Abstract
Species have been adapted to specific niches optimizing survival and reproduction; however, urbanization by humans has dramatically altered natural habitats. Artificial light at night (LAN), termed ‘light pollution’, is an often overlooked, yet increasing disruptor of habitats, which perturbs physiological processes that rely on precise light information. For example, LAN alters the timing of reproduction and activity in some species, which decreases the odds of successful breeding and increases the threat of predation for these individuals, leading to reduced fitness. LAN also suppresses immune function, an important proxy for survival. To investigate the impact of LAN in a species naive to light pollution in its native habitat, immune function was examined in Siberian hamsters derived from wild-caught stock. After four weeks exposure to dim LAN, immune responses to three different challenges were assessed: (i) delayed-type hypersensitivity (DTH), (ii) lipopolysaccharide-induced fever, and (iii) bactericide activity of blood. LAN suppressed DTH response and reduced bactericide activity of blood after lipopolysaccharide treatment, in addition to altering daily patterns of locomotor activity, suggesting that human encroachment on habitats via night-time lighting may inadvertently compromise immune function and ultimately fitness.
Keywords: light pollution, delayed-type hypersensitivity, bactericide, lipopolysaccharide, Phodopus sungorus
1. Introduction
Species have been adapted to specific temporal and spatial niches optimizing survival and reproduction; however, urbanization by humans has dramatically altered habitats. Artificial light at night (LAN), termed ‘light pollution’, is rapidly pervading the environment. For many organisms, this is problematic because it disrupts physiological processes, which rely on precise light information, such as timing of daily rhythms and seasonal adaptations. For example, songbirds living near streetlights have altered mating calls and lay eggs earlier than those living deep in the forest [1], beach mice living near populated coastlines have altered foraging behaviour [2], and bats near streetlights change their commuting patterns, with no evidence of habituation over time [3]. Altering the timing of reproduction and activity exposes these individuals to a greater risk of climatic challenges, predation and disease, which potentially decreases fitness. In a laboratory setting, LAN suppresses cell-mediated and humoral immune function in Japanese quail [4], cockerels [5] and rats [6]. Outside of the laboratory, with the demands of limited resources and other harsh environmental conditions, reduced immune function could seriously impact survival.
In the present study, we exposed Siberian hamsters to dim light throughout the night (5 lux) for four weeks; this level of illumination is approximately five times brighter than maximal moonlight, comparable to the levels of light pollution in areas surrounding urban centres, and is sufficient to suppress melatonin in hamsters [7]. We then assessed immune responses (as a proxy for survival) to three different challenges: (i) delayed-type hypersensitivity (DTH), (ii) lipopolysaccharide-induced fever responses, and (iii) bactericide activity of blood plasma.
2. Material and methods
(a). Animals
Eighteen individually-housed adult (greater than eight weeks of age) male Siberian hamsters (Phodopus sungorus) were obtained from our breeding colony at The Ohio State University and maintained in polypropylene cages (30 × 15 × 14 cm) at a constant temperature (22 ± 2°C) and relative humidity (50 ± 5%). Food (Harlan Teklad 8640, Indianapolis, IN, USA) and filtered tap water were available ad libitum. Hamsters were exposed to either a standard 16 L : 8 D cycle (LD; 150 lux : 0 lux) or a 16 : 8 light-dim light cycle (LdimL; 150 lux : 5 lux), with bright lights illuminated from 23.00 to 15.00 h eastern standard time (EST). Both the bright and dim lights were standard fluorescent bulbs emitting ‘cool white’ light composed of wavelengths distributed across the visible spectrum, and light intensity was measured at cage level. The same individuals were used for each test procedure. All experiments were approved by The Ohio State University Institutional Animal Care and Use Committee and performed in accordance with NIH guidelines.
