Abstract
Giant pandas (Ailuropoda melanoleuca) are an iconic conservation species, but despite significant research effort, do we understand what they really need? Estimating and mapping suitable habitat play a critical role in conservation planning and policy. But if assumptions about ecological needs are wrong, maps with misidentified suitable habitat will misguide conservation action. Here, we use an information-theoretic approach to analyse the largest, landscape-level dataset on panda habitat use to date, and challenge the prevailing wisdom about panda habitat needs. We show that pandas are associated with old-growth forest more than with any ecological variable other than bamboo. Other factors traditionally used in panda habitat models, such as topographic slope, are less important. We suggest that our findings are disparate from previous research in part because our research was conducted over a larger ecological scale than previous research conducted over more circumscribed areas within individual reserves. Thus, extrapolating from habitat studies on small scales to conservation planning on large scales may entail some risk. As the Chinese government is considering the renewal of its logging ban, it should take heed of the panda's dependency on old growth.
Keywords: conservation policy, ecological scale, habitat suitability, giant panda
1. Introduction
The giant panda is at a high risk of extinction and will require informed management for recovery. Panda conservation science has come a long way in recent years; now it is time to apply our increasingly sophisticated knowledge of pandas to conservation management strategies [1]. Although the giant panda genome has now been sequenced [2], we are still struggling to fully understand the panda's ecological requirements.
Estimating and mapping suitable habitat play a critical role in conservation planning and policy, but if assumptions about habitat suitability are wrong, conservation action will be misguided [3,4]. Ecological scale, perhaps the most fundamental concept in all of ecology [5], intersects with conservation when research conducted on one scale is used for conservation planning on another scale [3]. For the iconic endangered giant panda, models of habitat suitability have been used to infer rates of habitat loss, estimate amount of suitable habitat remaining inside and outside reserves, and design reserve systems linked by ecologically relevant corridors [6–8]. But maps and measures of habitat suitability are only as good as the underlying biological assumptions, which are sometimes influenced by the scale over which data are obtained. Modellers of panda habitat have not ignored the available ecological data, but have been forced to rely on data collected over limited temporal and spatial scales. What happens if the resulting habitat models are wrong?
Here, we analyse the largest, landscape-level dataset on panda habitat use, challenge prevailing wisdom about panda habitat needs, and provide direction to conservation policy and planning at the national level. We use data collected by dozens of field teams throughout Sichuan during the Chinese State Forestry Administration's Third National Survey on the giant panda, representing one of the most intensive efforts to survey any endangered species. This massive undertaking produced by far the largest dataset—in scope, sample size and range—of habitat use by giant pandas.
2. Material and methods
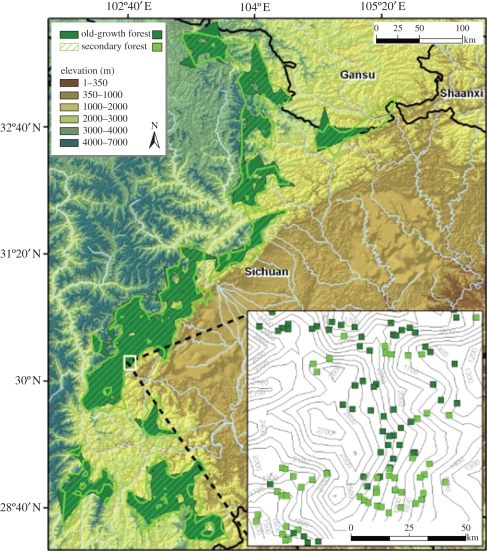
From 1999 to 2003, field observers recorded ecological variables associated with panda signs, matched with control plots where panda signs were not observed. The data included in our analysis were collected across the giant panda range in Sichuan province, including 19 reserves in the four primary mountain ranges: Minshan, Qionglai, Liangshan and Xiaoxiangling (figure 1; electronic supplementary material). This multi-year survey—across seasons and much of the landscape available to pandas today—avoids the potential limitations arising from data collected over small spatial and temporal scales.
