Abstract
Despite having a profound effect on population dynamics, the reasons that animals disperse are poorly understood. Evolutionary explanations have focused on inbreeding and competition, where the potential cost of philopatry is negated through dispersal. Such scenarios lead to the prediction that less successful individuals preferentially disperse, termed ‘fitness-associated dispersal’. Since heterozygosity is associated with fitness, we assessed whether dispersed animals had less observed heterozygosity (HO) than residents. We tested this prediction using both genetic and population-monitoring data of mountain goats (Oreamnos americanus). Individuals classified as dispersers through cross-assignment had the lowest mean HO, followed by residents, and then admixed individuals. Dispersed individuals had 6.3 per cent less HO than their subpopulation of origin. In the long-term study of the mountain goat herd at Caw Ridge, Alberta, immigrants had the lowest HO; however, the opposite pattern was seen in emigrants, which may be related to density dependence. This study is the first to provide empirical evidence that heterozygosity is associated with dispersal.
Keywords: dispersal, cross-assignment, heterozygosity, fitness, mountain goat
1. Introduction
The evolution of dispersal is a contentious topic. Ultimate explanations for dispersal have included competition for mates, competition for resources and inbreeding avoidance [1,2], none of which are mutually exclusive [3]. These ideas lead to the recognition of ‘fitness-associated dispersal’ ([4] and references therein), which is the preferential dispersal of less successful individuals. Since individual heterozygosity is also positively associated with fitness components in wild populations [5], this leads us to hypothesize that dispersed individuals have less heterozygosity than residents.
Here, we assessed whether dispersal was related to heterozygosity in a North American alpine ungulate, the mountain goat (Oreamnos americanus). The mountain goat is ideal for testing this hypothesis for two reasons: (i) higher individual heterozygosity is associated with increased survival [6] and (ii) mountain goat populations are spatially structured [7], which allows for dispersal to be detected through genetic assignment tests [8]. Using data from a range-wide population structure study [7] and a long-term study of individually marked goats at Caw Ridge, Alberta, Canada [9], we tested for an association between observed individual multi-locus heterozygosity (HO) and dispersal at two hierarchical levels, subpopulation and herd, and discuss the implications for the evolution of dispersal.
2. Material and methods
Detailed genotyping methods are available in Shafer et al. [7] and Mainguy et al. [6]. Briefly, 876 mountain goats were sampled across their native range in western North America [7] as well as 311 individuals from the Caw Ridge mountain goat herd in Alberta, Canada [6]. All individuals were genotyped at the 19 microsatellite loci used in Shafer et al. [7]. Although dispersal is male-biased, both sexes were included as they each disperse [7,9].
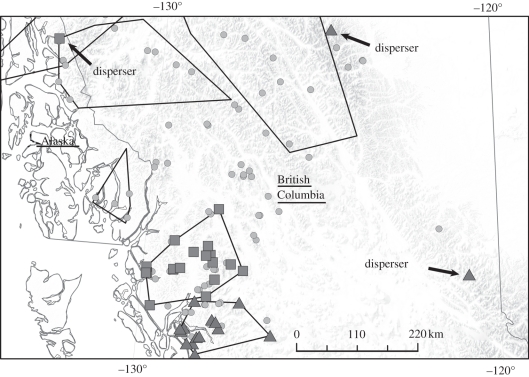
Subpopulations were identified using STRUCTURE 2.2 [10] as described in Shafer et al. [7]. Cross-assigned individuals or ‘dispersers’, defined as individuals genetically from one subpopulation but physically found in another (or clearly outside their own), were identified as follows: (i) polygons were constructed around individuals assigned to a subpopulation with a q > 0.80, where q is the proportion of the genotype originating in that subpopulation, that were confined to specific mountain range(s) using ARCGIS 9.0. These polygons constituted the ‘core’ populations (sensu [11]) and contained residents—this criterion was based on empirical data showing that mountain ranges help delineate subpopulation boundaries in alpine ungulates [7,12]; (ii) individuals with a q > 0.80 not found within the polygon and on a separate mountain range were considered cross-assigned (figure 1); and (iii) individuals with a q < 0.80 were considered to have admixed ancestry [13,14]. This produced three categories: dispersers (D), residents (R) and admixed (A). To ensure that the results were not biased by the STRUCTURE algorithm (i.e. failure to detect heterozygous dispersers), we simulated three pairs of populations of 25 individuals per population with varying degrees of differentiation (FST of 0.05, 0.15, 0.30) typed at loci with allele frequencies from Shafer et al. [7]. For each simulation, we measured the covariance between q and HO. We then randomly designated individuals with q > 0.80 as cross-assigned from each population, with the remainder of individuals with q > 0.80 classified as residents, and calculated HO for both categories (additional details in the electronic supplementary material).
