Abstract
Falciparum malaria is an important cause of acute symptomatic seizures in children admitted to hospitals in sub-Saharan Africa, and these seizures are associated with neurological disabilities and epilepsy. However, it is difficult to determine the proportion of seizures attributable to malaria in endemic areas since a significant proportion of asymptomatic children have malaria parasitaemia. We studied children aged 0–13 years who had been admitted with a history of seizures to a rural Kenyan hospital between 2002 and 2008. We examined the changes in the incidence of seizures with the reduction of malaria. Logistic regression was used to model malaria-attributable fractions for seizures (the proportion of seizures caused by malaria) to determine if the observed decrease in acute symptomatic seizures was a measure of seizures that are attributable to malaria. The overall incidence of acute symptomatic seizures over the period was 651/100 000/year (95% confidence interval 632–670) and it was 400/100 000/year (95% confidence interval 385–415) for acute complex symptomatic seizures (convulsive status epilepticus, repetitive or focal) and 163/100 000/year (95% confidence interval 154–173) for febrile seizures. From 2002 to 2008, the incidence of all acute symptomatic seizures decreased by 809/100 000/year (69.2%) with 93.1% of this decrease in malaria-associated seizures. The decrease in the incidence of acute complex symptomatic seizures during the period was 111/100 000/year (57.2%) for convulsive status epilepticus, 440/100 000/year (73.7%) for repetitive seizures and 153/100 000/year (80.5%) for focal seizures. The adjusted malaria-attributable fractions for seizures with parasitaemia were 92.9% (95% confidence interval 90.4–95.1%) for all acute symptomatic seizures, 92.9% (95% confidence interval 89.4–95.5%) for convulsive status epilepticus, 93.6% (95% confidence interval 90.9–95.9%) for repetitive seizures and 91.8% (95% confidence interval 85.6–95.5%) for focal seizures. The adjusted malaria-attributable fractions for seizures in children above 6 months of age decreased with age. The observed decrease in all acute symptomatic seizures (809/100 000/year) was similar to the predicted decline (794/100 000/year) estimated by malaria-attributable fractions at the beginning of the study. In endemic areas, falciparum malaria is the most common cause of seizures and the risk for seizures in malaria decreases with age. The reduction in malaria has decreased the burden of seizures that are attributable to malaria and this could lead to reduced neurological disabilities and epilepsy in the area.
Keywords: acute seizures, Africa, children, falciparum malaria, malaria-attributable fractions
Introduction
It is estimated that Plasmodium falciparum caused over 500 million episodes of clinical malaria in 2002, and 70% of these were in Africa (Snow et al., 2005). Falciparum malaria is thought to be the most common cause of acute seizures (Waruiru et al., 1996; Idro et al., 2008) and convulsive status epilepticus (Crawley et al., 1996; Idro et al., 2008; Sadarangani et al., 2008) in children admitted to hospitals in malaria endemic regions of sub-Saharan Africa. Most of these seizures are likely to be acute symptomatic seizures rather than febrile seizures since the infected erythrocyte adheres to the post-capillaries vessels in the brain (Idro et al., 2005) and over half of these seizures occur when the child is afebrile (Waruiru et al., 1996). The seizures are often associated with complex features (focal, repetitive or prolonged) and status epilepticus (Waruiru et al., 1996; Idro et al., 2008; Sadarangani et al., 2008), and these features are associated with subsequent neurocognitive impairments (Mung'ala-Odera et al., 2004; Carter et al., 2005; Idro et al., 2007) and the development of epilepsy (Carter et al., 2004; Ngoungou and Preux, 2008; Birbeck et al., 2010). Through this mechanism, falciparum malaria may be a major cause of neurological disability in these areas.
Over the past decade, there has been a significant reduction in malaria transmission and admissions on the Kenyan coast (O'Meara et al., 2008). Since malaria is an important cause of acute seizures (Waruiru et al., 1996; Idro et al., 2008), the significant decrease in the transmission of malaria provides an opportunity to determine seizures that are attributable to malaria. We hypothesized that the decrease in admissions with acute symptomatic seizures during a period of a reduction in malaria transmission would provide an appropriate measure of seizures that are attributable to malaria. We also hypothesized that most of the decrease in acute symptomatic seizures would occur in admissions with a positive blood-slide for malaria or malaria-associated seizures (MAS) compared with admissions with a negative blood-slide for malaria or non-malaria-associated seizures (non-MAS). The observed decrease in acute symptomatic seizures between 2002 and 2008 can be compared with the proportion of seizures that are attributable to malaria at the beginning of the study to determine if the observed decrease represents the seizures that are attributable to malaria. However, some admissions with MAS at the beginning of the study would not be attributable to malaria since the prevalence of asymptomatic parasitaemia in the community was as high as 30% during the study period (Mwangi et al., 2005). The seizures that are attributable to malaria at the beginning of the study, which predicts the decline that should follow the reduction of malaria, can be estimated by multiplying the malaria-attributable fractions for seizures (the proportion of seizures caused by malaria) with the incidence of MAS at the beginning of the study (O'Meara et al., 2008). An observed decrease that is similar to the predicted decline estimated by malaria-attributable fractions for seizures would suggest that the decrease in seizures observed is a measure of seizures that are attributable to malaria.