(b). Delayed-type hypersensitivity
After four weeks of housing in either an LD or LdimL condition, DTH, an ecologically valid in vivo assay of cell-mediated immune function [8] was assessed as previously described [9]. Briefly, DTH was induced by sensitization to, and later challenge with, the antigen 2,4-dinitro-1-fluorobenzene (DNFB; Sigma). Responses to this challenge reflect cell-mediated immune function, including T-cell-mediated inflammation and antigen processing and presentation [8]. On days 1 and 2, hamsters were sensitized by applying 25 µl of DNFB (0.5% wt/volume in 4 : 1 acetone to olive oil vehicle) to the dorsum. Seven days later baseline pinnae thickness was measured with a constant loading dial micrometer (Mitutoyo, Tokyo), and then hamsters were challenged on the right pinna with 20 µl of 0.2% (wt/volume) DNFB in vehicle, while the left pinna was treated with the vehicle solution alone. The thicknesses of both pinnae were measured every 24 h for the next 5 days by the same investigator (T.A.B.). All measurements were made between 07.00 and 08.30 h EST and animals were brought into the procedure room individually to minimize potential stressors.
(c). Lipopolysaccharide-induced fever
Procedures were performed as previously described [10] approximately eight weeks following DTH measurements. Briefly, hamsters were implanted intraperitoneally with radiotelemetric transmitters (Mini-Mitter, Sunriver, OR, USA) under isoflurane anaesthesia and allowed to recover for 5 days. Homecages were placed on TR-3000 receiver boards and connected to DP-24 DataPorts (Mini-Mitter), which continuously collected activity and temperature data in 15 min bins. At the beginning of the dark/dim phase (15.00 h), each hamster was given an intraperitoneal (IP) injection of saline to establish the baseline activity and temperature information. Twenty-four hours after saline injection, lipopolysaccharide (LPS; 400 µg kg−1), a component of Gram-negative bacteria cell walls, was administered IP to induce fever. Temperature and activity data were collected through to 19 h post-LPS.
(d). Bactericidal capacity of blood plasma
Blood samples both before DTH and 19 h post-LPS injection were collected under isoflurane anaesthesia through sterile, heparinized microcapillary tubes from the retro-orbital sinus. Samples were immediately centrifuged at 4°C for 30 min at 3300g and plasma aliquots were stored at −80°C until assayed [11]. Under a laminar flow hood, plasma samples were diluted 1 : 20 in CO2-independent media (Gibco, Carlsbad, CA, USA). A standard number of colony-forming units (CFUs) of Escherichia coli (Epower 0483E7, Fisher Scientific) was added to each sample in a ratio of 1 : 10. Plasma-bacteria mixtures were then incubated for 30 min at 37°C, and plated in duplicate onto tryptic-soy agar plates using a sterile technique. Two plates were spread with diluted bacteria alone as positive controls, and two were spread with media alone as negative controls. All plates were incubated at room temperature for 24 h, and then total CFUs were quantified by an experimenter unaware of lighting conditions. Total CFUs were averaged across the duplicates for each animal and then compared with the average of the positive control plates to calculate the per cent of bacteria killed. Neither negative control plate contained CFUs.
(e). Statistical analyses
Repeated-measures ANOVA was used to analyse DTH and fever response data between LD and LdimL groups, with day post-challenge as the repeated measure in both cases. Two-way ANOVA was used to analyse bactericide, body mass and home-cage activity data with lighting condition (LD versus LdimL) and pre- versus post-LPS, start versus end weight, and light versus dark as the independent variables, respectively. One outlier was removed from analysis of the bactericide assay and two were removed from activity analyses. All significant main effects were followed up with Fisher's post hoc comparisons. Statistics were performed using Statview 5.0.1 for Windows PC and mean differences were considered statistically significant when p < 0.05.
