Abstract
Abnormalities in motor sequence learning have been observed in non-manifesting carriers of the DYT1 dystonia mutation. Indeed, motor sequence learning deficits in these subjects have been associated with increased cerebellar activation during task performance. In the current study, we determined whether similar changes are also present in clinically manifesting DYT1 carriers as well as in carriers of other primary dystonia mutations such as DYT6. Additionally, we determined whether sequence learning performance and associated brain activation in these subjects correlate with previously described genotype-related abnormalities of cerebellar pathway integrity and striatal D2 dopamine receptor binding. Nineteen DYT1 carriers (10 non-manifesting DYT1: 51.5 ± 15.1 years; nine manifesting DYT1: 46.1 ± 15.1 years) and 12 healthy control subjects (42.8 ± 15.3 years) were scanned with H215O positron emission tomography while performing controlled sequence learning and reference tasks. Eleven DYT6 carriers (four non-manifesting DYT6: 38.0 ± 22.1; seven manifesting DYT6: 35.3 ± 14.2 years) were evaluated during task performance without concurrent imaging. DYT1 and DYT6 carriers also underwent diffusion tensor magnetic resonance imaging for the assessment of tract integrity and 11C-raclopride positron emission tomography to measure caudate/putamen D2 receptor binding. These imaging measures were correlated with sequence learning performance and associated activation responses. Sequence learning deficits of similar magnitude were observed in manifesting and non-manifesting DYT1 carriers. In contrast, learning deficits were not detected in DYT6 carriers, irrespective of clinical penetrance. Affected DYT1 carriers exhibited significant increases in sequence learning-related activation in the left lateral cerebellar cortex and in the right premotor and inferior parietal regions. Increases in premotor cortical activation observed in the mutation carriers correlated with reductions in cerebellar pathway integrity measured using magnetic resonance diffusion tensor imaging and probabilistic tractography. Additionally, the cerebellar tract changes correlated with reductions in dentate nucleus activation recorded during task performance. Sequence learning performance and task-related activation responses did not correlate with striatal D2 receptor binding. In summary, we found that sequence learning deficits and concomitant increases in cerebellar activation are specific features of the DYT1 genotype. The close relationship between reduced cerebellar pathway integrity and increased learning-related activation of the premotor cortex is compatible with the view of DYT1 dystonia as a neurodevelopmental circuit disorder.
Keywords: sequence learning, PET, DYT1 dystonia, DYT6 dystonia, cerebellum
Introduction
Primary torsion dystonia, a hyperkinetic movement disorder, is characterized by co-contractions of agonist and antagonist as well as overflow activity in muscles unrelated to the attempted movement (Hallett, 1998). The most frequent genetic variant associated with primary torsion dystonia is the GAG deletion in the DYT1 gene at 9q34 (Bressman, 2006; Breakefield et al., 2008). Another genetic variant, termed DYT6 was recently identified as a missense mutation of the THAP1 gene (Fuchs et al., 2009). Both mutations are inherited as autosomal dominant conditions with incomplete clinical penetrance (Saunders-Pullman et al., 2007). In patients with primary torsion dystonia with the DYT1 genotype, we previously found abnormal metabolic increases in the putamen, cerebellum and supplementary motor area (Eidelberg et al., 1998; Trost et al., 2002). In contrast, metabolic reductions in these regions were evident in carriers of the DYT6 mutation (Carbon et al., 2004b). Nonetheless, primary torsion dystonia carriers of either mutation have been found to express an abnormal dystonia-related metabolic covariance pattern (Trost et al., 2002; Carbon and Eidelberg, 2009), as well as significant reductions in striatal D2 receptor binding (Carbon et al., 2009).
The presence of metabolic alterations in brain regions involved in sequence learning (e.g. Doyon and Benali, 2005) prompted us to investigate this cognitive process in clinically non-manifesting DYT1 carriers (Ghilardi et al., 2003; Carbon et al., 2008). In the first study, we reported significant deficits in explicit sequence learning in these subjects, with intact motor execution and normal performance on an implicit visuomotor learning task (Ghilardi et al., 2003). In that study, and in a subsequent comparison of gene carriers and control subjects scanned at equi-performance (Carbon et al., 2008), sequence learning-related activation responses were found to be abnormally elevated in the pre-supplementary motor area and occipital association cortex, and lateral cerebellum of the mutation carriers. Subsequent magnetic resonance diffusion tensor imaging studies of pathway connectivity in DYT1 and DYT6 gene carriers have suggested that penetrance is regulated by a combination of neurodevelopmental abnormalities involving cerebellothalamic and thalamocortical projections (Argyelan et al., 2009). Importantly, these studies showed a close association between abnormal connectivity in these pathways and motor activation responses measured in the same subjects. Indeed, cerebellar outflow pathway integrity in DYT1 carriers was found to correlate with increased motor activation responses in the primary motor cortex and supplementary motor area.
In aggregate, these findings point to a discrete abnormality of the structure and function of sensorimotor networks in dystonia gene carriers (Carbon et al., 2010). Nonetheless, a number of issues remain concerning the relationship of these changes to the impairment of sequence learning observed in these subjects. Specifically, it is not known whether non-manifesting and manifesting DYT1 carriers differ with regard to learning performance and related neural activation responses. It is also unclear whether the sequence learning deficits encountered in DYT1 carriers are also present in other dystonia genotypes. Lastly, it is not known how the sequence learning deficits relate to other traits associated with dystonia mutation carrier status, such as reduced striatal dopaminergic neurotransmission (Carbon et al., 2009) and altered cerebello-thalamo-cortical pathway integrity (Argyelan et al., 2009).
In this study, we address these questions in a combined behavioural and neuroimaging investigation of affected and non-manifesting DYT1 and DYT6 carriers. To control for the potential confounding effects of involuntary movements occurring during the performance of the motor tasks, we also studied these subjects while they performed a non-motor visual sequence learning (VSEQ) task. In addition to comparing learning performance and task-related activation responses in mutation carriers with corresponding values from control subjects, we correlated these measures with cerebello-thalamo-cortical pathway connectivity and striatal D2 binding values obtained in the same subjects.
