Abstract
Carotenoid-based sexual ornaments are hypothesized to be reliable signals of male quality, based on an allocation trade-off between the use of carotenoids as pigments and their use in antioxidant defence against reactive oxygen species. Carotenoids appear to be poor antioxidants in vivo, however, and it is not clear whether variation in ornament expression is correlated with measures of oxidative stress (OXS) under natural conditions. We used single-cell gel electrophoresis to assay oxidative damage to erythrocyte DNA in the common yellowthroat (Geothlypis trichas), a sexually dichromatic warbler in which sexual selection favours components of the males' yellow ‘bib’. We found that the level of DNA damage sustained by males predicted their overwinter survivorship and was reflected in the quality of their plumage. Males with brighter yellow bibs showed lower levels of DNA damage, both during the year the plumage was sampled (such that yellow brightness signalled current OXS) and during the previous year (such that yellow brightness signalled past OXS). We suggest that carotenoid-based ornaments can convey information about OXS to prospective mates and that further work exploring the proximate mechanism(s) linking OXS to coloration is warranted.
Keywords: oxidation handicap hypothesis, good genes, epigamic signalling
1. Introduction
Over a decade ago, von Schantz et al. [1] proposed that a simple allocation trade-off between the signalling and antioxidant functions of carotenoids may underlie good-genes sexual selection for colourful traits. Assuming that carotenoids are both limiting and an important component of an individual's total antioxidant defence, and recognizing that oxidative damage to DNA and other cell constituents can have negative consequences at the level of the whole organism, von Schantz et al. [1] hypothesized that the deposition of carotenoids in inert, ornamental structures imposes an oxidative handicap on males, preventing poor-quality individuals from efficiently coping with the reactive oxygen species (ROS) produced during aerobic metabolism and immune activation. By preferring colourful males, females choose individuals that can most afford to divert carotenoids away from antioxidant defence and towards display; that is, they select healthy males in a favourable oxidative state.
The idea that ROS mediate sexual selection on colourful traits is compelling, in part because ROS may lie at the nexus of critical life-history decisions in animals. For example, investment in reproductive activities generates ROS, yet this investment may come at the expense of defence and repair mechanisms, yielding a cost of reproduction in the form of oxidative stress (OXS) that decreases survivorship, increases the rate of senescence or both [2]. As pathways regulating ROS appear to be heritable [3], ornaments revealing OXS are potential targets of good-genes sexual selection.
Despite significant recent attention, ROS-mediated sexual selection remains controversial. For example, experimental manipulation of carotenoid supply often has little direct effect on ROS, antioxidant capacity or measures of oxidative damage, leading to the general conclusion that carotenoids are poor antioxidants in vivo [4]. However, supplementation with other antioxidants appears to free (or protect) carotenoids for eventual incorporation into ornaments [5], suggesting that coloration can signal total antioxidant capacity. Several recent studies have demonstrated changes in ornamentation with increased OXS [6–8], but there are exceptions [9].
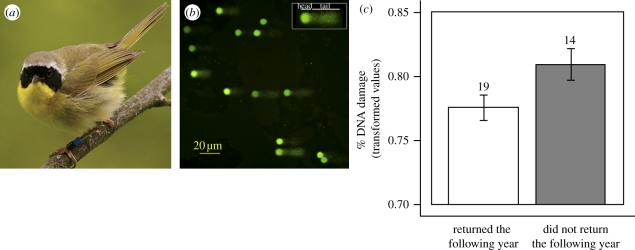
Here, we use single-cell gel electrophoresis (SCGE) of erythrocytes to measure oxidative damage to DNA and relate this damage to male ornamentation in the common yellowthroat. Male common yellowthroats possess a carotenoid-based, yellow ‘bib’ (figure 1a) that is a condition-dependent signal of quality preferred by females in our population [10,11].
Figure 1.
(a) Male common yellowthroat (Geothlypis trichas). (b) Common yellowthroat erythrocyte DNA subjected to SCGE and stained with SYBR Green. (c) Mean ± s.e. DNA damage (arcsine square-root transformed values) for males that did and did not return to the study area the following year.
2. Material and methods
We studied common yellowthroats nesting along power line and riparian corridors in Saratoga County, NY, USA from 2005 to 2009. Males were captured in mist-nests soon after arrival and filmed in standardized posture using digital video. We quantified the size (area) of the mask and bib using ImageJ as described in Freeman-Gallant et al. [11]. Although our analyses focus on the bib, we included the melanin-based mask because the mask is both condition-dependent and a target of female choice in some populations [12]. Colourimetrics were obtained using ultra-violet (UV)-vis spectrometry (Ocean Optics 2000, Dunedin, FL, USA) performed in the laboratory on feather samples collected at random from the centre of each male's bib. We quantified yellow brightness, carotenoid chroma (Ccar) and UV saturation (table 1; see [11] for details). Ccar provides a measure of yellow saturation that is positively correlated with feather carotenoid concentration in some species [9].