3. Results
(a). Immune responses
(i). DTH
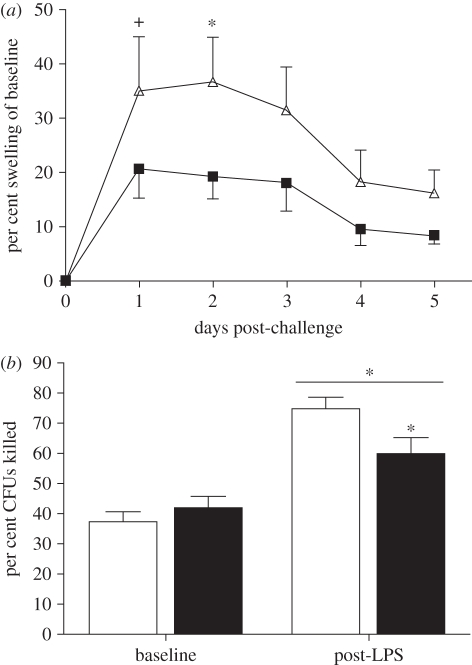
DNFB challenge-induced swelling in the right pinnae of both groups (F1,16 = 3.27, p < 0.05); however, exposure to dim LAN impaired the inflammatory response (F1,16 = 10.88, p < 0.01; figure 1a). Post hoc comparisons of individual days revealed LdimL-hamsters significantly reduced swelling compared with LD-hamsters on day 2 (p < 0.05).
Figure 1.
Immune responses to two different challenges. (a) LdimL-hamsters had a suppressed DTH response to challenge with DNFB. (b) Blood plasma bactericidal activity was equivalent at baseline; however, it was enhanced in both groups after LPS-treatment, but to a lesser extent in LdimL-hamsters. Mean ± s.e.m; n = 8–9 per group; *p < 0.05; +p = 0.09. (a) Triangles with solid line, LD; squares with solid line, LdimL. (b) White bars, LD; black bars, LdimL.
(ii). Blood plasma bactericidal capacity
Both groups had greater blood bactericidal activity post-LPS compared with baseline (F1,16 = 45.94, p < 0.0001) and there was a significant lighting treatment by baseline versus post-LPS interaction effect (F1,16 = 5.77, p < 0.05). Plasma from LD hamsters post-LPS killed more than twice as many CFUs as pre-LPS, with a similar induction (143%) in the LdimL group post-LPS. This induction in the LdimL group, however, was only 71 per cent of that in the LD group. Post hoc comparisons confirmed post-LPS LdimL-hamsters killed significantly fewer CFUs compared with LD-hamsters (p < 0.05; figure 1b).
(b). Response to LPS
(i). LPS-induced fever
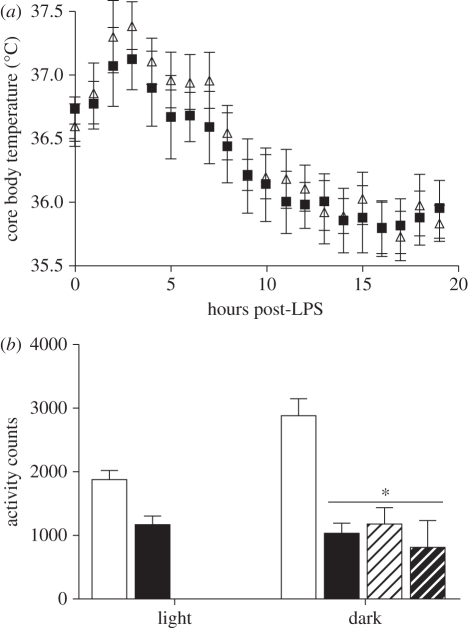
All hamsters showed a distinct peak in core body temperature shortly after LPS injection (F1,19 = 23.42, p < 0.0001; figure 2a); however, LD- versus LdimL-hamsters did not differ in response as there was no effect of lighting condition and no interaction (p > 0.05).
Figure 2.
Response to LPS-administration. (a) Both groups mounted an equivalent fever response to LPS. Total home-cage locomotor activity was reduced in LdimL-hamsters during both light and dark phases and LD-hamsters reduced activity to LdimL-hamster levels after LPS-treatment. Mean ± s.e.m; n = 8–9 per group; *p < 0.05. (a) Triangles, LD; squares, LdimL. (b) Open bars, LD saline; filled bars, LdimL saline; open bars with hatched lines, LD post-LPS; filled bars with hatched lines, LdimL post-LPS.