Materials and methods
Subjects
The following groups of right-handed subjects were included in the study:
Twenty-four DYT1 carriers [age: 47.3 ± 15.4 years (mean ± SD)] including 13 non-manifesting DYT1 (52.5 ± 14.1 years) and 11 manifesting DYT1 (42.7 ± 15.6 years) carriers] participated in the behavioural studies. Of these, 19 DYT1 carriers (48.9 ± 14.5 years) also participated in the H215O PET activation experiments. The imaging group was comprised of 10 non-manifesting DYT1 (51.5 ± 15.1 years) and nine manifesting DYT1 (46.1 ± 15.1 years) mutation carriers.
Eleven DYT6 carriers (36.3 ± 16.4 years) including four non-manifesting DYT6 (38.0 ± 22.1 years) and seven manifesting DYT6 (35.3 ± 14.1 years) carriers participated in the behavioural studies. These subjects did not participate in the H215O PET studies.
Twenty healthy control subjects (42.2 ± 13.5 years). Twelve of these subjects (44.7 ± 12.7 years) served as controls for both the behavioural and imaging components of the study. The remaining eight subjects participated only in the behavioural part of the study.
As previously discussed, subtle age differences are inherent to studies comparing manifesting- and non-manifesting-mutation carriers (Ghilardi et al., 2003; Carbon et al., 2008). In this study, there was no difference in age across the five subgroups of participants in the behavioural studies (i.e. the full cohort of non-manifesting DYT1, manifesting DYT1, non-manifesting DYT6, manifesting DYT6 and control subjects) although a non-significant trend was identified (P = 0.12, ANOVA). Pre-specified pairwise contrasts of the mutation cohorts with the control group were non-significant (P > 0.2, Dunnett's tests). Analogous comparison of age in the subjects who also participated in the imaging studies revealed no group difference (P = 0.30, ANOVA).
The DYT1 and DYT6 carriers were recruited and genetically tested through the Mirken Department of Neurology at Beth Israel Medical Centre in New York. The control cohort consisted of gene-negative relatives of the carriers and unrelated healthy volunteer subjects. Informed consent was obtained from all participants under protocols approved by the Institutional Review Boards of the participating institutions. The clinical features of the affected subjects and information on participation in the different imaging studies are presented in Table 1. Exclusion criteria for all subjects included: (i) past history of neurological illnesses other than dystonia; (ii) prior or current exposure to neuroleptic agents or drug use; (iii) past medical history of hypertension, cardiovascular disease or diabetes mellitus; and (iv) abnormal MRI. For controls and non-manifesting subjects, the following additional exclusion criteria were applied: (i) abnormal neurological examination; (ii) past history of dystonic symptoms; and (iii) current use of psychotropic medication.
Table 1.
Clinical characteristics of manifesting gene carriersa
| Group | Participation | Age, years | Symptom duration, years | Distribution (body part) | Burke–Fahn– Marsden motor score | Medications | MSEQ/CCW pace (Hz) |
|---|---|---|---|---|---|---|---|
| DYT1 | |||||||
| 1 | 1 | 23 | 13 | Generalized (Lg, rA) | 17 | THX, baclofen | 1 |
| 2 | – | 24 | 14 | Generalized (C, A, Lg, T) | 68 | Ethopropazine, baclofen | 2 |
| 3 | 1,2,3 | 28 | 11 | Focal (rA) | 1 | THX | 1 |
| 4 | 3 | 31 | 15 | Focal (rA) | 4 | None | 1 |
| 5 | 1,2,3 | 39 | 31 | Generalized (A, Lg, T) | 33 | THX, baclofen, zonisamide | 1 |
| 6 | 1,2,3 | 41 | 32 | Generalized (C, A, Lg, T) | 54 | THX | 1 |
| 7 | 1,2,3 | 42 | 36 | Generalized (C, A, Lg, T) | 17 | THX, levodopa, zonisamide | 1 |
| 8 | 1,2 | 57 | 50 | Generalized (C, rA, rLg) | 23 | Topiramate | 1.16 |
| 9 | 1,2,3 | 57 | 45 | Generalized (C, rA, Lg) | 6.5 | Levodopa | 1.3 |
| 10 | 1,2,3 | 64 | 54 | Generalized (C, A, Lg, T) | 35 | THX | 2 |
| 11 | 1,2,3 | 65 | 57 | Multifocal (A, Lg) | 19 | None | 1 |
| DYT6 | |||||||
| 1 | 2,3 | 19 | 11 | Generalized (C, A, Lg, T) | 23 | THX, BTX | 1 |
| 2 | 2 | 19 | 3 | Focal (C) | 2 | None | 1 |
| 3 | 2,3 | 23 | 11 | Generalized (C, rA, Lg) | 10.5 | THX, BTX | 1 |
| 4 | 2,3 | 45 | 20 | Segmental (A) | 5 | None | 1 |
| 5 | 2,3 | 45 | 34 | Generalized (C, A, Lg, T) | 51 | Pregabalin, | 1 |
| 6 | 2,3 | 48 | 28 | Focal (C) | 6 | THX | 1 |
| 7 | 2 | 49 | 14 | Segmental (A) | 6 | None | 1 |
a All subjects participated in psychophysical testing. 1 = H215O PET study; 2 = raclopride PET study and 3 = 3T diffusion tensor imaging MRI. A = arm(s); BTX = botulinum; C = craniofacial; l = left; Lg = leg(s); r = right; T = trunk; THX = trihexyphenidyl.
Behavioural assessments
Sequence learning tasks
Sequence learning was assessed in all study participants during the performance of the following tasks, described in detail elsewhere (Ghilardi et al., 2003; Carbon et al., 2010).
For motor sequence learning (MSEQ), eight targets were presented in a pseudo-randomized, but repeating sequence of eight elements. Subjects were informed that a sequence was to be presented and were instructed to learn the order of the sequence while reaching for the targets, to anticipate successive targets, and then to reach each target in synchrony with the tone. Additionally, they reported the order of the sequence verbally at the end of the 90 s trial block.
The observational sequence for the visual sequence learning task was performed by the manifesting DYT1 carriers and by the entire DYT6 cohort. The purpose of this task was to assess sequence learning performance without the potential confounds of abnormal movements in the affected gene carriers. As in motor sequence learning, eight targets were presented in a pseudo-randomized repeating sequence of eight elements throughout the duration of the 90 s trial block. Subjects were asked to learn the sequence order by attending to the display without moving. They reported the order of the sequence verbally at the end of each trial block.