Table 1.
Colourimetrics based on reflectance spectrometry.
| yellow brightness | average reflectance (R) across 550–625 nm | average (R550–625) |
| UV saturation | proportion of reflectance (R) across 320–700 nm attributed to reflectance in the UV (320–400 nm) | ∑ (R320–400)/∑ (R320–700) |
| carotenoid chroma | relative extent to which yellow reflectance (at R700) exceeds blue-green reflectance (at R450) | (R700−R450)/R700 |
In 2008–2009, we performed SCGE on erythrocytes to quantify oxidative damage to DNA. At the time of capture, we diluted 50 µl of whole blood from the brachial vein in 1.0 ml of ice-cold buffer (10% DMSO, 90% Newborn Bovine Serum) and stored samples on ice until cryopreservation at −80°C. After thawing at 37°C for 2 min, erythrocytes were pelleted, washed in 1X phosphate buffered saline, and then mixed with low melting point agarose to achieve a final suspension of 10 cells µl−1. Two 75 µl gels were poured onto a Trevigen (Gaithersburg, MD, USA) CometSlide and subjected to SCGE in 1X tris-borate-EDTA for 10 min at 35 V after first lysing cells and then denaturing DNA in alkaline solution (200 mM NaOH, 1 mM ethylenediaminetetraacetic acid (EDTA)). Following SCGE, gels were washed in 70 per cent ethanol and air dried. To visualize DNA, slides were stained with SYBR Green and digitally imaged at 25×. Comets representing erythrocyte nuclear DNA (in the ‘head’) and any DNA degraded through single and double-strand breaks (in the ‘tail’) were analysed using Comet Score v. 1.5 (figure 1b). Per cent DNA in the tail was averaged over all 204 ± 53 (s.d.) comets scored for each male and square-root arcsine transformed prior to analysis. Average per cent DNA in the tail was repeatable across the two gels examined for each male (ANOVA, F67,68 = 9.8, p < 0.0001; repeatability = 0.82; [13]). Early validation work in our laboratories showed no effect of cryopreservation on DNA damage (difference in mean damage between fresh and cryopreserved cells <1%; paired t-test; t13 = 1.07, p = 0.31). We use DNA damage as an index of OXS because it combines both free radical production and attack as well as antioxidant and repair mechanisms. See Collins [14] for discussion of SCGE and the comet assay as a measure of DNA damage caused by ROS and other sources.
In 2008, we obtained information on OXS and ornamentation for 17 males new to our study sites (‘inexperienced males’) and 16 males with a prior history of breeding (‘experienced males’). In 2009, we studied 17 inexperienced and 19 experienced males. We examined the relationship between DNA damage and ornamentation separately for inexperienced and experienced males because patterns of selection and condition-dependence are different in the two experience classes [11] and there are significant experience-by-ornament interactions in analyses of OXS pooling over males (ANCOVA, r2 = 0.34, p < 0.01, n = 48; experience × bib size, experience × yellow brightness, both p < 0.04). To avoid pseudoreplication, we used the most recent data for males that were present in both years. Sample sizes vary where incomplete data forced the exclusion of some males.
3. Results
OXS increased significantly with sampling date within a season, but there is no evidence that OXS increased with male age, either in cross-sectional analysis comparing inexperienced, first-time breeders (at our study sites) with experienced males (ANCOVA with year as a fixed effect, n = 50; date: F1,46 = 11.5, p = 0.001; experience: F1,46 = 0.01, p = 0.93), or in longitudinal comparisons of OXS across successive seasons for 19 returning birds (paired t18 = 1.1, p = 0.31). However, OXS was a significant predictor of survivorship from 2008 to 2009 (multiple logistic regression controlling for sampling date; OXS effect: Wald χ2 = 5.1, n = 33, p = 0.02; figure 1c). OXS was also reflected in the yellow coloration of the bib at the time of sampling. In multiple regressions of mask and bib traits on OXS, increasing OXS was associated with reduced yellow brightness (experienced males) and carotenoid chroma (inexperienced males) but not with mask size or bib UV coloration (table 2). Bib yellow brightness (in the year n) also revealed the level of OXS experienced by males the preceding year (in the year n − 1), when the plumage was obtained by males during moult (table 2).
Table 2.
