Abstract
Estimates of early-life traits of fishes (e.g. pelagic larval duration (PLD) and spawning date) are essential for investigating and assessing patterns of population connectivity. Such estimates are available for a large number of both tropical and temperate fish species, but few studies have assessed their variability in space, especially across multiple scales. The present study, where a Mediterranean fish (i.e. the white seabream Diplodus sargus sargus) was used as a model, shows that spawning date and PLD are spatially more variable at a scale of kilometres than at a scale of tens to hundreds of kilometres. This study indicates the importance of considering spatial variability of early-life traits of fishes in order to properly delineate connectivity patterns at larval stages (e.g. by means of Lagrangian simulations), thus providing strategically useful information on connectivity and relevant management goals (e.g. the creation of networks of marine reserves).
Keywords: life-history traits, spatial variability, white sea bream, Mediterranean Sea
1. Introduction
The life cycle of most coastal fish species is made up of a vagrant planktonic phase (corresponding to the stages of eggs and larvae) and a relatively sedentary phase (i.e. post-settlers to adults [1]). The spawning precedes the planktonic phase and its onset is related to environmental features [2]. The transition stage termed ‘settlement’ coincides temporally with the metamorphosis from the larva to the benthic juvenile and divides the life cycle into the two above-mentioned phases [3]. The pelagic larval duration (PLD (expressed as ‘number of days’)) corresponds to the period between hatching and settlement. PLD is regulated by both genetic [4] and environmental factors [5].
To understand where larvae produced in a given place (e.g. in a marine reserve) are exported to, it can be crucial to collate information relating to egg-release dates, PLD in open waters and direction and strength of currents within the temporal window corresponding to the larval stage. Data about PLD and spawning dates are thus important for estimating or modelling larval dispersal (e.g. by mean of Lagrangian models [6]) and, therefore, to investigate patterns of connectivity between marine populations [7]. Connectivity, from this perspective, is increasingly recognized as an important property for conservation issues and management of fisheries resources (see [7] for further discussion). Properly incorporating patterns of variability of PLD and spawning date, although untested so far, may help refine models of dispersal and improve estimates of spatial connectivity.
Estimates of PLD are available for a large number of both tropical [1] and temperate fish species [8], although the majority of these estimates are limited owing to the samples being taken at single places and/or within single temporal windows. Conversely, few studies have assessed variability of PLD in time [3] and space [1,9], especially across multiple scales [10]. The main implication of this is that available models of larval dispersal for many fish species [6] are often based on PLDs in terms of absolute and constant values (i.e. under the untested assumption that they would be constant across multiple spatial and temporal scales). The identification of spawning dates has been the subject of a number of studies that have seldom assessed variability in time (i.e. interannual variability, [11]) and space [2], but, as far as we know, no study has assessed variability over multiple scales.
Using the white sea bream Diplodus sargus sargus as a model species, the aim of this work is to investigate the potential variability in PLD and spawning dates at multiple spatial scales (i.e. from hundreds of metres to hundreds of kilometres) in order to shed light on these unassessed aspects of the life cycle and provide useful information for connectivity estimates.
2. Material and methods
The model species of this study (D. sargus sargus) is an ecologically and economically relevant species in Mediterranean sublittoral rocky reefs [12,13]. Adults are relatively sedentary and demersal [13], and produce pelagic eggs that hatch and release larvae after 3 days [14]. After metamorphosis, post-larvae settle into very shallow (0.5–1 m depth) coastal habitats [13].
Early post-larvae (settlers) were collected in June 2009, along approximately 200 km of the Apulian Adriatic coast, across the north–south axis (approx. 1°, from 41.2° N to 40.2° N). Seven sections of the coast were used as sample locations. Each location was roughly 8 km in length and locations were separated by 15–30 km. Within each location, two sites (represented by embayments with shallow rocky habitats alternated with sand patches) were randomly selected. A handnet was used to collect 10 specimens per site (n = 140). Specimens were stored in 95 per cent ethanol within several minutes of capture. All specimens were collected across locations within 8 days in order to prevent or reduce any relevant temporal bias.
The specimens sampled were measured (standard length, ±0.1 mm) before removing the otoliths. In fishes, spawning date and PLD can be assessed through the analysis of daily rings (or growth increments) on otoliths [3]. Otoliths develop around a primordium, which forms during the embryonic development, and grow by apposition of daily rings. PLD can therefore be accurately assessed by counting the number of daily rings between the primordium and the settlement mark (i.e. the first major transitional point; for details about otolith microstructure see [15]). Spawning date can be identified by back-calculations from the post-settlement age.
One sagittal otolith was removed from each specimen and processed following a standard procedure [15]. The daily rings were read using a high-powered microscope. For each specimen, the hatching date was back-calculated by subtracting the number of growth increments from the sampling date. The spawning date was then calculated by subtracting 3 days (corresponding to the time between spawning and larval release in D. sargus sargus [14]) from the estimated hatching date.