(ii). Locomotor activity
There was a main effect of light versus dark phase (F1,14 = 4.88, p < 0.05), showing that hamsters performed more of their daily activity during the dark phase. After saline treatment, LdimL-hamsters had reduced activity in both the dark and light phases relative to LD-hamsters; there was a main effect of lighting treatment (F1,14 = 42.32, p < 0.0001) and a significant interaction effect (F1,14 = 8.07, p < 0.01). Post LPS-treatment, LD-hamsters and LdimL-hamsters had equivalently low levels of locomotor activity (p > 0.05; figure 2b).
4. Discussion
Chronic exposure to ambient light levels found in urban environments at night disrupts circadian activity patterns and alters immune function in Siberian hamsters derived from wild-caught stock. Four weeks of 5 lux LAN was sufficient to alter both cell-mediated immunity and bactericidal capacity, without affecting febrile response to LPS. Alteration of circadian activity patterns demonstrates that a single environmental factor, chronic dim light exposure at night, is sufficient to alter physiology and behaviour in this species.
In vivo T-cell-mediated immune responses, as assayed by DTH [9], are sensitive to melatonin concentrations, as melatonin enhances antigen presentation and amplifies T-cell proliferation [12]. Suppressed DTH response in LdimL-hamsters in the current study could potentially be a result of dim-light suppression of pineal melatonin synthesis [7]. Results of this assay reflect altered immune function, an effect that may have fitness consequences by either damaging host defence or shunting energy towards other processes. It must be noted that DTH responses may change over the course of a day owing to many factors, but we restricted our assay to one morning timepoint, at the nadir of the cortisol and activity rhythm. This minimized variables that could produce inconsistent measurements, but also limits the scope of our conclusions since we do not know how the response changes over time. Thus, while we cannot say LAN produces an overall immunocompromise, we may make the more specific point that the immune system is sensitive to low lux LAN. In contrast to DTH, blood bactericidal activity is an in vitro measure of non-specific, innate immune response, predominately mediated by soluble plasma proteins involving the complement cascade [13]. In ringdoves, administration of melatonin, or its metabolic precursor tryptophan, increases bactericidal activity of blood [14]. LdimL-hamsters, in comparison with LD-hamsters, displayed no basal differences in blood bactericidal activity; however, post-LPS plasma from LdimL-hamsters reduced bactericidal capacity. Activation of the immune system appears to be necessary to reveal the effects of LAN on this parameter. LPS-induced fever did not differ between LdimL- compared with LD-hamsters. Consistent with these data, removing melatonin by pinealectomy in Siberian hamsters has no impact on LPS-induced fever [15], which may explain why LAN did not affect a fever response. LPS-treatment did, however, reduce activity levels in LD- but not LdimL-hamsters. Whether this difference represents altered sickness behaviours in LdimL-hamsters, or whether this response is simply masked by the general reduction in activity of LdimL-hamsters remains unspecified.
Taken together, these data suggest that dim light, consistent with typical levels of light pollution from urban development, alters immune function and circadian activity patterns, which could potentially compromise survival. Our study emphasizes the ecological relevance of light pollution on immune function, an important proxy for survival. Under natural conditions, resource limitations and thermoregulatory demands can interact to compromise immune function. Further alterations in immune function by exposure to LAN could potentially reduce the odds of survival. Thus, night-time light exposure should be considered an important contributing factor in species decline. Future studies should address the mechanisms underlying these phenomena and the ultimate consequences of artificial light on ecosystem stability.
Acknowledgements
All experiments were approved by the Ohio State University Institutional Animal Care and Use Committee and performed in accordance with NIH guidelines.
This work was supported by the U.S. National Science Foundation (IOS-04-16897 and IOS-08-38098). T. A. B. was supported by the U. S. Department of Defense through the National Defense Science and Engineering Graduate Fellowship (NDSEG) program. We thank Dr Z.M. Weil for helpful comments in the preparation of this manuscript.