The malaria-attributable fractions for seizures is a predictive measure of the proportion of children not admitted with seizures if malaria was completely eliminated (Smith et al., 1994; Koram and Molyneux, 2007). Although malaria-attributable fractions for seizures can be calculated by comparing the parasite prevalence in seizure cases with that of other admissions that did not develop seizures (Cole and MacMahon, 1971; Smith et al., 1994), this method is imprecise because many children in the community (as high as 30%) have asymptomatic malaria parasitaemia and thus, some children presenting to hospital with MAS may have their seizures caused by other illnesses but the parasitaemia is co-incidental (Mwangi et al., 2005; Koram and Molyneux, 2007). Since there is no one ‘gold standard’ test to determine ‘true’ malaria (Bejon et al., 2007), malaria-attributable fractions for seizures calculated on the basis of a positive blood-slide for malaria alone are biased towards sensitivity but are non-specific. Logistic regression fully uses the detailed information on parasitaemia distributions in both cases and comparison group and therefore has been suggested as a valuable alternative for modelling malaria-attributable fractions in malaria endemic areas (Smith et al., 1994; Mwangi et al., 2005; Bejon et al., 2007). The logistic regression model can be modified to allow for non-continuity between negative and positive blood-slides for malaria (Smith et al., 1994; Koram and Molyneux, 2007) and adjusts the lack of monotonicity in the relationship between malaria symptoms and parasitaemia that is reported in some studies (Smith et al., 1994). The model can be extended to allow for inclusion of other variables such as age and period of study, alongside the parasitaemia when modelling the malaria-attributable fractions for seizures (Smith et al., 1994).
The logistic regression model uses parasitaemia from children admitted to hospital with severe symptoms such as acute seizures and from a comparison group to model the relation between parasitaemia and the symptoms of malaria (Smith et al., 1994; Mwangi et al., 2005; Bejon et al., 2007). The parasitaemia used in the model was assessed with optical microscopy. The logistic regression technique models malaria-attributable fractions for various clinical features at different thresholds of parasitaemia and uses the modelled malaria-attributable fractions (i.e. the proportion of cases within a threshold of parasitaemia) to calculate the sensitivities (defined as a measure of the proportion of seizures with a positive blood-slide that can be positively identified as attributable to malaria) and specificities (defined as a measure of the proportion of seizures with a positive blood-slide that can be negatively identified as attributable to malaria) associated with each threshold of parasitaemia (Smith et al., 1994; Mwangi et al., 2005; Bejon et al., 2007). The thresholds of parasitaemia used for deriving malaria-attributable fractions depend on the use of the case definition (Smith et al., 1994) and previous studies have observed that malaria-attributable fractions derived at higher parasitaemia levels are likely to be associated with high sensitivities and specificities acceptable for case definitions (Mwangi et al., 2005; Bejon et al., 2007).
We examined the changes in the incidence of admissions with seizures over a 7-year period and used the decrease in the incidence of malaria to calculate malaria-attributable fractions for seizures to determine if the observed decrease in seizures was a measure of the childhood admissions with seizures that are attributable to malaria.
Materials and methods
Patients and procedures
We used an online database of admissions to identify all children aged between 0 and 13 years admitted to Kilifi District Hospital on the Kenyan coast, from 2002 to 2008, with a history of seizures. We reviewed their clinical notes to extract information on malaria status and seizures. Kilifi District Hospital is located in a regularly enumerated demographic surveillance system area and serves a rural community of about 100 000 children (Sadarangani et al., 2008). There are two annual peaks of malaria related to the two rainy seasons. The prevalence of parasitaemia in children admitted with trauma (as a measure of background prevalence in the non-febrile community) has reduced from >20% in 2002 to <1% in 2007 (O'Meara et al., 2008). The decline in malaria has been attributed to the scaling-up of malaria control interventions such as the distribution of insecticide-treated nets, introduction of effective anti-malarial drugs and health education (Nevill et al., 1996; Marsh et al., 1999; Amin et al., 2007; Okiro et al., 2007). The relationship with control measures is, however, unclear and there is evidence that transmission was falling before the interventions were introduced (O'Meara et al., 2008). In 1998 sulphadoxine-pyrimethamine replaced chloroquine and in 2004 artemether–lumefantrine was introduced (Amin et al., 2007). The introduction of Haemophilus influenzae type B vaccine, introduced into the Kenya Expanded Programme on Immunization in 2001, has helped reduce the incidence of invasive haemophilus disease in this area (Cowgill et al., 2006) and this may reduce the incidence of seizures, particularly in young infants without parasitaemia.
Clinical management of patients
For every child admitted to the hospital, clinical and laboratory data are entered into a standard database. The medical history included the parental description of episodes of seizures, number and duration of seizures. Full blood count, malaria parasitaemia, plasma glucose, venous blood gases and blood culture were done on all children at admission, including the comparison group comprising trauma patients. Severe malaria was defined according to the WHO criteria as a child with asexual malaria parasitaemia and either Blantyre Coma Score ≤2 (i.e. cerebral malaria), respiratory distress and/or haemoglobin <5 g/dl (Molyneux et al., 1989; World Health Organization, 2000). Severe malaria was treated with parenteral quinine and when the child was able to take treatment orally, it was completed with sulphadoxine–pyrimethamine (until September 2006) or artemether–lumefantrine thereafter.
Clinicians and nurses at the hospital provide 24-h clinical cover. Nursing staff were trained to record details of seizures on a standard proforma. These details included data for the time of onset and cessation of seizures, anti-epileptic drugs administered and the level of consciousness at the end of a seizure, as determined by the Blantyre Coma Score (Molyneux et al., 1989). A standard protocol for seizure management was used. A child with a seizure lasting ≥5 min was given intravenous diazepam (0.3 mg/kg) and if still convulsing 10 min later was given intramuscular paraldehyde (0.4 mg/kg). Intravenous phenobarbital (15 mg/kg) was given for prolonged seizures (20–30 min). If these seizures did not respond to these two anti-epileptic drugs, they were termed refractory seizures and were treated with intravenous phenytoin (18 mg/kg). Children with brief repetitive seizures (≥3 seizures in 1 h lasting >5 min) were given phenobarbital (15 mg/kg).