Figure 1.
Example of cross-assigned mountain goats. Individuals assigned (q > 0.80) to their subpopulations are in squares and triangles, with the dispersers labelled. Grey circles represent admixed individuals. For clarity, all polygons are drawn but only two subpopulations are shown.
Immigrants and emigrants were identified from the long-term Caw Ridge study population where animals aged 1–3 disperse [9]. This is the only mountain goat herd with such data available. All 2-year-old males in the herd have been fitted with a radio-collar since 2001 to track long-distance movements. Emigrants from Caw Ridge (DE) were individuals for whom departure from Caw Ridge was confirmed using live radio signal via helicopter fly-over or visual means. Individuals that appeared to have emigrated but were found deceased were grouped separately. Immigrants to Caw Ridge (DI) were easily identified through population monitoring (figure 2) since they were unmarked.
Figure 2.
All mountain goats at Caw Ridge are uniquely marked, and males are fitted with radio collars allowing us to document both emigration and immigration. Here is a uniquely marked female and her soon to be marked kid.
We compared HO between dispersal categories (D, R and A) across subpopulations, and at Caw Ridge. We compared the average HO across categories using a Kruskal–Wallis test. Wilcoxon tests were conducted between all pairs of categories. HO of D individuals was compared with the HO and the expected heterozygosity (HE) of their subpopulation of origin using a sign test. For the Caw Ridge herd, we compared DE and DI with R using a Wilcoxon test and a sign test. Means are presented ±s.e. and tests were one-tailed. All statistical analyses were done using the freeware R 2.8.0. (http://www.r-project.org), and raw data are provided in the electronic supplementary material.
3. Results
A total of 803 animals with sufficient geo-referencing were tested for cross-assignment. Of these, 30 were classified as D, 436 as R and 309 as A. Assignment of dispersal was unambiguous as D individuals were clearly disjunct (figure 1). HO differed among the D (0.38 ± 0.02), R (0.43 ± 0.01) and A (0.46 ± 0.01) groups (Kruskal–Wallis test; χ2 = 10.4, d.f. = 2, p < 0.01). HO was lower in D than in R (pair-wise test; W = 5065.5, p = 0.02), in D than in A (W = 3088.5, p<0.01) and in R than in A (W = 61 592.5, p = 0.02). Dispersers averaged 6.3 per cent (±2.4%) less HO from that of their subpopulation of origin (s = 10, p = 0.05). The same pattern was observed relative to subpopulation HE (s = 6, p < 0.01). In the simulated data, there was no relationship between q and HO (mean Pearson coefficient 0.0005 ± 0.0007; electronic supplementary material, figure S1), and the HO of dispersers was not different from that of residents (W = 730, p = 0.58).
Over the past 23 years at Caw Ridge, 266 animals lived to at least 1 year of age, of which 15 were classified as DE, four as DI and the remainder as R. Mean HO for DI, DE and R at Caw Ridge were 0.45 (±0.03), 0.55 (±0.03) and 0.50 (±0.01), respectively. A trend of lower HO in DI compared with DE (W = 11, p = 0.03) and R (W = 343, p = 0.15) was observed, but R was lower than DE (W = 1321, p = 0.03). There was no difference between pooled DI and DE from residents (W = 2669, p = 0.84) and between the HO of DE and those individuals that died while emigrating (W = 37.5, p = 0.48). The four DI individuals averaged 5.7 per cent (±2.6%) less HO than the herd average (s = 0, p = 0.06) and were assigned by STRUCTURE to the S3 subpopulation in Shafer et al. [7], which encompasses Caw Ridge.