To test for potential spatial variability in PLD and spawning dates, two analysis of covariance (ANCOVA) were run, where ‘location’ (Lo) was treated as a random factor with seven levels, ‘site’ (Si) was used as a random factor nested within Lo, with two levels, and the fish ‘standard length’ (SL) as the covariate. Ten otoliths (replicates) were read from each site. The test for covariate effect was performed to prevent fish size (possibly different from site to site) affect on spatial comparisons of PLDs and spawning dates. In other words, only conditioning on fish size, any observed difference is attributable to ‘pure’ spatial patterns.
3. Results
Fish size ranges between 0.9 and 1.4 cm SL (1.11 ± 0.01 cm, mean ± s.e.).
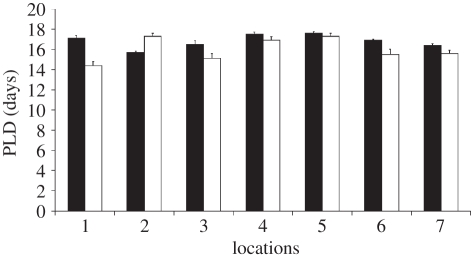
PLD values ranged on the whole from 13 to 19 days. The average PLD value per site varied from 14.4 ± 0.4 (mean ± s.e.) to 17.6 ± 0.16 (mean ± s.e.; figure 1). Coefficients of variation were quite low and ranged from 0.03 to 0.11. On the whole, back-calculated spawning dates ranged from 4 to 24 May 2009. No significant effect of the covariate ‘fish size’ was detected on PLD or spawning dates. PLD and dates of spawning significantly varied over the scale of sites, whereas no significant differences were detected among locations (table 1).
Figure 1.
PLD values for each of the seven locations (mean ± s.e.). Black bars indicate site 1 and white bars indicate site 2 in each location.
Table 1.
ANCOVA on PLD and spawning dates. (Standard length (SL) was set as covariate. n.s., not significant; Res, residuals; MS, mean squares. See text for factor labels. ***p < 0.001.)
| PLD |
spawning dates |
||||
|---|---|---|---|---|---|
| source | d.f. | MS | pseudo-F | MS | pseudo-F |
| SL | 1 | 21.94 | 4.80 n.s. | 1.06 | 0.03 n.s. |
| Lo | 6 | 7.75 | 0.85 n.s. | 71.614 | 0.79 n.s. |
| Si(Lo) | 6 | 9.34 | 9.12*** | 92.359 | 16.11*** |
| Res | 125 | 1.02 | 5.733 | ||
| total | 139 | 139 | |||
4. Discussion
The present study provides the evidence that spawning date and PLD are spatially variable at a scale of kilometres. It is not easy to interpret this outcome on the basis of the available literature, where spatial variability has been investigated exclusively at small (tens of kilometres [2,8]) or large scales (hundreds–thousands of kilometres [1,7]), without any spatial hierarchy in the adopted sampling designs. From this perspective, the present study provides for the first time to our knowledge, a multiple-scale assessment of spatial variability in spawning dates and PLD. On a relatively small spatial scale (kilometres), the observed differences in spawning dates and PLD could be attributed to small-scale variability in environmental and oceanographic features and processes (e.g. small areas of retention, coastal gyres) and/or in the food availability and growth conditions, which are known to influence PLD and the onset of spawning [2,3,5]. Strictly, with respect to the variability in PLD, this point is particularly important for species facing, at the larval stage, the strong environmental variability typical of coastal waters (e.g. for species with coastal larvae like D. sargus sargus), where freshwater inputs, sewage discharges, etc. may locally represent more or less suitable conditions for settlement.
Estimates of PLD and spawning dates are being used in a variety of applications [16,17], including the estimate or modelling of population connectivity of fishes [9]. The significant small-scale spatial variability detected in the present study for PLD and spawning dates suggests that modelling dispersal and connectivity without taking into account variability at multiple scales carries the risk of producing unreliable models that may fail to generalize patterns of dispersal/connectivity (traditionally based on single-value estimates without any proper spatial assessment). Defining variability in spawning date and PLD at multiple scales (both temporal and spatial), therefore, can be pivotal to proper delineate connectivity patterns at larval stages and provide effective clues for management (i.e. design of marine reserve networks, [11]).
Acknowledgements
Both authors have adhered to the Italian legal requirements and the experiment was approved by Total Foundation and the Italian MIUR.
This research was funded by Total Foundation and the Italian MIUR (PRIN Project: protocol no. 2008E7KBAE). Authors thank Dr Chris McCullough (University of Plymouth) and two anonymous referees for critically reviewing an early draft of the article.