Case definitions for the study
The definitions for seizures were based on those of the International League against Epilepsy (ILAE, 1989). Acute symptomatic seizures were defined as seizures in the current illness and within 1 week prior to admission. The three main phenotypes of acute symptomatic seizures studied were convulsive status epilepticus (defined as convulsions lasting >30 min, or intermittent convulsions without regaining consciousness for 30 min), focal seizures (defined as seizures starting at one part of the body) and repetitive symptomatic seizures (defined as more than one seizure in the same illness). Seizures that were repetitive, focal or fulfilled the definition of convulsive status epilepticus were termed acute complex symptomatic seizures and simple symptomatic seizures as a generalized seizure without any of these features. We further classified convulsive status epilepticus into definite, probable or possible categories based on the information that was available from the history (Table 1). Febrile seizures were defined as seizures in children aged between 1 month and 6 years who had a febrile illness without malaria parasitaemia or evidence of bacterial meningitis (Berkley et al., 2001) or encephalitis (cerebrospinal fluid white cell count >50/µl).
Table 1.
Case definitions for children admitted with acute symptomatic seizures and the number of cases admitted from the demographic surveillance system area
| Case category | Criteria | Number of casesa |
|---|---|---|
| Acute seizures | Seizures in the current illness and within 1 week prior to admission | 4486 |
| Slide-positive seizures | Seizures in children admitted with malaria parasitaemia | 2762 |
| Slide-negative seizures | Seizures in children admitted without malaria parasitaemia | 1724 |
| Repetitive seizures | More than one seizure in the current illness | 2181 |
| Focal seizures | Convulsions localized to one part of the body | 512 |
| Definite convulsive status epilepticus | Seizures lasting >30 min or intermittent seizures for >30 min with a Blantyre Coma Score ≤2 on admission and documented by nursing or medical staff | 219 |
| Probable convulsive status epilepticus | Convulsions on the way to hospital until admission | 462 |
| A Blantyre Coma Score ≤2 on admission and definite history of a seizure in last 30 minutes or definite history of >10 seizures in last 24 h | ||
| Use of phenytoin or phenobarbital to stop uncontrolled seizures | ||
| Possible convulsive status epilepticus | A Blantyre Coma Score ≤2 on admission and either a definite history of a seizure in last 30 min or possible history of 5–10 seizures in last 24 h | 266 |
| Definite history of a seizure lasting 30 min | ||
| Definite history of >5 seizures in last 24 h | ||
| Non-malaria attributable seizures | Sum of malaria slide-negative seizures and slide-positive seizures not attributable to malaria | 1844 |
| Malaria attributable seizures | Malaria slide-positive seizures attributable to malaria | 2642 |
| Febrile seizures | Seizures in children aged 1 month to 6 years who had a febrile illness without evidence of parasitaemia or bacterial meningitis or encephalitis | 1126 |
| Total person-years | The total population figures for children were made at the midpoint of the years of study by fitting a log-linear regression line through the observed population counts. | 689 053 |
aThe total numbers identified and summed together for each case category. Blantyre Coma Score (Molyneux et al., 1989).
MAS were defined as admissions to hospital with a history of seizures and malaria parasitaemia detected on thick and thin blood slides stained with 10% Giemsa. Non-MAS were defined as admissions to hospital with a history of seizures and no parasitaemia detected on the blood slides. Parasite density was quantified by count of the number of parasites per 100 white cell counts or 500 red cell counts (if count per 100 white cell count is missing or >100/100 white cell counts) and multiplying these figures by whole white cell counts or red cell counts, respectively. If whole blood cell counts data were missing, parasitaemia was estimated on the basis of a uniform white cell count of 8000/µl (Rogers et al., 2006).
Data management and statistical analysis
The patient’s data were entered and tabulated in Filemaker Pro 9 Advanced (Filemaker Inc.). All analyses were computed using STATA (version 11; Stata Corp). A probability level of <0.05 was considered significant.
Deriving malaria-attributable fraction for seizures
We applied logistic regression methods to model the risk of seizures as a continuous function of malaria parasitaemia by comparing all admissions with seizures (n = 4486) and a comparison group (n = 1411) [children aged 0–13 years admitted with heterogeneous non-infectious diagnoses such as trauma (not involving head region), burns and snake bites]. The comparison group had no seizures on admission and the parasitaemia seen in these children is representative of the background prevalence of P. falciparum in asymptomatic children in the community.
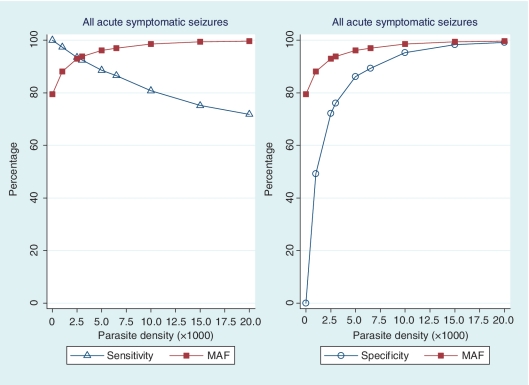
The logistic regression model used was: log(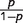 ) = α + βxז (Smith et al., 1994), where p is the probability of admission with seizures (where the admissions with seizures are treated as ‘positive’ and the comparison group as ‘negative’), x is the parasite density and ז is the power function that improves the numerical stability of the maximum likelihood estimation. The coefficient (β) in the logistic model can be used to calculate the risk of seizures caused by malaria for each child. The risks of a group are averaged to estimate the proportion of the group whose seizures are caused by ‘true’ malaria rather than another illness with coincidental parasitaemia. The averaged risks give the positive predictive value or malaria-attributable fractions for seizures for the group analysed (Smith et al., 1994). Sensitivity and specificity can then be calculated using the malaria-attributable fractions for seizures or the proportion of seizure cases within each level of parasitaemia (Fig. 1). For this study, malaria-attributable fractions for seizures were reported at levels of parasitaemia ≥2500/µl since this threshold of parasitaemia is associated with high sensitivity and specificity for case definitions in this area (Mwangi et al., 2005; Bejon et al., 2007).