Relationship between male ornamentation and oxidative stress (OXS) measured as per cent DNA damage to erythrocytes using SCGE. (Ornaments were measured in the year (n) and OXS in the year (n) and (n − 1). F-statistics are for multiple linear regressions. Effect sizes are for data that have been standardized to a mean of zero with unit variance; bold terms are significant at p < 0.05).
| overall model | oxidative stress in the year n |
oxidative stress in the year n−1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| inexperienced malesa |
experienced malesb |
experienced malesc |
|||||||
|
r2 = 0.29, p = 0.02 |
r2 = 0.44, p = 0.01 |
r2 = 0.41, p = 0.05 |
|||||||
| effect | F | p | effect | F | p | effect | F | p | |
| bib size | 0.02 | 4.7 | 0.04 | 2.6 | 0.13 | 0 | 0.61 | ||
| bib yellow brightness | 1.6 | 0.22 | −0.04 | 6.7 | 0.02 | −0.03 | 5.4 | 0.04 | |
| bib carotenoid chroma | −0.02 | 4.2 | 0.05 | 1.4 | 0.26 | 1.9 | 0.19 | ||
| bib UV saturation | 1.2 | 0.27 | 0.3 | 0.57 | 0 | 0.99 | |||
| mask size | 0.2 | 0.70 | 0.6 | 0.45 | 0.9 | 0.38 | |||
| sampling date | 0.03 | 10.1 | 0.004 | 3.2 | 0.09 | 0.03 | 9.2 | 0.01 | |
an = 33 males from the year 2008 and 2009.
bn = 23 males from the year 2008 and 2009 (using most recent data for each male to avoid pseudoreplication).
cn = 18 males from the year 2009.
4. Discussion
We found that elements of a carotenoid-based plumage ornament reflect OXS, as measured by SCGE and the comet assay. Experienced males with brighter yellow plumage and inexperienced males with greater carotenoid chroma showed reduced OXS, which, in turn, was linked to greater overwinter survivorship in our population. We have previously shown that sexual selection favours increased carotenoid chroma among inexperienced (but not experienced) males and that, at the population level, males with greater yellow brightness achieve higher mating success. By selecting brighter bibs, females mate with older [11] and healthier males [12] who also show reduced OXS (this study).
Although our results suggest an important role for ROS in sexual signalling, OXS need not be revealed to prospective mates via direct, carotenoid-allocation trade-offs. For example, if androgens are both pro-oxidants and important for the exaggeration of secondary sexual traits, an oxidative cost to ornament development may produce a relationship between OXS and the extent of exaggeration even if carotenoids are themselves irrelevant to ROS surveillance [15]. Additionally, the pro-oxidant consequences of infection coupled with a role for carotenoids in immune stimulation [16] may generate complex associations between OXS, health and ornamentation that do not necessarily rely on an antioxidant function for carotenoids. Attention to such indirect linkages has already been proved fruitful [6,7].
Different aspects of yellow coloration revealed more OXS in inexperienced versus experienced males. In addition, increasing bib size was correlated with DNA damage only among inexperienced males. To the extent that inexperienced males new to our study areas were younger than males with a known breeding history, these results suggest that the proximate mechanisms linking OXS to male ornamentation may change with male age. Age-related changes in sexual signalling have been found in other systems [17] but they are rarely studied in the context of ROS-mediated sexual selection (but see [6,8,18]), perhaps contributing to the mixed results in the literature.
To the best of our knowledge, this is the first study that relates DNA damage to sexual signalling in a vertebrate. Recent tests of ROS-mediated sexual selection measure the extent of lipid peroxidation when quantifying OXS, but ROS clearly affect other cell constituents [2]. DNA damage caused by ROS may be particularly important, as this damage contributes to high rates of telomere shortening, abnormal patterns of gene regulation and the etiology of many diseases [19]. Indeed, the significance of DNA damage for proper cell function is revealed by the existence of a large number of complex damage recognition and repair pathways [19]. Our results suggest that oxidative damage to DNA should be considered when assessing the information content of ornaments and ROS-related costs of sexual signalling.
Acknowledgements
This work was approved by the Institutional Animal Care and Use Committee at Skidmore College.
This work was supported by the National Science Foundation (IBN-0412746) and, in the field, by R. Schneider and M. Garfinkel.