Reference tasks
In the motor execution task, targets appeared in a predictable counter-clockwise order. Subjects had to reach the target in synchrony with the tone. Thus, they had to initiate each movement before the corresponding target and tone were presented.
In the audiovisual control condition, subjects remained immobile but experienced comparable sensory stimuli as during the activation tasks. Screen targets, cursor images and tones were presented to the subjects asynchronously and irregularly in equal numbers to those used in the motor tasks.
All subjects were trained to perform the tasks outside the scanner 1–7 days prior to the imaging session.
Performance measures
Task performance measures were quantified during the scanning for all those participating in the PET imaging component of the study, whereas for those participating in the behavioural part only, data were acquired outside the scanner. The subjects were also rated according to the Burke–Fahn–Marsden Dystonia Rating Scale at the time of the study. For the affected subjects, the anti-dystonic and psychotropic medications were discontinued for at least 12 h before testing. To quantify learning performance during motor sequence learning, we calculated the number of correct anticipatory movements per cycle. All the movements that were initiated below the lowest onset time during a random reaction time task performed outside the scanner were considered anticipatory (Nakamura et al., 2001). Thus, in motor sequence learning, correct movements reflect anticipation of target appearance and successful retrieval of previously acquired targets. The percentage of correctly anticipated targets in each trial block (% CorrectMSEQ) was used to quantify learning performance during motor sequence learning. For visual sequence learning, the number of total correct target locations verbally reported by each subject at the end of a trial block (from 0 unawareness of a repeating sequence, to 8 complete correct sequence) represented the declarative score (declarative scoresVSEQ), a descriptor of the explicit learning that was achieved in the trial block.
All behavioural measures were analysed using one-way ANOVA comparing the five groups of subjects (non-manifesting DYT1, manifesting DYT1, non-manifesting DYT6, manifesting DYT6 and control). This was followed by pairwise contrasts of each gene-positive group with control values using Dunnett's tests. We also directly compared manifesting and non-manifesting mutation carriers collapsed across genotypes. This comparison was also performed separately for the DYT1 carriers. However, because of limited sample size, this comparison was not performed in the DYT6 carriers.
Neuropsychological testing
Mutation carriers also underwent neuropsychological testing after the imaging sessions; the manifesting subjects remained off their medication during the cognitive assessments. The details of the neuropsychological battery have been provided elsewhere (Feigin et al., 2007). Raw scores from psychometric tests were converted into t-scores based on normative samples for the comparison with age-matched controls from our database.
Positron emission tomography
Scanning was performed using the GE Advance tomograph at North Shore University Hospital as described in detail previously (Nakamura et al., 2001; Carbon and Eidelberg, 2002). For each task run, relative regional cerebral blood flow was estimated using a slow bolus method in which 10 mCi of H215O (Ferrieri et al., 1994) in 4 ml saline was injected by automatic pump over 16 s (15 ml/min) followed by a manual 3 ml saline flush. Dynamic 3D PET data acquisition began at the time of radioactivity arrival in the brain and continued for 80 s thereafter. Reconstructed PET images were corrected for random coincidences, electronic dead time and tissue attenuation by transmission scans. A single scalar correction was used to compensate for scatter effects.
Manifesting DYT1 and control subjects were scanned while performing the motor sequence learning, visual sequence learning, motor execution task and audiovisual control condition tasks two to three times in randomized order. Non-manifesting DYT1 carriers underwent the same imaging protocol, but did not perform the visual sequence learning task. The subjects performed the motor sequence learning and motor execution tasks with the dominant right arm. Different target sequences were employed for each learning trial; psychophysical recording of learning performance was acquired for every run.
Image analysis
Data processing were performed using SPM5 software (Institute of Neurology http://www.fil.ion. ucl.ac.uk/spm/software/spm5/). Standard preprocessing (realignment, spatial normalizing, smoothing with 10 × 10 × 10 mm) was applied followed by univariate analyses. Voxel-wise comparisons were performed using the flexible factorial model implemented in SPM5. We controlled for the effect of potential global blood flow differences by a global normalization to 50 ml/min/dl. SPM(t) maps were generated to assess the effects of task (motor sequence learning, visual sequence learning, motor execution task, audiovisual control condition) and group (non-manifesting DYT1, manifesting DYT1, control), as well as the interactions between these factors. To control for potential confounds resulting from inter-subject variability in movement pace during task performance, the inter-tone interval was entered as a covariate of no interest, as was subject age. Group contrasts were specified a priori to test hypotheses relating to genotypic and phenotypic effects in the imaging data (see above). All comparisons utilized pairing of learning tasks (motor sequence learning, visual sequence learning) with the corresponding reference tasks (motor execution task, audiovisual control condition).
Structure–function relationships
Correlations with cerebellar pathway connectivity
Ten DYT1 carriers (age: 47.1 ± 12.9 years; four non-manifesting DYT1) underwent magnetic resonance diffusion tensor imaging with probabilistic tractography in addition to the H215O PET activation studies. In a prior diffusion tensor imaging study (Argyelan, 2009), we reported consistent reductions in the integrity of the cerebellar outflow pathways bilaterally in this group of DYT1 carriers. Right cerebello-thalamic connectivity values from these subjects were additionally found to correlate with movement-related activation responses measured in the ventrolateral thalamus and motor cortical areas of the same individuals. In this study, we sought to determine whether these diffusion tensor imaging changes also correlated with sequence learning-related brain activation. Given that group comparisons revealed abnormally increased task-related responses lateralized to the right cerebral hemisphere, we specifically interrogated the activation scans for the presence of significant correlations with left cerebellar connectivity values. This was done using the multiple regression model in SPM. Because of potential selection bias leading to overestimations of effect size in such voxel-based correlational searches (Kriegeskorte et al., 2009; Vul et al., 2009), we employed a split sample approach in this and related analyses (Argyelan et al., 2009; Kriegeskorte et al., 2010). Furthermore, to exclude the potential confounding effect of the tight correlations that have been demonstrated between cerebellar outflow pathway connectivity and motor activation responses (Argyelan et al., 2009), the search for significant regional correlations with sequence learning-related activation was conducted using the non-movement visual sequence learning scans. Interaction effects were identified at the voxel level using the multiple regression model in SPM and were considered significant for P < 0.05, corrected at the cluster level. The resulting brain regions were analysed independently in the motor sequence learning scans using spherical (10 mm diameter) volumes-of-interest centred on the peak voxel of each significant cluster. For each subject, the values for the individual motor sequence learning scans were plotted with respect to the corresponding cerebellar pathway connectivity measure.