) = α + βxז (Smith et al., 1994), where p is the probability of admission with seizures (where the admissions with seizures are treated as ‘positive’ and the comparison group as ‘negative’), x is the parasite density and ז is the power function that improves the numerical stability of the maximum likelihood estimation. The coefficient (β) in the logistic model can be used to calculate the risk of seizures caused by malaria for each child. The risks of a group are averaged to estimate the proportion of the group whose seizures are caused by ‘true’ malaria rather than another illness with coincidental parasitaemia. The averaged risks give the positive predictive value or malaria-attributable fractions for seizures for the group analysed (Smith et al., 1994). Sensitivity and specificity can then be calculated using the malaria-attributable fractions for seizures or the proportion of seizure cases within each level of parasitaemia (Fig. 1). For this study, malaria-attributable fractions for seizures were reported at levels of parasitaemia ≥2500/µl since this threshold of parasitaemia is associated with high sensitivity and specificity for case definitions in this area (Mwangi et al., 2005; Bejon et al., 2007).
Figure 1.
Sensitivity and specificity of parasite density associated with malaria-attributable fractions (MAF) for acute symptomatic seizures in children admitted to Kilifi District Hospital between 2002 and 2008. The overall MAF for seizures were reported or derived at thresholds of parasitaemia ≥2500/μl since this threshold is associated with malaria illnesses in this area (Mwangi et al., 2005) and are associated with high sensitivities and specificities that are acceptable for case definitions in this study (Smith et al., 1994). The malaria-attributable fractions for seizures were used to determine if the observed percentage decrease in seizures during a period of a reduction in transmission of malaria was a measure of seizures attributable to malaria.
Age and year of study were entered as covariates of parasitaemia in the logistic regression model because the two factors are most likely to influence the malaria-attributable fractions modelled (Smith et al., 1994). The model was repeated in different phenotypes of acute symptomatic seizures and various age groups. Since parasitaemia is uncommon in children younger than 6 months of age (Idro et al., 2008), we did not model malaria-attributable fractions for seizures for this group. Confidence intervals around the malaria-attributable fractions for seizures were estimated by bootstrapping with 1000 iterations.
Comparison between groups
Student’s t-test was used to compare age between admissions with MAS and those with non-MAS. All categorical data were compared using the Pearson’s chi-squared test. The trends in mean age at admission by year were investigated with linear regression for both admissions with MAS and those with non-MAS.
Calculating the incidence of seizures
The incidence analysis considered a child’s first hospital admission with seizures and any other subsequent admission was excluded. The incidence of hospital admissions with acute symptomatic seizures was calculated for all children who lived in the demographic surveillance study area. The incidences were calculated using the denominator as the annual population figures for children, which were made at the midpoint of the years of study by fitting a log-linear regression line through the observed population counts. Additionally, the annual incidence of MAS and non-MAS was calculated for all admissions with acute symptomatic seizures. The declining trends in incidence of seizures by year were investigated using linear regression.
Estimating the decrease in the incidence of seizures
The predicted decline in acute symptomatic seizures that are attributable to malaria (seizures that should decrease following the reduction of malaria) was the incidence of MAS at 2002 (the beginning of the study) multiplied by the malaria-attributable fractions for seizures (O'Meara et al., 2008). The observed decrease in the incidence of acute symptomatic seizures was the difference in the incidence between 2002 and 2008. The percentage decrease in the incidence of seizures was the observed decrease in the incidence of seizures expressed as a percentage of the incidence at 2002. Ethical permission for the study was obtained from the Kenya Medical Research Institute (KEMRI) National Ethical Review committee.
Results
General description
Over the 7-year period, a total of 34 057 children aged between 0 and 13 years were admitted to Kilifi District Hospital, of whom 7150 (21%) had asexual P. falciparum parasites detected on admission and 5580 (16%) had a history of seizures (Table 2). Of the 7150 children admitted with malaria parasitaemia, 4370 (61.1%) did not have a history of seizures during their illness leading to hospitalization. Of the 5580 children with a history of seizures, 1007 (18%) did not live in the study area, and were excluded from the analysis. The analysis data set, therefore, comprised 4573 children. The data for 9% of the 4573 children were not available from the hospital archives and thus we could not obtain full description of the seizures. Malaria parasitaemia was detected in 140 (10%) of the 1411 comparison group and the prevalence of parasitaemia decreased consistently between 2002 and 2008 (Table 2).
Table 2.
Secular trends in paediatric admissions to Kilifi District Hospital between 2002 and 2008 and the incidence of acute symptomatic seizures by malaria association
| Admission profiles | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | Total |
|---|---|---|---|---|---|---|---|---|
| Number of admissions from within and outside the DSS | 4851 | 5539 | 5056 | 4751 | 5016 | 4496 | 4348 | 34 057 |
| Number of admissions with history of seizures from within and outside DSS | 1310 | 1521 | 964 | 430 | 433 | 530 | 392 | 5580 |
| Number of MAS from the DSSa | 734 | 1028 | 392 | 230 | 207 | 79 | 110 | 2780 |
| Number of admissions with non-MAS from the DSSa | 271 | 246 | 326 | 200 | 219 | 249 | 282 | 1793 |
| Mean age (standard error) in months for MAS from the DSS | 30.2 (0.9) | 29.9 (0.7) | 29.7 (1.0) | 31.8 (1.3) | 35.1 (1.6) | 40.1 (2.7) | 32.9 (2.0) | |
| Mean age (standard error) in months for non-MAS from the DSS | 29.3 (1.6) | 30.1 (1.6) | 29.4 (1.7) | 26.5 (1.9) | 25.2 (1.7) | 27.1 (1.9) | 30.0 (1.6) | |
| Parasite prevalence (%) in the comparison group for this study | 16 | 46 | 33 | 24 | 18 | 6 | 6 | |
| Person-years | 85 827 | 89 592 | 97 099 | 1 00 303 | 1 03 492 | 1 05 289 | 1 07 451 | 6 89 053 |
| Previous study’s incidence of slide-positive admissions per 1000 (O’Meara et al., 2008) b | 17.2 | 18.4 | 11.3 | 7.3 | 5.8 | 3.4 | – | |
| Previous study’s parasite prevalence (%) in trauma patients (O’Meara et al., 2008)b | 19 | 24 | 13 | 11 | 7 | 1 | – | |
| Annual incidence of admissions with acute symptomatic seizures/100 000/year | ||||||||
| All MAS | 855 | 1147 | 404 | 229 | 200 | 75 | 102 | |
| All non-MAS | 316 | 275 | 316 | 197 | 206 | 200 | 260 | |
| CSE MAS | 139 | 180 | 117 | 61 | 52 | 26 | 28 | |
| CSE non-MAS | 54 | 40 | 89 | 47 | 53 | 49 | 55 | |
| Repetitive MAS | 432 | 569 | 192 | 119 | 101 | 39 | 50 | |
| Repetitive non-MAS | 164 | 132 | 159 | 75 | 96 | 89 | 107 | |
| Focal MAS | 136 | 101 | 38 | 25 | 25 | 5 | 7 | |
| Focal non-MAS | 54 | 26 | 30 | 29 | 30 | 13 | 30 | |
a Admission numbers as contained in the online admission database, of which 69 and 18 children did not show any evidence of seizures on review of clinical for the MAS and non-MAS groups, respectively. b These figures were part of another analysis (O′Meara et al., 2008). The annual population figures for children were made at the midpoint of the years of study by fitting a log-linear regression line through the observed population counts. CSE = Convulsive status epilepticus; DSS = the demographic surveillance system.