4. Discussion
Dispersing mountain goats appear to be less heterozygous than non-dispersers. Across subpopulations, dispersers had lower HO than residents, and at the herd level, immigrants to Caw Ridge had the lowest HO. Simulations indicated that the difference was not due to bias in the assignments. However, the opposite trend was observed in emigrants from Caw Ridge. This latter result suggests that alternative mechanisms may be influencing dispersal from Caw Ridge. One scenario we considered was whether Caw Ridge emigrant survival was related to heterozygosity (i.e. less heterozygous individuals were emigrating but had poorer survival); but HO was not different between DE and emigrants that were only found deceased. A possible explanation could be related to density. The Caw Ridge herd recently doubled in size [9] and is showing signs of density dependence [15]. In shrews, Hanski et al. [16] found that as density increased, dispersers tended to be more highly ranked individuals. Maternal condition was hypothesized to influence dispersal in such instances ([16]; sensu [17]). In mountain goats, the relationship between heterozygosity, rank, maternal condition and dispersal, has yet to be examined but may shed additional light on the factors promoting dispersal.
The evolution of dispersal is clearly multi-factorial [3]. As a result, models simulating dispersal must take into account a diverse array of costs and processes that often lack realistic assumptions (e.g. condition-dependent strategies; [1]). Because multi-locus heterozygosity can be a proxy for inbreeding [18], the pattern of lower heterozygosity could be attributed to increased coancestry. Models have shown that inbreeding depression could select for dispersal, resulting in a balance between the costs of coancestry and dispersal [19,20]. In both inbreeding and fitness scenarios, selection could have favoured the dispersal of individuals with reduced genetic heterozygosity because the disperser's fitness would benefit from future heterosis [20–22] and it provides a means of removing deleterious mutations [4]. Heterosis occurs when individuals from divergent populations reproduce, and is most pronounced in highly differentiated populations with low HO. In spatially structured species like the mountain goat [7], admixture results from matings between dispersers and residents. The distribution of HO across D, R and A categories suggests that heterosis could be occurring, as the outbred–admixed individuals have the highest HO. The level to which HO in A is confounded by historic dispersal events and subsequent backcrosses is unclear, but the cost of philopatry versus dispersal in a fitness- or inbreeding-related context should still consider the potential benefit of heterosis. Future research should also use genomic resources (i.e. [23]) to identify chromosomal regions and ultimately the genes responsible for dispersal.
Acknowledgements
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) grants to D.W.C. and S.D.C., and the Alberta Conservation Association. Both J.P. and A.B.A.S. were funded by NSERC and the Alberta Ingenuity fund.
References
- 1.Bowler D. E., Benton T. G. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225 10.1017/S1464793104006645 (doi:10.1017/S1464793104006645) [DOI] [PubMed] [Google Scholar]
- 2.Clobert J., Danchin E., Dhondt A. A., Nichols J. D. (eds) 2001. Dispersal. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Dobson F. S., Jones W. T. 1985. Multiple causes of dispersal. Am. Nat. 126, 855–858 10.1086/284457 (doi:10.1086/284457) [DOI] [Google Scholar]
- 4.Hadany L., Eshel I., Motro U. 2004. No place like home: competition, dispersal, and complex adaptation. J. Evol. Biol. 17, 1328–1336 10.1111/j.1420-9101.2004.00768.x (doi:10.1111/j.1420-9101.2004.00768.x) [DOI] [PubMed] [Google Scholar]
- 5.Chapman J. R., Nakagawa S., Coltman D. W., Slate J., Sheldon B. C. 2009. A quantitative review of heterozygosity-fitness correlations in animal populations. Mol. Ecol. 18, 2746–2765 10.1111/j.1365-294X.2009.04247.x (doi:10.1111/j.1365-294X.2009.04247.x) [DOI] [PubMed] [Google Scholar]