Correlations with striatal D2 receptor binding
Seventeen DYT1 carriers (age: 50.2 ± 13.9 years; eight non-manifesting DYT1) underwent 11C- raclopride PET imaging in addition to the activation studies. Significant reductions in striatal D2 receptor binding have been described in manifesting and non-manifesting dystonia mutation carriers (Carbon et al., 2009). In the current study, we determined whether individual differences in caudate/putamen D2 binding in DYT1 carriers correlated with sequence learning performance and/or task-related activation responses recorded in these subjects. Sequence learning performance was quantified in 26 mutation carriers [age: 43.2 ± 15.8 years; 16 DYT1 (nine non-manifesting DYT1); 10 DYT6 (three non-manifesting DYT6)] who underwent raclopride PET imaging in addition to behavioural testing. To assess correlations between caudate D2 receptor binding and learning performance, % CorrectMSEQ measures were entered as covariates in a voxel-based regression analysis of the raclopride PET images. As described above, we employed a split sample approach for all voxel-wise correlation analyses. Given the possibility of latent sub-threshold effects in the data (i.e. type II error), the regression model was also applied to the entire cohort.
For all voxel-based analyses, results were considered significant at P < 0.05 corrected for multiple comparisons at the cluster level with a cluster size cut-off of 50 voxels. For within-group correlations of sequence learning-related activation with task performance or cerebello-thalamo-cortical pathway connectivity, results were reported at P < 0.05, false discovery rate corrected if conforming a priori to the known spatial topography of the neural activation responses associated with sequence learning (c.f. Doyon and Benali, 2005; Bapi et al., 2006). The results of conjunction analyses were considered significant at P < 0.05, family wise error-corrected without spatial constraint (Friston et al., 2005). Coordinates were reported in the standard anatomical space developed at the Montreal Neurological Institute.
Results
Behavioural data
Motor sequence learning
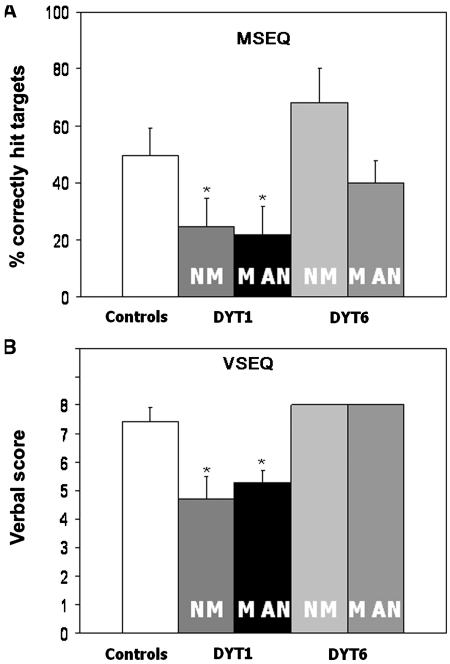
During motor sequence learning (Fig. 1A), the percentage of correctly anticipated targets (%CorrectMSEQ) differed across the five groups [F(4, 80) = 7.5; P < 0.0001; ANOVA], with significant reductions in the manifesting- and non-manifesting DYT1 groups relative to controls (manifesting DYT1: 21.7 ± 5.2; non-manifesting DYT1: 24.7 ± 5.0; control: 49.4 ± 5.0; P < 0.05, Dunnett's tests). In contrast, manifesting- and non-manifesting DYT6 subject performance did not differ from normal (manifesting DYT6: 40.0 ± 8.6; non-manifesting DYT6: 68.2 ± 12.1; P > 0.5). There was no effect of penetrance on learning performance in DYT1 carriers (manifesting- versus non-manifesting DYT1; P > 0.5).
Figure 1.
Sequence learning performance measures. (A) Motor sequence learning (MSEQ): bar graph of group mean values (±SE) for the learning achieved during task performance. Subject performance was represented by the percentage of correctly hit targets averaged across trials. Significant reductions compared to controls (P < 0.05 Dunnett's test) are denoted by an asterisk. (B) Visual sequence learning (VSEQ): bar graph of group mean values (±SE) for verbal scores (range: 0–8) obtained after each trial block. The standard error for the DYT6 carriers was zero because all subjects reported the observation sequence correctly. NM = non-manifesting; MAN = manifesting.
Visual sequence learning
Comparison of the declarative scores (declarative scoresVSEQ) obtained at the end of the visual sequence learning trial (Fig. 1B) revealed analogous group differences [F(4, 80) = 6.5; P = 0.0002, ANOVA]. Post hoc comparison with controls showed abnormal reductions in manifesting- and non-manifesting DYT1 carriers (manifesting DYT1: 5.3 ± 0.4; non-manifesting DYT1: 4.7 ± 0.8; control: 7.4 ± 0.5; P < 0.05, Dunnett's tests). In contrast, declarative scoresVSEQ for the manifesting- and non-manifesting DYT6 groups did not differ from normal (manifesting DYT6: 8.0 ± 0.6; non-manifesting DYT6: 8.0 ± 0.9; P > 0.5, Dunnett's tests). There was no effect of penetrance on visual sequence learning performance in DYT1 carriers (manifesting- versus non-manifesting DYT1; P > 0.5, Wilcoxon signed rank test), or in the combined mutation carriers cohort (all manifesting versus all non-manifesting; P > 0.5).