Details of the Third National Survey are available elsewhere [9]. The known and potential range of giant pandas was divided into sections measuring 2–6 km2 and field teams established transects along altitudinal gradients, ensuring that each transect sampled all representative habitat types. The presence of panda along transects was determined by signs (primarily faeces, but also foraging sites and dens) and vegetation was sampled in 20 m × 20 m plots. A sign more than 200 m from another sign merited establishing another plot. Data collected for each plot included topography, altitude, slope, forest type, forest age, tree diameter at breast height (DBH), canopy coverage, shrub cover, shrub height and bamboo presence. Control plots were established at every 200 m change in elevation along the transect and following transitions between forest types, to ensure that all habitat types encountered were sampled. After excluding marginal habitats, such as monocultured forests and data from nine reserves with inadequate records, we had 4908 plots for analysis (1116 panda sign plots and 3792 control plots).
We used an information-theoretic approach [10] to determine the suite of factors that best predicted the presence of panda signs. Pearson's (for continuous variables) or Kendall's (for discrete variables) correlation analysis was first conducted to test independence between variables. For those variables with a correlation coefficient above 0.5, we only kept the variable with clear biological meaning in the subsequent analysis in order to weaken multi-collinearity. We constructed a full model set, including global models with all meaningful explanatory variables, and calculated the Akaike information criterion (AIC) to evaluate model fit. Using the differences in AIC values (Δi) between the lowest scoring model and each candidate model, we calculated the Akaike weights and evidence ratios for each of the models.
3. Results
Multiple models (i.e. 11 of the 256) had an evidence ratio less than 10 and differences between candidate models tended to be small (table 1). However, the first three models in table 1, including bamboo presence, slope, forest age, tree DBH and understory height, were approximately 1.5, 2.1 and 2.6 times more likely to explain the presence of panda signs than the models that included those same variables plus shrub cover, tree canopy and elevation, respectively.
Table 1.
Summary of the top logistic regression model sets (evidence ratio greater than 10) predicting the presence of panda sign, out of a total of 256 models resulting from the full model set. The models are in descending order indicating the most to least supported based on Akaike information criteria (AIC). K, number of predictors in the model; ΔAIC, difference in AIC value from that of the best model; Wi, Akaike weight of the ith model, representing the probability that the ith model is the best model in the candidate group; W1/Wi, evidence ratio, comparing the model with the highest Akaike weight (w1) to the ith model (wi) in the candidate group.
| model construction | K | AIC | ΔAIC | AIC weights (Wi) | evidence ratios (W1/Wi) |
|---|---|---|---|---|---|
| bamboo presence, slope, forest age, tree DBH, understory height | 6 | 4830.85 | 0 | 0.214 | 1.00 |
| bamboo presence, slope, forest age, tree DBH, understory height, shrub cover | 7 | 4831.64 | 0.79 | 0.143 | 1.48 |
| bamboo presence, slope, forest age, tree canopy, tree DBH, understory height | 7 | 4832.37 | 1.52 | 0.099 | 2.14 |
| bamboo presence, elevation, slope, forest age, tree DBH, understory height | 7 | 4832.76 | 1.91 | 0.082 | 2.60 |
| bamboo presence, slope, forest age, tree canopy, tree DBH, understory height, shrub cover | 8 | 4833.23 | 2.38 | 0.065 | 3.29 |
| bamboo presence, elevation, slope, forest age, tree DBH, understory height, shrub cover | 8 | 4833.58 | 2.73 | 0.055 | 3.92 |
| bamboo presence, elevation, slope, forest age, tree canopy, tree DBH, understory height | 8 | 4834.31 | 3.46 | 0.038 | 5.64 |
| bamboo presence, slope, forest age, tree DBH | 5 | 4834.32 | 3.47 | 0.038 | 5.67 |
| bamboo presence, elevation, forest age, tree canopy | 5 | 4834.42 | 3.57 | 0.036 | 5.96 |
| bamboo presence, forest age, tree DBH, understory height | 5 | 4834.72 | 3.87 | 0.031 | 6.93 |
| bamboo presence, elevation, slope, forest age, tree canopy, tree DBH, understory height, shrub cover | 9 | 4835.19 | 4.34 | 0.024 | 8.76 |
Our analyses reveal presence of bamboo and forest age (old growth versus secondary growth) as the ecological variables best predicting panda habitat use. Of the top models (evidence ratios less than 10), bamboo and forest age are the only variables common to all models. Following model selection, we estimated the relative importance of each of the contributing variables by calculating the sum of the Akaike weights for all models that included the variable under consideration. Although the presence of bamboo ranked first, old-growth forest was essentially equivalent (Akaike weights = 1.010 and 1.009, respectively), followed closely by tree DBH (0.958), a measure that is undoubtedly related to forest age (as well as site productivity). Slope, a variable frequently used in habitat models for pandas [7,8], ranked much lower (0.844), no more important than understory height (0.824), which rarely factors into panda habitat suitability models. The remaining variables—shrub cover, elevation and tree canopy—had poor predictive power (less than 0.500).