Over the surveillance period, the mean age of admissions with MAS appeared to increase (test for trend; P = 0.001), but that of non-MAS was unchanged (test for trend; P = 0.366) (Table 2). Overall, children admitted with MAS were older than those admitted with non-MAS (mean age 30.8 months versus 28.4 months; P = 0.001). The proportion of acute complex symptomatic seizures was not different between the admissions with MAS and those with non-MAS for convulsive status epilepticus [566/2762 (20%) versus 381/1724 (22%); P = 0.199] and focal seizures [308/2762 (11%) versus 204/1724 (12%); P = 0.485]. However, the proportion of recurrent seizures was different between admissions with MAS and those with non-MAS [1385/2762 (48%) versus 796/1764 (41%); P = 0.01].
Malaria-attributable fractions for seizures
The levels of parasitaemia ≥2500/µl that were used for reporting malaria-attributable fractions for all acute symptomatic seizures in this study were associated with high sensitivities (>90%) and specificities (>70%) (Fig. 1).The adjusted malaria-attributable fractions for seizures in children admitted with acute symptomatic seizures and parasitaemia was 92.9% [95% confidence interval (95% CI) 90.4–95.1%]. The adjusted malaria-attributable fractions for acute complex symptomatic seizures were similar: 92.9% (95% CI 89.4–95.5%) for convulsive status epilepticus, 93.6% (95% CI 90.9–95.9%) for repetitive seizures and 91.8% (95% CI 85.6–95.4% for focal seizures). The adjusted malaria-attributable fractions for seizures in children older than 6 months of age admitted with parasitaemia decreased with age and were 98.3% (95% CI 96.7–99.0%) in 6–23 months old, 95.1% (95% CI 93.4–96.8%) in 24–59 months old and 93.5% (95% CI 92.1–95.1%) in 59–156 months old children.
The burden of acute seizures
The overall incidence of acute symptomatic seizures was 651/100 000/year (95% CI 632–670) and it was 400/100 000/year (95% CI 385–415) for acute complex symptomatic seizures. The incidence of MAS was 401/100 000/year (95% CI 368–416) and that of non-MAS was 250/100 000/year (95% CI 237–262). The overall incidence of acute complex symptomatic seizures was 137/100 000/year (95% CI 129–147) for convulsive status epilepticus, 316/100 000/year (95% CI 304–330) for repetitive seizures and 74/100 000/year (95% CI 68–81) for focal seizures. The annual incidence of acute complex MAS is shown in Table 2.
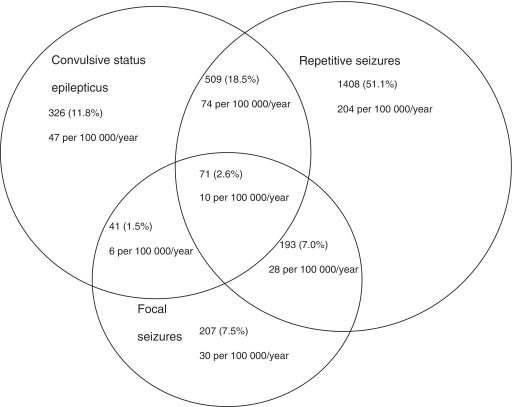
The overall incidence of admissions with an overlap of the three phenotypes of seizures (convulsive status epilepticus, repetitive and focal) was 10/100 000/year (95% CI 8–13) (Fig. 2). The greatest overlap was between repetitive seizures and convulsive status epilepticus [74/100 000/year (95% CI 58–70)]. The incidence of admissions with an overlap between focal seizures and repetitive seizures was 28/100 000/year (95% CI 24–32) while that for focal seizures and convulsive status epilepticus was 5/100 000/year (95% CI 5–9). The overall incidence of admissions with simple generalized seizures was 258/100 000/year (95% CI 246–270).
Figure 2.
The proportion of acute complex symptomatic seizures documented during the study period. There was an overlap between the three seizure phenotypes. The greatest overlap was between convulsive status epilepticus and repetitive seizures, followed by repetitive seizures and focal seizures; focal seizures, convulsive status epilepticus and repetitive seizures; and focal seizures and convulsive status epilepticus. The absolute estimates are expressed as a percentage of the total acute complex symptomatic seizures (n = 2755). The corresponding incidences per 100 000 per year are also provided.