Neuropsychological data
Performance measures from the gene carrier cohorts were compared with the corresponding values from the healthy control group. Deficits in aspects of executive functioning [Symbol Digit Modality test; F(4, 38) = 4.1, P = 0.009] and visuomotor construction [copy portion of the Rey Complex Figure; F(4, 38) = 3.5, P = 0.022] were noted across the groups; performance was significantly abnormal only in the manifesting DYT1 cohort (Symbol Digit Modality test: P = 0.034; Rey copy: P = 0.036, post hoc tests). Significant performance abnormalities were not evident in non-manifesting DYT1 carriers or in clinically penetrant or in non-penetrant DYT6 carriers. Self-reported measures of depression fell in the normal range for all mutation carrier groups.
Imaging
Brain activation: effect of sequence learning
Significant sequence learning-related activation responses were present in all groups (non-manifesting DYT1, manifesting DYT1, control) and performance modalities (motor sequence learning, visual sequence learning) in the dorsal premotor cortex (left > right, Brodmann area 6) and the parietal association region (Brodmann area 39/40/7) bilaterally, and in the left anterior cingulate cortex (Brodmann area 32) [MSEQControl > motor execution task (CCWControl) ∩ MSEQmanifesting DYT1 > CCWmanifesting DYT1 ∩ MSEQnon-manifesting DYT1 > CCWnon-manifesting DYT1 ∩ VSEQC > audiovisual (AVControl) ∩ VSEQmanifesting DYT1>AVmanifesting DYT1; SPM Pcorr < 0.05; Supplementary Table 1]. Within-group activation patterns of modality independent sequence learning brain activation responses are presented in Fig. 2.
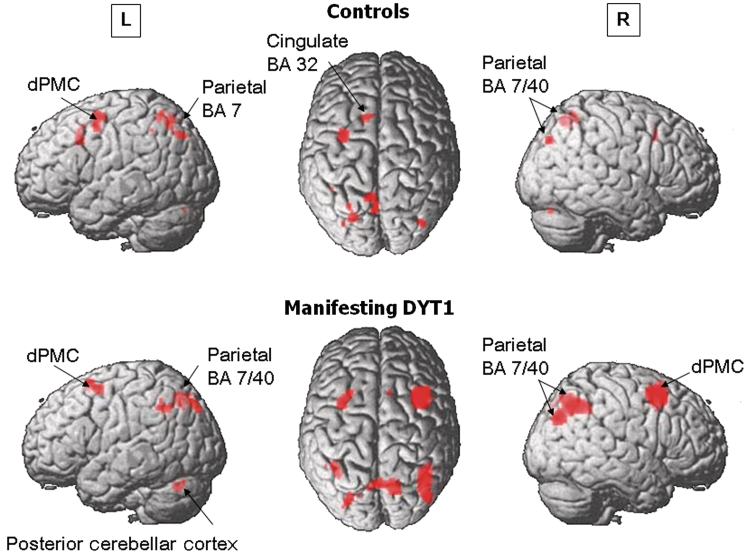
Figure 2.
Within-group analyses of brain activation responses. Top: Healthy control subjects exhibited modality independent sequence learning-related activation responses in the left dorsal premotor (dPMC), anterior cingulate [Brodmann area (BA) 32] and superior parietal cortex, as well as in the medial parietal region (Brodmann area 7) bilaterally (see Supplementary Table 2). Bottom: Manifesting DYT1 carriers showed modality independent bilateral activation responses in the dorsal premotor cortex and inferior and medial parietal regions, as well as in the left posterior cerebellar cortex. [The surface rendering of the statistical map (SPM5 canonical template, T > 3.0)].
In control subjects, significant activation during visual sequence learning was present in the right dorsal premotor cortex (Brodmann area 6) and left precuneus (Brodmann area 7) and cingulate cortex (Brodmann area 32). During motor sequence learning, significant activation was found bilaterally in the dorsal premotor cortex, pre-supplementary motor area (Brodmann area 6), parietal association cortex (Brodmann areas 39, 40, 7) and temporo-occipital regions (Brodmann area 19/37). Additional contributions were present in the left dorsolateral prefrontal cortex (Brodmann area 9/46) and cingulate cortex (Brodmann area 32). Shared effects of visual sequence learning and motor sequence learning were found in the dorsal premotor cortex as well as in lateral and medial parietal association cortex (Fig. 2 and Supplementary Table 2).
In manifesting DYT1 carriers, the motor and visual sequence learning tasks were also associated with significant prefrontal and parietal activation (Table 2). Moreover, sequence learning-related activation responses for the two tasks [(motor sequence learning > motor execution task) versus (visual sequence learning > audiovisual control condition)] did not differ in the manifesting DYT1 carriers. Sequence learning-related activation responses common to both modalities [(motor sequence learning > motor execution task) ∩ (visual sequence learning > audiovisual control condition)] were evident (SPM, Pcorr < 0.05) bilaterally in the dorsal premotor cortex (right > left, Brodmann area 6), parietal association cortex (Brodmann area 39/40/7) and in the left posterior cerebellum (lobule VI). The overall sequence learning-related activation pattern [(motor sequence learning > motor execution task) + (visual sequence learning > audiovisual control condition)]manifesting DYT1 is presented in Fig. 2.
Table 2.