References
- 1.Kempenaers B., Borgstrom P., Loes P., Schlicht E., Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739 10.1016/j.cub.2010.08.028 (doi:10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 2.Bird B. L., Branch L. C., Miller D. L. 2004. Effects of coastal lighting on foraging behavior of beach mice. Conserv. Biol. 18, 1435–1439 10.1111/j.1523-1739.2004.00349.x (doi:10.1111/j.1523-1739.2004.00349.x) [DOI] [Google Scholar]
- 3.Stone E. L., Jones G., Harris S. 2009. Street lighting disturbs commuting bats. Curr. Biol. 19, 1123–1127 10.1016/j.cub.2009.05.058 (doi:10.1016/j.cub.2009.05.058) [DOI] [PubMed] [Google Scholar]
- 4.Moore C. B., Siopes T. D. 2000. Effects of lighting conditions and melatonin supplementation on the cellular and humoral immune responses in Japanese quail Coturnix coturnix japonica. Gen. Comp. Endocrinol. 119, 95–104 10.1006/gcen.2000.7496 (doi:10.1006/gcen.2000.7496) [DOI] [PubMed] [Google Scholar]
- 5.Kirby J. D., Froman D. P. 1991. Research note: evaluation of humoral and delayed hypersensitivity responses in cockerels reared under constant light or a twelve hour light : twelve hour dark photoperiod. Poultry Sci. 70, 2375–23781754552 [Google Scholar]
- 6.Oishi K., Shibusawa K., Kakazu H., Kuriyama T., Ohkura N., Machida K. 2006. Extended light exposure suppresses nocturnal increases in cytotoxic activity of splenic natural killer cells in rats. Biol. Rhyth. Res. 37, 21–35 10.1080/09291010500386774 (doi:10.1080/09291010500386774) [DOI] [Google Scholar]
- 7.Brainard G. C., Richardson B. A., Petterborg L. J., Reiter R. J. 1982. The effect of different light intensities on pineal melatonin content. Brain Res. 233, 75–81 10.1016/0006-8993(82)90931-3 (doi:10.1016/0006-8993(82)90931-3) [DOI] [PubMed] [Google Scholar]
- 8.Nelson R. J., Denlinger D. L., Somers D. E. 2010. Photoperiodism: the biological calendar, pp. 470–481 New York, NY: Oxford University Press [Google Scholar]
- 9.Bilbo S. D., Nelson R. J. 2003. Sex differences in photoperiodic and stress-induced enhancement of immune function in Siberian hamsters. Brain Behav. Immunol. 17, 462–472 10.1016/S0889-1591(03)00063-1 (doi:10.1016/S0889-1591(03)00063-1) [DOI] [PubMed] [Google Scholar]
- 10.Bilbo S. D., Drazen D. L., Quan N., He L., Nelson R. J. 2002. Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proc. R. Soc. Lond. B 269, 447–454 10.1098/rspb.2001.1915 (doi:10.1098/rspb.2001.1915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin L. B., Weil Z. M., Nelson R. J. 2007. Immune defense and reproductive pace of life in Peromyscus mice. Ecology 88, 2516–2528 10.1890/07-0060.1 (doi:10.1890/07-0060.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pioli C., Caroleo M. C., Nistico G., Doria G. 1993. Melatonin increases antigen presentation and amplifies specific and non specific signals for T-cell proliferation. Int. J. Immunopharmacol. 15, 463–468 10.1016/0192-0561(93)90060-C (doi:10.1016/0192-0561(93)90060-C) [DOI] [PubMed] [Google Scholar]
- 13.Tieleman I. B., Williams J. B., Ricklefs R. E., Klasing K. C. 2005. Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proc. R. Soc. B 272, 1715–1720 10.1098/rspb.2005.3155 (doi:10.1098/rspb.2005.3155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terron M. P., Delgado J., Paredes S. D., Barriga C., Reiter R. J., Rodriguez A. B. 2009. Effect of melatonin and tryptophan on humoral immunity in young and old ringdoves (Streptopelia risoria). Exp. Gerontol. 44, 653–658 10.1016/j.exger.2009.07.005 (doi:10.1016/j.exger.2009.07.005) [DOI] [PubMed] [Google Scholar]
- 15.Wen J. C., Dhabhar F. S., Prendergast B. J. 2007. Pineal-dependent and -independent effects of photoperiod on immune function in Siberian hamsters (Phodopus sungorus). Horm. Behav. 51, 31–39 10.1016/j.yhbeh.2006.08.001 (doi:10.1016/j.yhbeh.2006.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]