The overall incidence of admissions with febrile seizures was 163/100 000/year (95% CI 154–173). The incidence of febrile convulsive status epilepticus was 12/100 000/year (95% CI 9–15) and that for febrile focal seizures and febrile repetitive seizures was 7/100 000/year (95% CI 5–9) and 52/100 000/year (95% CI 47–58), respectively. The incidence of simple generalized febrile seizures was 70/100 000/year (95% CI 64–77).
The incidence of febrile seizures with an overlap of all the three phenotypes of acute complex symptomatic seizures was 2/100 000/year (95% CI 1–3). The overlap of febrile seizures with complex features was highest between repetitive seizures and convulsive status epilepticus [14/100 000/year (95% CI 12–17)]. The incidence of febrile seizures with both focal and convulsive status epilepticus features was 1/100 000/year (95% CI 0.5–2) while that for those with both focal and repetitive features was 6/100 000/year (95% CI 4–8).
Changes in the incidence of seizures
Declining trends
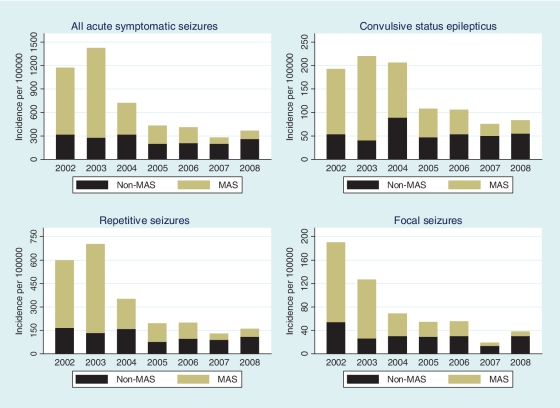
From 2002 to 2008, the incidence of all admissions with acute symptomatic seizures demonstrated a significant declining trend (P < 0.001). Similarly, the three types of acute complex symptomatic seizures showed a declining trend during the period [convulsive status epilepticus (test for declining trend; P < 0.001), repetitive seizures (test for declining trend; P < 0.001) and focal seizures (test for declining trend; P < 0.001)]. The decrease for the incidence of MAS was greater than that of non-MAS for all types of acute symptomatic seizures (Fig. 3).
Figure 3.
The changes in the incidence of admissions with acute symptomatic seizures between 2002 and 2008. All acute symptomatic seizures decreased during the period and most of the decrease occurred in malaria-associated seizures (MAS) compared with non-MAS (P < 0.001). Similarly, most of the decrease observed in the three types of acute complex symptomatic seizures occurred in MAS compared with non-MAS.
Observed decrease
From 2002 to 2008, all acute symptomatic seizures decreased by 809/100 000/year (69.2%) with 93.1% of the decrease occurring in MAS (Table 3). During the same period, convulsive status epilepticus decreased by 111/100 000/year (57.2%) and all (100%) of the decrease was seen in MAS. From 2002 to 2008, repetitive seizures decreased by 440/100 000/year (73.7%) and 86.8% of this decrease occurred in MAS. During the study period, focal seizures decreased by 153/100 000/year (80.5%) with 84.3% of this decline in MAS.
Table 3.
The malaria-attributable fractions for seizures, the predicted and the observed decrease in the incidence of seizures and the proportion of the observed decrease that occurred in malaria-associated seizures
| Type of seizures | Adjusted malaria- attributable fractions for seizures (95% CI) | Predicted decline in seizures using malaria-attributable fractions/100 000/year | Observed decrease in seizures/100 000/year (percentage decrease) | Proportion of the observed decrease that occurred in MAS |
|---|---|---|---|---|
| All acute symptomatic seizures | 92.9% (90.4–95.1%) | 794 | 809 (69.2%) | 753/100 000/year (93.1%) |
| Convulsive status epilepticus | 92.9% (89.4–95.5%) | 129 | 111 (57.2%) | 111/100 000/year (100%) |
| Repetitive seizures | 93.6% (90.9–95.9%) | 404 | 440 (73.7%) | 382/100 000/year (86.8%) |
| Focal seizures | 91.8% (85.6–95.4%) | 125 | 153 (80.5%) | 129/100 000/year (84.3%) |
The predicted decline in the incidence of seizures was the incidence of MAS in 2002 (the beginning of the study) multiplied by the malaria-attributable fractions for seizures. Annual incidences of seizures are provided in Table 2. The observed decrease in the incidence of seizures was the difference in the incidence between 2002 and 2008 (the end of study). Percentage decrease was the decrease in the incidence expressed as a percentage of the incidence at 2002.
Predicted decline
The predicted decline estimated by multiplying the incidence of MAS at the beginning of the study by malaria-attributable fractions for seizures indicated that acute symptomatic seizures would decrease by 794/100 000/year following the reduction of malaria (Table 3). During the same period, acute complex symptomatic seizures were predicted to decline by 129/100 000/year for convulsive status epilepticus, 404/100 000/year for repetitive seizures and 125/100 000/year for focal seizures.
The observed decrease (809/100 000/year) in all acute symptomatic seizures was similar to the predicted decline (794/100 000/year) that was estimated using malaria-attributable fractions for seizures at the beginning of the study (Table 3). Additionally, the observed decrease in the three types of acute symptomatic seizures were similar to the predicted decline estimated using malaria-attributable fractions for seizures at the beginning of the study. Nevertheless, the observed decrease was slightly larger than the predicted decline calculated using malaria-attributable fractions for seizures for all types of acute symptomatic seizures except convulsive status epilepticus.
Discussion
We found that the overall incidence of acute symptomatic seizures was 651/100 000/year (95% CI 632–670) and it was 400/100 000/year (95% CI 385–415) and 163/100 000/year (95% CI 154–173) for acute complex symptomatic seizures and febrile seizures, respectively. The adjusted malaria-attributable fractions for acute symptomatic seizures in all admissions with parasitaemia were relatively high and decreased with age in children older than 6 months of age. From 2002 to 2008, all acute symptomatic seizures decreased by 809/100 000/year (69.2%) with 93.1% of this decrease occurring in MAS. The observed decrease (809/100 000/year) in all acute symptomatic seizures was similar to the predicted decline (794/100 000/year) that was estimated using malaria-attributable fractions for seizures.
