assessment Model and the Sequential Assessment Model. Larger muscles in successful attackers indicate that defenders may be subject to detrimental effects but they also receive information from the pattern of shell rapping. Current models assume that contests are symmetrical, where opponents fight in the same way, but this is not always the case. Indeed, the effect of relative muscle mass on RHP varies between the two roles in these fights.
Acknowledgements
We thank Kath Sloman for advice on the protein assay and Ann Torr for technical support. We thank Robert Elwood, Duncan Irschick and Stephen Votier for their constructive comments on the manuscript. This work was funded by a University of Plymouth research studentship.
References
- 1.Briffa M., Sneddon L. U. 2010. Contest behaviour. In Evolutionary behavioral ecology (eds Westneat D. F., Fox C. W.), pp. 246–265 Oxford, UK: Oxford University Press [Google Scholar]
- 2.Payne R. J. H., Pagel M. 1996. Escalation and time costs in displays of endurance. J. Theor. Biol. 183, 185–193 10.1006/jtbi.1996.0212 (doi:10.1006/jtbi.1996.0212) [DOI] [Google Scholar]
- 3.Schuett G. W., Grober M. S. 2000. Post-fight levels of plasma lactate and corticosterone in male copperheads, Agkistrodon contortrix (Serpentes, Viperidae): differences between winners and losers. Physiol. Behav. 71, 335–341 10.1016/S0031-9384(00)00348-6 (doi:10.1016/S0031-9384(00)00348-6) [DOI] [PubMed] [Google Scholar]
- 4.Briffa M., Elwood R. W. 2001. Decision rules, energy metabolism and vigour of hermit-crab fights. Proc. R. Soc. Lond. B 268, 1841–1848 10.1098/rspb.2001.1752 (doi:10.1098/rspb.2001.1752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowles S. L., Cotton P. A., Briffa M. 2010. Whole-organism performance capacity predicts resource holding potential in the hermit crab Pagurus bernhardus. Anim. Behav. 80, 277–282 10.1016/j.anbehav.2010.05.004 (doi:10.1016/j.anbehav.2010.05.004) [DOI] [Google Scholar]
- 6.Perry G., Levering K., Girard I., Garland T. J. 2004. Locomotor performance and social dominance in male Anolis cristatellus. Anim. Behav. 67, 37–47 10.1016/j.anbehav.2003.02.003 (doi:10.1016/j.anbehav.2003.02.003) [DOI] [Google Scholar]
- 7.Mowles S. L., Cotton P. A., Briffa M. 2009. Aerobic capacity influences giving-up decisions in fighting hermit crabs: does stamina constrain contests? Anim. Behav. 78, 735–740 10.1016/j.anbehav.2009.07.003 (doi:10.1016/j.anbehav.2009.07.003) [DOI] [Google Scholar]
- 8.Enquist M., Leimar O. 1983. Evolution of fighting behaviour: decision rules and assessment of relative strength. J. Theor. Biol. 102, 387–410 10.1016/0022-5193(83)90376-4 (doi:10.1016/0022-5193(83)90376-4) [DOI] [Google Scholar]
- 9.Payne R. J. H. 1998. Gradually escalating fights and displays: the cumulative assessment model. Anim. Behav. 56, 651–662 10.1006/anbe.1998.0835 (doi:10.1006/anbe.1998.0835) [DOI] [PubMed] [Google Scholar]
- 10.Huyghe K., Vanhooydonck B., Scheers H., Molina-Borja M., Van Damme R. 2005. Morphology, performance and fighting capacity in male lizards, Gallotia galloti. Funct. Ecol. 19, 800–807 10.1111/j.1365-2435.2005.01038.x (doi:10.1111/j.1365-2435.2005.01038.x) [DOI] [Google Scholar]
- 11.Sneddon L. U., Huntingford F. A., Taylor A. C., Orr J. F. 2000. Weapon strength and competitive success in the fights of shore crabs (Carcinus maenas). J. Zool. 250, 397–403 10.1111/j.1469-7998.2000.tb00783.x (doi:10.1111/j.1469-7998.2000.tb00783.x) [DOI] [Google Scholar]
- 12.Vanhooydonck B., Herrel A. Y., Van Damme R., Irschick D. J. 2005. Does dewlap size predict male bite performance in Jamaican Anolis lizards? Funct. Ecol. 19, 38–42 10.1111/j.0269-8463.2005.00940.x (doi:10.1111/j.0269-8463.2005.00940.x) [DOI] [Google Scholar]
- 13.Lailvaux S. P., Hathway J., Pomfret J., Knell R. J. 2005. Horn size predicts physical performance in the beetle Euoniticellus intermedius (Coleoptera: Scarabaeidae). Funct. Ecol. 19, 632–639 10.1111/j.1365-2435.2005.01024.x (doi:10.1111/j.1365-2435.2005.01024.x) [DOI] [Google Scholar]
- 14.Briffa M., Elwood R. W., Dick J. T. A. 1998. Analysis of repeated signals during shell fights in the hermit crab Pagurus bernhardus. Proc. R. Soc. Lond. B 265, 1467–1474 10.1098/rspb.1998.0459 (doi:10.1098/rspb.1998.0459) [DOI] [Google Scholar]
- 15.Briffa M., Elwood R. W. 2002. Power of shell-rapping signals influences physiological costs and subsequent decisions during hermit crab fights. Proc. R. Soc. Lond. B 269, 2331–2336 10.1098/rspb.2002.2158 (doi:10.1098/rspb.2002.2158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briffa M., Elwood R. W. 2000. The power of shell rapping influences rates of eviction in hermit crabs. Behav. Ecol. 11, 288–293 10.1093/beheco/11.3.288 (doi:10.1093/beheco/11.3.288) [DOI] [Google Scholar]
- 17.Dowds B. M., Elwood R. W. 1985. Shell wars II: the influence of relative size on decisions made during shell fights. Anim. Behav. 33, 649–656 10.1016/S0003-3472(85)80088-9 (doi:10.1016/S0003-3472(85)80088-9) [DOI] [Google Scholar]
- 18.Lohuis T. D., Harlow H. J., Beck T. D. I. 2007. Hibernating black bears (Ursus americanus) experience skeletal muscle protein balance during winter anorexia. Comp. Biochem. Phys. B 147, 20–28 10.1016/j.cbpb.2006.12.020 (doi:10.1016/j.cbpb.2006.12.020) [DOI] [PubMed] [Google Scholar]
- 19.Bradford M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 10.1016/0003-2697(76)90527-3 (doi:10.1016/0003-2697(76)90527-3) [DOI] [PubMed] [Google Scholar]
- 20.Briffa M., Elwood R. W. 2010. Repeated measures analysis of contests and other dyadic interactions: problems of semantics, not statistical validity. Anim. Behav. 80, 443–449 10.1016/j.anbehav.2010.06.009 (doi:10.1016/j.anbehav.2010.06.009) [DOI] [Google Scholar]
- 21.Bradbury J. W., Vehrencamp S. L. 1998. Principles of animal communication. Sunderland, MA: Sinnauer Associates [Google Scholar]
- 22.Sadava D., Heller H. C., Orians G. H., Purves W. K., Hillis D. M. 2008. Life, the science of biology, 8th edn. Sunderland, MA: Sinauer Associates, Inc [Google Scholar]
- 23.Briffa M., Elwood R. W. 2004. Use of energy reserves in fighting hermit crabs. Proc. R. Soc. Lond. B 271, 373–379 10.1098/rspb.2003.2633 (doi:10.1098/rspb.2003.2633) [DOI] [PMC free article] [PubMed] [Google Scholar]