Sequence learning-related activation responses in manifesting DYT1 carriers
| Contrast | MNI coordinates |
Z-max | Cluster size | ||
|---|---|---|---|---|---|
| Brain region | x | y | z | ||
| MSEQManifesting-DYT1 > CCWManifesting-DYT1 | |||||
| Right dorsal premotor cortex (BA 6) | 18 | 16 | 56 | 5.16 | 486 |
| Left dorsal premotor cortex (BA 6) | −30 | 6 | 62 | 4.19 | 160 |
| Right inferior parietal lobule (BA 40/7) | 42 | −62 | 44 | 4.48 | 156 |
| Left superior parietal lobule (BA 7/40) | −26 | −66 | 50 | 3.90 | 91 |
| Right anterior cingulate cortex (BA 32) | 2 | 26 | 38 | 4.33 | 196 |
| Right ventral prefrontal (BA 10, BA 46)a | 40 | 50 | 12 | 4.53 | 94 |
| Right ventral prefrontal (BA 10)a | 28 | 64 | 2 | 4.36 | 85 |
| Left cerebellar hemisphere (lobule VI)a | −32 | −66 | −26 | 4.56 | 99 |
| VSEQManifesting-DYT1 > AVManifesting-DYT1 | |||||
| Right dorsal premotor cortex (BA 6) | 46 | 14 | 52 | 4.61 | 402 |
| Right inferior parietal lobule (BA 40/7) | 40 | −50 | 46 | 5.29 | 395 |
| Left inferior parietal lobule (BA 40/7) | −38 | −50 | 52 | 5.83 | 414 |
| Right Precuneus (BA 7) | 10 | −66 | 52 | 4.60 | 228 |
| Right temporo-parietal cortex (BA 19, BA 39) | 36 | −78 | 36 | 4.02 | 149 |
| Left cerebellar hemisphere (lobule VI/crus I) | −46 | −72 | −28 | 4.34 | 183 |
| (MSEQManifesting-DYT1 > CCWManifesting-DYT1) ∩ (VSEQManifesting-DYT1 > AVManifesting-DYT1) | |||||
| Right inferior parietal lobule (BA 40/7) | 40 | −60 | 44 | 6.33 | 139 |
| Left inferior parietal lobule (BA 40) | −38 | −54 | 40 | 5.20 | 10 |
| Right dorsal premotor cortex (BA 6) | 40 | 12 | 52 | 6.31 | 291 |
| Left dorsal premotor cortex (BA 6) | −32 | 6 | 58 | 5.26 | 12 |
| Left dorsal premotor cortex (BA 6) | −26 | 12 | 64 | 5.02 | 4 |
| Right precuneus (BA 7) | 12 | −68 | 50 | 5.61 | 51 |
| Right precuneus, angular gyrus (BA 7/39) | 42 | −76 | 34 | 5.30 | 23 |
| Left superior parietal lobule (BA 7) | −28 | −80 | 42 | 4.95 | 5 |
| Left posterior cerebellum (lobule VI) | −26 | −66 | −28 | 5.02 | 5 |
a False discovery rate corrected. P < 0.05; AV = audiovisual; BA = Brodmann area.
Within- and between-group visual sequence learning deactivation responses are summarized in Supplementary Table 3. Both manifesting DYT1 and healthy control subjects exhibited significant learning-related deactivation responses [(motor sequence learning < motor execution task ∩ (visual sequence learning < audiovisual control condition)] in the middle temporal gyrus.
Abnormal sequence learning-related brain activation: DYT1 carriers
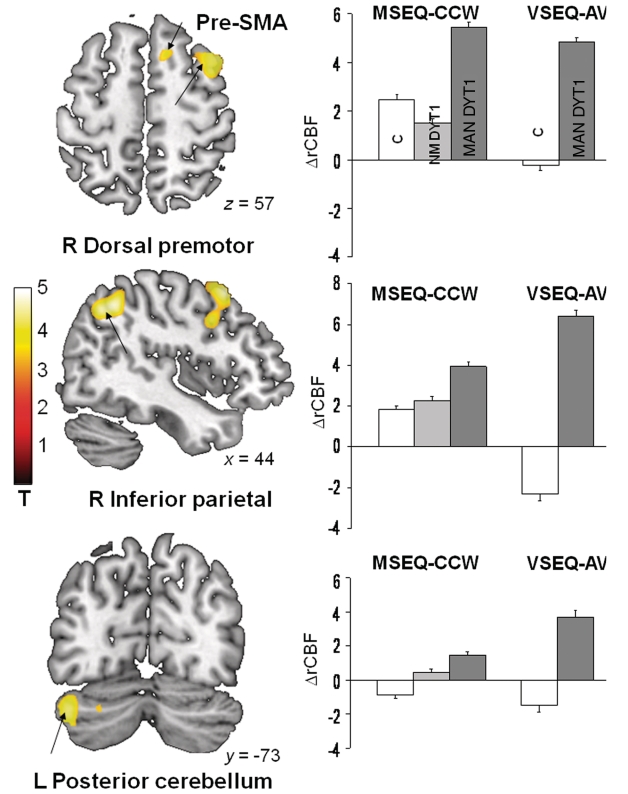
Sequence learning-related activation responses in manifesting DYT1 carriers [(motor sequence learning > motor execution task) + (visual sequence learning > audiovisual control condition)]manifesting DYT1 were compared with analogous modality-independent activation responses in control subjects [(motor sequence learning > motor execution task) + (visual sequence learning > audiovisual control condition)control]. Abnormally increased activation was present in the manifesting DYT1 carriers (SPM Pcorr < 0.05) in the right dorsal premotor cortex and inferior parietal cortex, and in the left cerebellar cortex (Fig. 3 and Table 3). Significant reductions in learning-related activation were not discerned in the manifesting DYT1 carriers relative to control subjects. Likewise, no significant differences in motor sequence learning-related activation were present in comparisons of manifesting- and non-manifesting DYT1 carriers.
Figure 3.
Between-group comparison of sequence learning-related activation responses. Brain regions in which task-related activation responses during motor and visual sequence learning were elevated in manifesting (MAN) DYT1 carriers relative to controls (Table 3). SPM(t) maps (left) were superimposed on a single-subject MRI T1 template. The position of each slice is indicated by x, y or z coordinates in MNI space. Bar diagrams (right) illustrate changes in adjusted regional cerebral blood flow (ΔrCBF) during motor sequence learning (MSEQ-CCW) and during visual sequence learning (VSEQ-AV) in the respective cluster (mean ± SE) for manifesting (MAN, dark grey) and non-manifesting (NM, light grey) DYT1 carriers, and healthy control subjects (white). Increased learning-related activation was present in the right rostral supplementary motor area (pre-SMA) (top), in the right dorsal premotor and inferior parietal regions (middle), and in the left posterior cerebellar cortex (bottom). The clusters were identified using the flexible factorial model in SPM5 for the contrast of the manifesting DYT1 and the healthy control groups. The corresponding regional values for the non-manifesting DYT1 cohort are presented for comparison. The colour stripe represents T-values thresholded at 4.55 (P = 0.05, corrected for multiple comparisons).
Table 3.