- 6.Mainguy J., Côté S. D., Coltman D. W. 2009. Multilocus heterozygosity, parental relatedness and individual fitness components in a wild mountain goat, Oreamnos americanus population. Mol. Ecol. 18, 2297–2306 10.1111/j.1365-294X.2009.04197.x (doi:10.1111/j.1365-294X.2009.04197.x) [DOI] [PubMed] [Google Scholar]
- 7.Shafer A. B. A., Côté S. D., Coltman D. W. 2011. Hot spots of genetic diversity descended from multiple Pleistocene refugia in an alpine ungulate. Evolution 65, 125–138 10.1111/j.1558-5646.2010.01109.x (doi:10.1111/j.1558-5646.2010.01109.x) [DOI] [PubMed] [Google Scholar]
- 8.Waser P. M., Strobeck C. 1998. Genetic signatures of interpopulation dispersal. Trends Ecol. Evol. 13, 43–44 10.1016/S0169-5347(97)01255-X (doi:10.1016/S0169-5347(97)01255-X) [DOI] [PubMed] [Google Scholar]
- 9.Festa-Bianchet M., Côté S. D. 2008. Mountain goats: ecology, behavior, and conservation of an alpine ungulate. Washington, DC: Island Press [Google Scholar]
- 10.Pritchard J. K., Stephens M., Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155, 945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bélichon S., Clobert J., Massot M. 1996. Are there different fitness components between philopatric and dispersing individuals? Acta Oecol. 17, 503–517 [Google Scholar]
- 12.Worley K., Strobeck C., Arthur S., Carey J., Schwantje H., Veitch A., Coltman D. W. 2004. Population genetic structure of North American thinhorn sheep (Ovis dalli). Mol. Ecol. 13, 2545–2556 10.1111/j.1365-294X.2004.02248.x (doi:10.1111/j.1365-294X.2004.02248.x) [DOI] [PubMed] [Google Scholar]
- 13.Bergl R. A., Vigilant L. 2007. Genetic analysis reveals population structure and recent migration within the highly fragmented range of the Cross River gorilla (Gorilla gorilla diehli). Mol. Ecol. 16, 501–516 10.1111/j.1365-294X.2006.03159.x (doi:10.1111/j.1365-294X.2006.03159.x) [DOI] [PubMed] [Google Scholar]
- 14.Lecis R., Pierpaoli M., Biro Z. S., Szemethy L., Ragni B., Vercillo F., Randi E. 2006. Bayesian analyses of admixture in wild and domestic cats (Felis silvestris) using linked microsatellite loci. Mol. Ecol. 15, 119–132 10.1111/j.1365-294X.2005.02812.x (doi:10.1111/j.1365-294X.2005.02812.x) [DOI] [PubMed] [Google Scholar]
- 15.Hamel S., Côté S. D., Festa-Bianchet M. 2010. Maternal characteristics and environment affect the costs of reproduction in female mountain goats. Ecology 91, 2034–2043 10.1890/09-1311.1 (doi:10.1890/09-1311.1) [DOI] [PubMed] [Google Scholar]
- 16.Hanski I., Peltonen A., Kaski L. 1991. Natal dispersal and social dominance in the common shrew Sorex araneus. Oikos 62, 48–58 10.2307/3545445 (doi:10.2307/3545445) [DOI] [Google Scholar]
- 17.Ims R. A. 1990. Determinants of natal dispersal and space use in grey-sided voles, Clethrionomys rufoccanus: a combined field laboratory experiment. Oikos 57, 106–113 10.2307/3565743 (doi:10.2307/3565743) [DOI] [Google Scholar]
- 18.Jensen H., Bremset E. M., Ringsby T. H., Sæther B.-E. 2007. Multilocus heterozygosity and inbreeding depression in an insular house sparrow metapopulation. Mol. Ecol. 16, 4066–4078 10.1111/j.1365-294X.2007.03452.x (doi:10.1111/j.1365-294X.2007.03452.x) [DOI] [PubMed] [Google Scholar]
- 19.Perrin N., Mazalov V. 1999. Dispersal and inbreeding avoidance. Am. Nat. 154, 282–292 10.1086/303236 (doi:10.1086/303236) [DOI] [PubMed] [Google Scholar]
- 20.Roze D., Rousset F. 2009. Strong effects of heterosis on the evolution of dispersal rates. J. Evol. Biol. 22, 1221–1233 10.1111/j.1420-9101.2009.01735.x (doi:10.1111/j.1420-9101.2009.01735.x) [DOI] [PubMed] [Google Scholar]
- 21.Guillaume F., Perrin N. 2006. Joint evolution of dispersal and inbreeding load. Genetics 173, 497–509 10.1534/genetics.105.046847 (doi:10.1534/genetics.105.046847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan M. T. 2002. Genome-wide deleterious mutation favors dispersal and species integrity. Heredity 89, 253–257 10.1038/sj.hdy.6800143 (doi:10.1038/sj.hdy.6800143) [DOI] [PubMed] [Google Scholar]
- 23.Poissant J., Shafer A. B. A., Mainguy J., Davis C. S., Côté S. D., Hogg J. T., Coltman D. W. 2009. Genome-wide cross-amplification of domestic sheep microsatellite loci in bighorn sheep and mountain goats. Mol. Ecol. Res. 9, 1121–1126 10.1111/j.1755-0998.2009.02575.x (doi:10.1111/j.1755-0998.2009.02575.x) [DOI] [PubMed] [Google Scholar]