4. Discussion
As an obligate bamboo specialist, it is no surprise that bamboo predicts panda habitat use. But it is surprising that old-growth forest should attain the same level of importance as bamboo. Our data do not allow us to discern what draws pandas to advanced stages of forest succession. One possibility is that the bamboo that grows underneath old growth is more nutritious [11]. Another intriguing possibility is that only old-growth trees grow large enough to form cavities suitable for maternity dens [12]. This raises the question: are birth dens a factor-limiting panda population size in reserves with a history of logging [1]?
Despite numerous studies based on data collected over a limited scale—typically a portion of a single reserve [6,13–15]—previous research has not clearly identified this strong association with old-growth forests. Although one single-reserve study indicated a positive relationship between forest age and panda presence [13], old growth did not assume the primacy it did in our findings. Lack of robust range-wide data and conflicting evidence among several studies over smaller areas has meant that old growth has not figured prominently in habitat mapping, policy and planning. By contrast, our dataset represents more than 70 per cent of the panda's current range, which allows us to make inferences across the entire panda landscape. This underscores the importance of ecological scale in inferring habitat selection: old growth emerges as an important factor influencing panda habitat preferences only when data are collected over a large enough scale. Our fine-grained study over large extent captured habitat relations that were not fully understood previously. Now, maps and models of suitable panda habitat should be revised to give priority to old-growth forest.
Our data point to a need for habitat modelling to be more sensitive to the issue of ecological scale, especially when scaling up from data collected on localized scales to plan conservation practice over much larger scales. Appropriate conservation planning often requires this type of range-wide landscape scale approach. We recognize that scale-appropriate ecological research will not always be easy and may require substantial effort over time and space. However, there are at times no good substitutes to real effort. While such endeavours in ecology are increasingly rare in an era when quick empirical studies trump investment in long-term ecological research, the pay-off can be substantial and irreplaceable [16].
More than a decade ago the Chinese government implemented a ban on all logging throughout the panda's range, an action that curtailed significant losses to the forest ecosystem on which giant pandas and other wildlife depend [17]. This logging moratorium expires in 2010 and the government must decide whether to extend the ban [17,18]. It is critical for the long-term future of the panda to identify the ecological variables important for their persistence now, when the government still has time to consider these variables while devising its timber-harvest policy. As the Chinese government decides whether or not to lift the ban on logging, it should consider this: it may be more cost-effective to protect the existing old growth than to open it up to logging while protecting an equivalent area of secondary growth forest.
Acknowledgements
This work was supported by grants from the National Basic Research Programme (973 Programme, 2007CB411600), National Natural Science Foundation of China (no. 30830020, 30970382), Sichuan Youth Fund (07ZQ026-017) and State Forestry Administration of China. We are grateful for Dr James Sheppard for creating figure 1.
Figure 1.
Distribution of old-growth forest (dark green fill) and secondary forest (light green stripe fill) in the mountain ranges of central China across 600 km of latitude. At regional scales there was a high degree of overlap between the two forest categories. At smaller local scales, the two forest categories were clustered in discrete patches (map inset: dark squares, old-growth forest 20 m × 20 m plots, light-green squares, secondary plots).