Abnormal modality independent increases in sequence learning-related activation in manifesting DYT1 carriers [(MSEQ > CCW and VSEQ > AV)manifesting-DYT1 > (MSEQ > CCW and VSEQ > AV)control]
| Brain region | MNI coordinates |
Z-max | Cluster size | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Right dorsal premotor cortex (BA 6) | 44 | 18 | 54 | 4.70 | 553 |
| Right inferior parietal, precuneus (BA 40) | 42 | −52 | 46 | 4.84 | 344 |
| Left cerebellar cortex, crus I | −44 | −74 | −34 | 4.32 | 382 |
Sequence learning-related brain activation: structure–function relationships
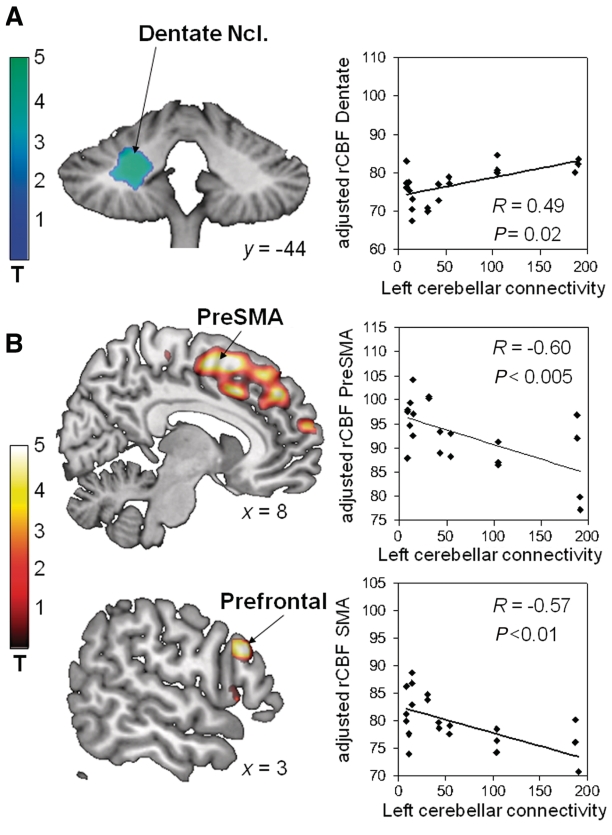
Voxel-wise multiple regression analysis of connectivity values from the left cerebellar outflow pathway of DYT1 carriers and cerebral blood flow scans acquired during visual sequence learning performance revealed a significant positive correlation (Table 4) in the left dentate nucleus (Fig. 4A). Significant negative cerebral blood flow correlations were identified (Table 4) in the lateral prefrontal cortex (Brodmann areas 9, 45) and in the medial frontal cortex, involving the supplementary motor area, pre-supplementary motor area, and the anterior cingulate cortex (Brodmann area 32) (Fig. 4B). For each of these regions, cerebral blood flow values were computed prospectively in the motor sequence learning scans of the manifesting- and non-manifesting DYT1 carriers. The presence of significant correlations with cerebellar outflow pathway connectivity (Table 4) was confirmed in each of the areas that were identified in the SPM analysis of the visual sequence learning scans (Fig. 4A and B).
Table 4.
Correlations between sequence learning-related activation and measures of left cerebellar tract connectivity in manifesting-DYT1 subjectsa
| Brain region | MNI coordinates |
Z-max | Cluster size | VOI-based correlation in MSEQ |
|||
|---|---|---|---|---|---|---|---|
| x | y | z | R | P-value | |||
| Positive correlation | |||||||
| Left dentate nucleus | −24 | −40 | −32 | 4.67 | 523 | 0.42 | 0.05 |
| Inverse correlation | |||||||
| Supplementary motor area, presupplementary motor area, cingulate gyrus (Right > Left; BA 6/32) | 2 | −22 | 60 | 5.68 | 3510 | −0.74 | <0.001 |
| Right lateral prefrontal cortex (BA 9, BA 45) | 60 | 22 | 26 | 5.74 | 184 | −0.57 | <0.01 |
a Left-hand columns represent the significant clusters identified using SPM voxel-wise regression of the regional cerebral blood flow scans acquired during VSEQ performance. Left cerebellothalamic connectivity values were entered as the covariate of interest in this analysis.
The right-most column represents the correlation coefficients (and P-values) for these regions computed using regional cerebral blood flow values for each region obtained from the corresponding MSEQ scans of these subjects. BA = Brodmann area; VOI = volume-of-interest.
Figure 4.
Correlations between cerebellar outflow pathway connectivity and regional cerebral blood flow measured during sequence learning. Regions are displayed in which regional cerebral blood flow (rCBF) recorded during visual sequence learning in manifesting DYT1 mutation carriers correlated with measures of left cerebello-thalamic pathway connectivity determined using probabilistic tractography (left). These correlations were validated prospectively (right) using volumes-of-interest centered on the peak voxel and placed on the co-registered motor sequence learning scans acquired in the same subjects. (A) A significant positive correlation was identified between cerebellar tract integrity and regional cerebral blood flow measured in the ipsilateral dentate nucleus (Ncl). (B) Significant negative correlations between these variables were present in the right supplementary motor area extending into the adjacent pre-supplementary motor area (preSMA) region (top), and in the right lateral prefrontal cortex (bottom). The colour stripe represents T-values thresholded at 4.55 (P = 0.05, corrected for multiple comparisons).
In contrast, no significant correlations were identified between caudate/putamen D2 receptor binding values measured with raclopride PET in gene carriers and sequence learning performance. Likewise, voxel-wise searches of cerebral blood flow scans acquired during motor sequence learning and visual sequence learning task performance did not reveal brain areas correlating significantly with caudate or putamen D2 binding, even at lenient statistical thresholds (P < 0.01 uncorrected).
Discussion
In this study, we demonstrate that the sequence learning deficits previously described in dystonia gene carriers represent an endophenotype of the DYT1 mutation but not of other dystonia mutations. Indeed, the DYT1 and DYT6 mutation carriers differed significantly with regard to learning performance. While the former showed clear sequence learning deficits, the latter performed normally. Despite significant motor impairment, affected subjects of either genotype performed at the levels of their non-manifesting counterparts, both during motor sequence learning and visual sequence learning. Thus, dystonic symptoms, although interfering substantially with motor performance (Carbon et al., 2010), did not affect sequence-learning performance in either of the genotypes.