Malaria-attributable fractions for seizures
We modelled malaria-attributable fractions for seizures to determine the proportion of seizures that would decrease following the reduction of malaria. We calculated malaria-attributable fractions for seizures with logistic regression since the high prevalence of asymptomatic parasitaemia in the community (Mwangi et al., 2005) can result in some seizures caused by other illnesses with coincidental parasitaemia being incorrectly classified as attributable to malaria if other methods were used (Smith et al., 1994). The adjusted malaria-attributable fractions for all acute symptomatic seizures were derived at parasitaemia levels ≥2500/µl since this threshold was shown to be associated with malaria illnesses in previous studies in this area (Mwangi et al., 2005; Bejon et al., 2007) and is associated with high sensitivities and specificities in this study. Malaria-attributable fractions for seizures were high and similar among the three acute complex symptomatic seizures with only repetitive seizures particularly attributable to malaria.
We found high malaria-attributable fractions for seizures in children >6 months and these attributable fractions decreased with age, which may reflect the increasing immunity and tolerance to malaria as children grow older. This finding, however, is predictive of what would normally be expected of the risk of seizures in malaria with age when the transmission intensity of malaria is stable. The mean age of children with MAS appeared to increase over the study period compared with that of children with non-MAS and supports the hypothesis that the pathogenesis of seizures in malaria is related to intrinsic factors of host age as well as the transmission intensity and levels of parasitaemia. A decrease in transmission of malaria impairs the development of immunity (Snow et al., 1997) and thus older children are admitted with severe complications such as cerebral malaria (O'Meara et al., 2008), which is strongly associated with seizures.
Changes in the incidence of seizures
Most of the observed decrease in the incidence of seizures occurred in MAS compared with non-MAS and supports the hypothesis that the decrease observed in seizures is associated with the reduction of malaria (O'Meara et al., 2008). The observed decrease in acute symptomatic seizures was similar to the predicted decline that was estimated by malaria-attributable fractions for seizures in 2002 and supports the hypothesis that the decrease in seizures during a period of a reduction in malaria transmission is an approximate measure of seizures that are attributable to malaria. Nevertheless, the observed decrease was larger than the predicted decline by 15/100 000/year, which could be attributable to other factors other than the reduction in malaria.
The observed decrease that was in excess of the predicted decline calculated using malaria-attributable fractions for seizures is probably caused by the improvement in health services rather than the malaria control interventions that led to the reduction of malaria. Such improvements include more widespread use of antimicrobials, the improvement in the treatment of seizures in the peripheral health facilities [although anti-epileptic drugs are not readily available in rural areas (Ahmad et al., 2006)] and the reduction in haemophilus meningitis following the introduction of H. influenzae type B vaccine (Cowgill et al., 2006). The finding that the decrease caused by the improvement in health services did not occur in convulsive status epilepticus could suggest that another non-malarial cause of status epilepticus, such as epilepsy (Sadarangani et al., 2008) was still high during the study period.
Since acute complex symptomatic seizures are important in the pathogenesis of severe malaria (Crawley et al., 1996) and are associated with neurocognitive impairments and development of epilepsy (Carter et al., 2005; Idro et al., 2007; Sadarangani et al., 2008), the decrease in the incidence of these seizures could lead to a reduction in the incidence and prevalence of neurological disabilities and mortality in this area.
Limitations
These incidence rates are conservative because almost all of the seizures detected were convulsive seizures and non-convulsive seizures may have been missed. Furthermore, we could not ascertain the proportion of patients whose seizures had been treated with anti-epileptics drugs at the peripheral prior referral since this may have reduced the number of referrals or admissions with seizures. However, very few patients, if any, will have received prior treatment for seizures before admission because anti-epileptic drugs such as diazepam and phenobarbital are not readily available in the community (Ahmad et al., 2006). Additionally, some seizure cases may have been missed because traditional healers only refer the severely sick children to Kilifi District Hospital (Waruiru et al., 1996), hospital user fee charges may reduce hospital use (Chuma et al., 2009) and some children with short seizures would not be referred to hospital (Sadarangani et al., 2008). The malaria-attributable fractions for seizures calculated from a single blood-slide does not account for the parasitaemia that may have been undetected by the less sensitive optical microscopy (Ochola et al., 2006). Furthermore, the density of parasitaemia can fluctuate significantly within hours and therefore use of parasitaemia from a single blood film complicates the modelling (Koram and Molyneux, 2007).
Conclusion
The high malaria-attributable fractions for seizures modelled in this study confirms that falciparum malaria is the most common cause of acute symptomatic seizures in children admitted to hospital with parasitaemia in a malaria endemic area and that the risk for seizures decreases with age. The observed decrease in the incidence of seizures was similar to the predicted decline that was estimated using the modelled malaria-attributable fractions for seizures and suggests that the decrease observed is an approximate measure of the childhood admissions with seizures that are attributable to malaria. The observed decrease in seizures could lead to reduced neurological disabilities and epilepsy associated with malaria in this area.
Funding
The Wellcome Trust (083744 to C.N. and 083579 to J.A.B.); the Kenya Medical Research Institute; Biomedical Research Centre in Oxford (to P.B.).
Acknowledgements
We thank the KEMRI clinicians and nurses, the mapping and census team and the clinical data managers who made this study possible. The support of the records management staff was invaluable in retrieving patient notes. We also thank Karren Visser for the editorial assistance. We are indebted to Dr Greg Fegan for statistical advice. This article is published with the permission of the director of KEMRI.