References
- 1.von Schantz T., Bensch S., Grahn M., Hasselquist D., Wittzell H. 1999. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. Lond. B 266, 1–12 10.1098/rspb.1999.0597 (doi:10.1098/rspb.1999.0597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monaghan P., Metcalfe N. B., Torres R. 2009. Oxidative stress as a mediator of life history tradeoffs: mechanisms, measurements and interpretation. Ecol. Lett. 12, 75–92 10.1111/j.1461-0248.2008.01258.x (doi:10.1111/j.1461-0248.2008.01258.x) [DOI] [PubMed] [Google Scholar]
- 3.Kim S.-Y., Noguera J. C., Morales J., Velando A. 2010. Heritability of resistance to oxidative stress in early life. J. Evol. Biol. 23, 769–773 10.1111/j.1420-9101.2010.01942.x (doi:10.1111/j.1420-9101.2010.01942.x) [DOI] [PubMed] [Google Scholar]
- 4.Costantini D., Møller A. P. 2008. Carotenoids are minor antioxidants for birds. Funct. Ecol. 22, 367–370 10.1111/j.1365-2435.2007.01366.x (doi:10.1111/j.1365-2435.2007.01366.x) [DOI] [Google Scholar]
- 5.Pike T. W., Blount J. D., Lindström J., Metcalfe N. B. 2007. Availability of non-carotenoid antioxidants affects the expression of a carotenoid-based sexual ornament. Biol. Lett. 3, 353–356 10.1098/rsbl.2007.0072 (doi:10.1098/rsbl.2007.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso-Alvarez C., Pérez-Rodríguez L., Garcia J. T., Viñuela J. 2009. Testosterone-mediated trade-offs in the old age: a new approach to the immunocompetence handicap and carotenoid-based sexual signaling. Proc. R. Soc. B 276, 2093–2101 10.1098/rspb.2008.1891 (doi:10.1098/rspb.2008.1891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mougeot F., Martínez-Padilla J., Webster L. M. I., Blount J. D., Pérez-Rodríguez L., Piertney S. B. 2009. Honest sexual signaling mediated by parasite and testosterone effects on oxidative balance. Proc. R. Soc. B 276, 1093–1100 10.1098/rspb.2008.1570 (doi:10.1098/rspb.2008.1570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres R., Velando A. 2007. Male reproductive senescence: the price of immune-induced oxidative damage on sexual attractiveness in the blue-footed booby. J. Anim. Ecol. 76, 1161–1168 10.1111/j.1365-2656.2007.01282.x (doi:10.1111/j.1365-2656.2007.01282.x) [DOI] [PubMed] [Google Scholar]
- 9.Isaksson C., Andersson S. 2008. Oxidative stress does not influence carotenoid mobilization and plumage pigmentation. Proc. R. Soc. B 275, 309–314 10.1098/rspb.2007.1474 (doi:10.1098/rspb.2007.1474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn P. O., Whittingham L. A., Freeman-Gallant C. R., DeCoste J. 2008. Geographic variation in the function of ornaments in the common yellowthroat. J. Avian Biol. 39, 66–72 10.1111/j.0908-8857.2008.04113.x (doi:10.1111/j.0908-8857.2008.04113.x) [DOI] [Google Scholar]
- 11.Freeman-Gallant C. R., Taff C. C., Morin D. F., Dunn P. O., Whittingham L. A. 2010. Sexual selection, multiple male ornaments, and age- and condition-dependent signaling in the common yellowthroat. Evolution 64, 1007–1017 10.1111/j.1558-5646.2009.00873.x (doi:10.1111/j.1558-5646.2009.00873.x) [DOI] [PubMed] [Google Scholar]
- 12.Dunn P. O., Garvin J. C., Whittingham L. A., Freeman-Gallant C. R., Hasselquist D. 2010. Carotenoid and melanin-based ornaments signal similar aspects of male quality in two populations of the common yellowthroat. Funct. Ecol. 24, 149–158 10.1111/j.1365-2435.2009.01606.x (doi:10.1111/j.1365-2435.2009.01606.x) [DOI] [Google Scholar]
- 13.Lessells C. M., Boag P. T. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 [Google Scholar]
- 14.Collins A. R. 2009. Investigating oxidative DNA damage and its repair using the comet assay. Mut. Res. 681, 24–32 [DOI] [PubMed] [Google Scholar]
- 15.Alonso-Alvarez C., Bertrand S., Faivre B., Chastel O., Sorci G. 2007. Testosterone and oxidative stress: the oxidation handicap hypothesis. Proc. R. Soc. B 274, 819–825 10.1098/rspb.2006.3764 (doi:10.1098/rspb.2006.3764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGraw K. J., Ardia D. R. 2003. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 162, 704–712 10.1086/378904 (doi:10.1086/378904) [DOI] [PubMed] [Google Scholar]
- 17.Badyaev A. V., Duckworth R. A. 2003. Context-dependent sexual advertisement: plasticity in development of sexual ornamentation throughout the lifetime of a passerine bird. J. Evol. Biol. 16, 1065–1076 10.1046/j.1420-9101.2003.00628.x (doi:10.1046/j.1420-9101.2003.00628.x) [DOI] [PubMed] [Google Scholar]
- 18.Cote J., Arnoux E., Sorci G., Gaillard M., Faivre B. 2010. Age-dependent allocation of carotenoids to coloration versus antioxidant defenses. J. Exp. Biol. 213, 271–277 10.1242/jeb.035188 (doi:10.1242/jeb.035188) [DOI] [PubMed] [Google Scholar]
- 19.Halliwell B., Gutteridge J. 2007. Free radicals in biology and medicine. Oxford, UK: Oxford University Press [Google Scholar]