References
- 1.Swaisgood R. R., Wei F., Wildt D. E., Kouba A. J., Zhang Z. 2010. Giant panda conservation science: how far we have come. Biol. Lett. 6, 143–145 10.1098/rsbl.2009.0786 (doi:10.1098/rsbl.2009.0786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R., et al. 2009. The sequence and de nova assembly of the giant panda genome. Nature 463, 311–317 10.1038/nature08696 (doi:10.1038/nature08696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobbs N. T. 2003. Challenges and opportunities in integrating ecological knowledge across scales. For. Ecol. Manage. 181, 223–238 10.1016/S0378-1127(03)00135-X (doi:10.1016/S0378-1127(03)00135-X) [DOI] [Google Scholar]
- 4.Orians G. H., Wittenberger J. F. 1991. Spatial and temporal scales in habitat selection. Am. Nat. 137, S29–S49 10.1086/285138 (doi:10.1086/285138) [DOI] [Google Scholar]
- 5.Levin S. A. 1992. The problem of pattern and scale in ecology: the Robert H. MacArthur award lecture. Ecology 73, 1943–1967 10.2307/1941447 (doi:10.2307/1941447) [DOI] [Google Scholar]
- 6.Feng T., Van Manen F. T., Zhao N., Li M., Wei F. 2009. Habitat assessment for giant pandas in the Qinling mountain region of China. J. Wildl. Manage. 73, 852–858 10.2193/2008-186 (doi:10.2193/2008-186) [DOI] [Google Scholar]
- 7.Liu J., Linderman M., Ouyang Z., An L., Yang J., Zhang H. 2001. Ecological degradation in protected areas: the case of Wolong Nature Reserve for giant pandas. Science 292, 98–101 10.1126/science.1058104 (doi:10.1126/science.1058104) [DOI] [PubMed] [Google Scholar]
- 8.Shen G., Feng C., Xie Z., Ouyang Z., Li J., Pascal M. 2008. Proposed conservation landscape for giant pandas in the Minshan Mountains, China. Conserv. Biol. 22, 1144–1153 10.1111/j.1523-1739.2008.01038.x (doi:10.1111/j.1523-1739.2008.01038.x) [DOI] [PubMed] [Google Scholar]
- 9.State Forestry Administration 2006. The third national survey report on giant panda in China. Beijing, China: Science Press [Google Scholar]
- 10.Burnham K., Anderson D. 2002. Model selection and multimodel inference: a practical information-theoretic approach. New York, NY: Springer-Verlag [Google Scholar]
- 11.Reid D. G., Taylor A. H., Hu J., Qin Z. 1991. Environmental influences on bamboo Bashania fangiana growth and implications for giant panda conservation. J. Appl. Ecol. 28, 855–868 10.2307/2404212 (doi:10.2307/2404212) [DOI] [Google Scholar]
- 12.Zhang Z., Swaisgood R. R., Wu H., Zhang Z., Li M., Yong Y., Hu J., Wei F. 2007. Factors predicting den use by maternal giant pandas. J. Wildl. Manage. 71, 2694–2698 10.2193/2006-504 (doi:10.2193/2006-504) [DOI] [Google Scholar]
- 13.Bearer S., Linderman M., Huang J., An L., He G., Liu J. 2008. Effects of fuelwood collection and timber harvesting on giant panda habitat use. Biol. Conserv. 141, 385–393 10.1016/j.biocon.2007.10.009 (doi:10.1016/j.biocon.2007.10.009) [DOI] [Google Scholar]
- 14.Linderman M., Bearer S., An L., Tan Y., Ouyang Z., Liu J. 2005. The effects of understory bamboo on broad-scale estimates of giant panda habitat. Biol. Conserv. 121, 383–390 10.1016/j.biocon.2004.05.011 (doi:10.1016/j.biocon.2004.05.011) [DOI] [Google Scholar]
- 15.Schaller G. B., Hu J., Pan W., Zhu J. 1985. The giant pandas of Wolong. Chicago, IL: University of Chicago Press [Google Scholar]
- 16.Swaisgood R. R., Terborgh J. T., Blumstein D. T. 2010. Funding should come to those who wait. Science 329, 276. 10.1126/science.329.5989.276-a (doi:10.1126/science.329.5989.276-a) [DOI] [PubMed] [Google Scholar]
- 17.Morell V. 2008. Letting 1000 forests bloom. Science 320, 1442–1443 10.1126/science.320.5882.1442 (doi:10.1126/science.320.5882.1442) [DOI] [PubMed] [Google Scholar]
- 18.Loucks C. J., Lu Z., Dinerstein E., Wang H., Olson D. M., Zhu C., Wang D. 2001. Giant pandas in a changing landscape. Science 294, 1465. 10.1126/science.1064710 (doi:10.1126/science.1064710) [DOI] [PubMed] [Google Scholar]