Glossary
Abbreviations
- MAS
malaria-associated seizures
References
- Ahmad S, Ellis JC, Kamwendo H, Molyneux E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: an open randomised trial. Lancet. 2006;367:1591–97. doi: 10.1016/S0140-6736(06)68696-0. [DOI] [PubMed] [Google Scholar]
- Amin A, Zurovac D, Kangwana B, Greenfield J, Otieno D, Akhwale W, et al. The challenges of changing national malaria drug policy to artemisinin-based combinations in Kenya. Mal J. 2007;6:72. doi: 10.1186/1475-2875-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejon P, Berkley JA, Mwangi T, Ogada E, Mwangi I, Maitland K, et al. Defining childhood severe falciparum malaria for intervention studies. PLoS Med. 2007;4:e251. doi: 10.1371/journal.pmed.0040251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley JA, Mwangi I, Ngetsa CJ, Mwarumba S, Lowe BS, Marsh K, et al. Diagnosis of acute bacterial meningitis in children at a district hospital in sub-Saharan Africa. Lancet. 2001;357:1753–7. doi: 10.1016/S0140-6736(00)04897-2. [DOI] [PubMed] [Google Scholar]
- Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol. 2010;9:1173–81. doi: 10.1016/S1474-4422(10)70270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JA, Neville BGR, White S, Ross AJ, Otieno G, Mturi N, et al. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45:978–81. doi: 10.1111/j.0013-9580.2004.65103.x. [DOI] [PubMed] [Google Scholar]
- Carter JA, Ross AJ, Neville BGR, Obiero E, Katana K, Mung'ala-Odera V, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10:3–10. doi: 10.1111/j.1365-3156.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- Chuma J, Musimbi J, Okungu V, Goodman C, Molyneux C. Reducing user fees for primary health care in Kenya: Policy on paper or policy in practice? Int J Equity Health. 2009;8:15. doi: 10.1186/1475-9276-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P, MacMahon B. Attributable risk percent in case-control studies. Brit J prev soc Med. 1971;25:242–44. doi: 10.1136/jech.25.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowgill KD, Ndiritu M, Nyiro J, Slack MP, Chiphatsi S, Ismail A, et al. Effectiveness of haemophilus influenzae type b Conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA. 2006;296:671–8. doi: 10.1001/jama.296.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. Seizures and status epilepticus in childhood cerebral malaria. Q J Med. 1996;89:591–7. doi: 10.1093/qjmed/89.8.591. [DOI] [PubMed] [Google Scholar]
- Idro R, Gwer S, Kahindi M, Gatakaa H, Kazungu T, Ndiritu M, et al. The incidence, aetiology and outcome of acute seizures in children admitted to a rural Kenyan district hospital. BMC Pediatr. 2008;8:5. doi: 10.1186/1471-2431-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–40. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- Idro R, Ndiritu M, Ogutu B, Mithwani S, Maitland K, Berkley JA, et al. Burden, features and outcome of neurological involvement in acute falciparum malaria in Kenyan children. JAMA. 2007;297:2232–40. doi: 10.1001/jama.297.20.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on classification and terminology of the international league against epilepsy. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Koram KA, Molyneux ME. When is "malaria" malaria? The different burdens of malaria infection, malaria disease, and malaria-like illnesses. Am J Trop Med Hyg. 2007;77:1–5. [PubMed] [Google Scholar]
- Marsh VM, Mutemi WM, Muturi J, Haaland A, Watkins WM, Otieno G, et al. Changing home treatment of childhood fevers by training shop keepers in rural Kenya. Trop Med Int Health. 1999;4:383–9. doi: 10.1046/j.1365-3156.1999.00403.x. [DOI] [PubMed] [Google Scholar]
- Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989;71:441–59. [PubMed] [Google Scholar]
- Mung'ala-Odera V, Snow RW, Newton RJC. The burden of the neurocognitive impairment associated with Plasmodium falciparum malaria in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71:64–70. [PubMed] [Google Scholar]
- Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191:1932–9. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevill CG, Some ES, Mung'ala VO, Mutemi W, New L, Marsh K, et al. Insecticide-treated bednets reduce mortality and severe morbidity from malaria among children on the Kenyan coast. Trop Med Int Health. 1996;1:139–46. doi: 10.1111/j.1365-3156.1996.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Ngoungou EB, Preux PM. Cerebral Malaria and epilepsy. Epilepsia. 2008;49:19–24. doi: 10.1111/j.1528-1167.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- O'Meara WP, Bejon P, Mwangi TW, Okiro EA, Peshu N, Snow RW, et al. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–62. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochola L, Vounatsou P, Smith T, Mabaso M, Newton CR. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect Dis. 2006;6:582–8. doi: 10.1016/S1473-3099(06)70579-5. [DOI] [PubMed] [Google Scholar]
- Okiro EA, Hay SI, Gikandi PW, Sharif SK, Noor AM, Peshu N, et al. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151. doi: 10.1186/1475-2875-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers WO, Atuguba F, Oduro AR, Hodgson A, Koram KA. Clinical case definitions and malaria vaccine efficacy. J Infect Dis. 2006;193:467–73. doi: 10.1086/499314. [DOI] [PubMed] [Google Scholar]
- Sadarangani M, Seaton C, Scott JA, Ogutu B, Edwards T, Prins A, et al. Incidence and outcome of convulsive status epilepticus in Kenyan children: a cohort study. Lancet Neurol. 2008;7:145–50. doi: 10.1016/S1474-4422(07)70331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl. 1):S1–90. [PubMed] [Google Scholar]
- Smith T, Schellenberg JA, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med. 1994;13:2345–58. doi: 10.1002/sim.4780132206. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero J-O, Palmer A, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. The Lancet. 1997;349:1650–4. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- Waruiru CM, Newton CR, Forster D, New L, Winstanley P, Mwangi I, et al. Epileptic seizures and malaria in Kenyan children. Trans R Soc Trop Med Hyg. 1996;90:152–5. doi: 10.1016/s0035-9203(96)90120-0. [DOI] [PubMed] [Google Scholar]