References
- 1.Thresher R. E., Colin P. L., Bell L. J. 1989. Planktonic duration, distribution and population structure of western and central Pacific damselfishes (Pomacentridae). Copeia 1989, 420–434 10.2307/1445439 (doi:10.2307/1445439) [DOI] [Google Scholar]
- 2.Vinagre C., Ferreira T., Matos L., Costa M. J., Cabral H. N. 2009. Latitudinal gradients in growth and spawning of sea bass, Dicentrarchus labrax, and their relationship with temperature and photoperiod. Estuar. Coast. Shelf Sci. 81, 375–380 10.1016/j.ecss.2008.11.015 (doi:10.1016/j.ecss.2008.11.015) [DOI] [Google Scholar]
- 3.Searcy S. S., Sponaugle S. 2000. Variable larval growth in a coral reef fish. Mar. Ecol. Prog. Ser. 206, 213–226 10.3354/meps206213 (doi:10.3354/meps206213) [DOI] [Google Scholar]
- 4.Bonhomme F., Planes S. 2000. Some evolutionary arguments about what maintains the pelagic interval in reef fishes. Environ. Biol. Fish. 59, 365–383 10.1023/A:1026508715631 (doi:10.1023/A:1026508715631) [DOI] [Google Scholar]
- 5.Sponaugle S., Grorud-Colvert K., Pinkard D. 2006. Temperature mediated variation in early life history traits and recruitment success of the coral reef fish Thalassoma bifasciatum in the Florida Keys. Mar. Ecol. Prog. Ser. 308, 1–15 10.3354/meps308001 (doi:10.3354/meps308001) [DOI] [Google Scholar]
- 6.Watson J. R., Mitarai S., Siegel D. A., Caselle J. E., Dong C., McWilliams J. C. 2010. Realized and potential larval connectivity in the Southern California Bight. Mar. Ecol. Prog. Ser. 401, 31–48 10.3354/meps08376 (doi:10.3354/meps08376) [DOI] [Google Scholar]
- 7.Almany G. R., Connolly S. R., Heath D. D., Hogan J. D., Jones G. P., McCook L. J., Mills M., Pressey R. L., Williamson D. H. 2009. Connectivity, biodiversity conservation, and the design of marine reserve networks for coral reefs. Coral Reefs 28, 339–351 10.1007/s00338-009-0484-x (doi:10.1007/s00338-009-0484-x) [DOI] [Google Scholar]
- 8.Macpherson E., Raventos N. 2006. Relationship between pelagic larval duration and geographic distribution of Mediterranean littoral fishes. Mar. Ecol. Prog. Ser. 327, 257–265 10.3354/meps327257 (doi:10.3354/meps327257) [DOI] [Google Scholar]
- 9.Bay L. K., Buechler K., Gagliano M., Caley M. 2006. Intraspecific variation in the pelagic larval duration of tropical reef fishes. J. Fish Biol. 68, 1206–1214 10.1111/j.0022-1112.2006.01016.x (doi:10.1111/j.0022-1112.2006.01016.x) [DOI] [Google Scholar]
- 10.McCormick M. I. 1994. Variability in age and size at settlement of the tropical goatfish Upeneus tragula (Mullidae) in the northern Great Barrier Reef lagoon. Mar. Ecol. Prog. Ser. 103, 1–15 10.3354/meps103001 (doi:10.3354/meps103001) [DOI] [Google Scholar]
- 11.Fox C. J., Geffen A. J., Taylor N., Davison P., Rossetti H., Nash R. D. M. 2007. Birth-date selection in early life stages of plaice Pleuronectes platessa in the eastern Irish Sea (British Isles). Mar. Ecol. Prog. Ser. 345, 255–269 10.3354/meps06967 (doi:10.3354/meps06967) [DOI] [Google Scholar]
- 12.Guidetti P. 2006. Marine reserves re-establish lost predatory interactions and cause community-wide changes in rocky reefs. Ecol. Appl. 16, 963–976 10.1890/1051-0761(2006)016[0963:MRRLPI]2.0.CO;2 (doi:10.1890/1051-0761(2006)016[0963:MRRLPI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 13.Harmelin-Vivien M. L., Harmelin J. G., Leboulleux V. 1995. Microhabitat requirements for settlement of juvenile sparid fishes on Mediterranean rocky shores. Hydrobiologia 300–301, 309–320 10.1007/BF00024471 (doi:10.1007/BF00024471) [DOI] [Google Scholar]
- 14.Ranzi S. 1933. Sparidae, Lobotidae in Uova, larve e stadi giovanili di Teleostei. Fauna Flora Golfo Napoli 38, 374–376 [Google Scholar]
- 15.Green B. S., Mapstone B., Carlos G., Begg G. A. 2009. Tropical fish otoliths: information for assessment, management and ecology. New York, NY: Springer [Google Scholar]
- 16.Shulman M. J., Bermingham E. 1995. Early life histories, ocean currents and the population genetics of coral reef fishes. Evolution 49, 879–910 10.2307/2410412 (doi:10.2307/2410412) [DOI] [PubMed] [Google Scholar]
- 17.Lester S. E., Ruttenberg B. I., Gaines S. D., Kinlan B. P. 2007. The relationship between dispersal ability and geographic range size. Ecol. Lett. 10, 745–758 10.1111/j.1461-0248.2007.01070.x (doi:10.1111/j.1461-0248.2007.01070.x) [DOI] [PubMed] [Google Scholar]