The overall regional activation pattern in DYT1 carriers performing visual and motor sequence learning tasks accorded with results of earlier imaging studies of the learning of sequential information in healthy human subjects (Bapi et al., 2006; Doyon, 2008). This cognitive process was consistently found to involve activation of a frontoparietal network in conjunction with the posterior cerebellum. However, relative to healthy subjects, DYT1 carriers exhibited greater learning-related cerebellar activation during both the motor and visual tasks. Moreover, within the frontoparietal network, activation was relatively increased in the right lateral premotor cortex and in the right inferior parietal cortex of manifesting DYT1 carriers.
The findings build upon our earlier investigations of sequence learning deficits in non-manifesting DYT1 mutation carriers (Ghilardi et al., 2003; Carbon et al., 2004c, 2008). Our earlier studies have raised the possibility of a functional reorganization of frontostriatal pathways with a shift from striatal to cerebellar processing during sequence learning. The prominent involvement of the cerebellum has been confirmed in the present study in two respects. First, we found that increased cerebellar cortex activation characterized sequence learning-related brain activation responses in DYT1 mutation carriers, regardless of whether concurrent motor network activation was also present or whether the subjects exhibited clinically manifest symptoms of dystonia. Abnormally increased cerebellar activation was present during visual sequence learning in the current cohort of manifesting DYT1 carriers, consistent with earlier studies of non-manifesting carriers of this mutation (Ghilardi et al., 2003; Carbon et al., 2008). Second, the results disclosed a significant association between impaired cerebellar pathway connectivity in DYT1 carriers and cortical activation responses recorded during sequence learning.
Abnormally increased activation of the cerebellar cortex was noted in our earlier studies of non-manifesting DYT1 carriers. Importantly, using an equi-performance study design, we have demonstrated that increased cerebellar activation does not relate to the presence of performance differences, but characterizes brain activation in the DYT1 genotype at control performance levels (Carbon et al., 2008). We have also shown that normal sequence learning-related activation networks (Carbon et al., 2003), which include dentate nucleus activation for target acquisition and right cerebellar cortex activation for target retrieval, are not expressed in the DYT1 genotype (Carbon et al., 2004c). Rather, the gene carriers were found to use a different activation network during sequence learning, which was characterized by pronounced bilateral contributions from the cerebellar cortex and from prefrontal regions. We note that the presence of cerebellar activation during sequence learning is not necessarily abnormal (Leggio et al., 2000; Doyon et al., 2002; Bapi et al., 2006; Steele and Penhune, 2010). Nevertheless, in health, activation of the left cerebellar cortex is a characteristic of the early motor phases of sequence learning, whereas after extended learning periods there is a transition of neural processing from the cerebellar cortex to the dentate nucleus (Doyon et al., 2002; Carbon et al., 2003; Penhune and Doyon, 2005).
Concurrent activation of the cerebellar cortex and dentate nucleus is thought to mediate sequence encoding, and is an essential step in the early consolidation of learning (Doyon et al., 2002; Penhune and Doyon, 2005). Our data raise the possibility that the abnormal sequence learning-related activation in the DYT1 carriers reflects an impairment of the transition from early to later stages of information processing. Our current study also disclosed effects of impaired cerebellar connectivity on cortical brain activation responses. Notably, the role of cerebro-cerebellar interaction in learning processes has not fully been elucidated, but clearly both the supplementary motor area and pre-supplementary motor area receive inputs that originate in the dentate nucleus of the cerebellum (Akkal et al., 2007). Associations between cerebellar input and plastic changes in the motor cortex have been demonstrated during the acquisition of a visuomotor skill (Doyon et al., 1996), and both lateral and medial premotor cortex activation responses co-vary with cerebellar cortical activation in healthy subjects during successful sequence learning (Carbon et al., 2003). The cerebello-thalamo-cortical pathway facilitates intracortical inhibition via thalamocortical projections to inhibitory interneurons in the sensorimotor cortex (Molinari et al., 2002; Daskalakis et al., 2004). Our data suggest the presence of a comparable effect of the cerebello-thalamo-cortical system on the medial premotor cortex during learning. However, the nature of cerebello–premotor interactions in normal subjects is not well understood, but recent data suggest that these functional interactions are complex, with distinct inhibitory and facilitatory effects on the motor cortex during learning, as opposed to simple movement (Torriero et al., 2010).
Altered cerebellar modulation of pre-supplementary motor area activity may be key to deficient motor programming in dystonia. This region has been characterized as the interface of motor control and cognition based upon: (i) measurements of local neuronal activity (Ikeda et al., 1999); (ii) a unique pattern of connectivity with prefrontal non-motor regions (Luppino et al., 1993); and (iii) its activation in relation to various cognitive challenges (Forstmann et al., 2008; Jung et al., 2010; van Gaal et al., 2010). Along these lines, it has recently been suggested that the key functions of the supplementary motor area and pre-supplementary motor area relate to the resolution of the conflicting neural signals associated with competing motor programmes (Nachev et al., 2008). According to this hypothesis, neural activity in both supplementary motor area and pre-supplementary motor area constitute the major determinants of the dorsal premotor cortex activation response during task performance. Thus, the observed abnormal increases in premotor and inferior parietal task-related activation can both be construed as downstream effects of deficient cerebellar output. Notably, in an earlier metabolic imaging study (Carbon et al., 2004b), we found that manifesting mutation carriers exhibited characteristic resting metabolic increases in the pre-supplementary motor area and parietal association regions.
In contrast to the cerebellum, the basal ganglia did not contribute to the activation changes seen in the mutation carriers. Striatal dopaminergic neurotransmission has been implicated in sequence learning in healthy human subjects (Carbon et al., 2004a; Badgaiyan et al., 2007; Karabanov et al., 2010), in patients with basal ganglia disorders (Carbon et al., 2004a; Argyelan et al., 2008; Kwak et al., 2010), and in experimental animal models (Matsumoto et al., 1999; Eckart et al., 2010). Moreover, striatal D2 receptor availability is abnormally reduced in the DYT1 genotype (Asanuma et al., 2005; Carbon et al., 2009). Nonetheless, there was no discernible correlation between caudate/putamen D2 receptor binding and sequence learning performance or associated neural activation responses. Of note, we have found a significant difference between striatal D2 binding across the two dystonia genotypes (Carbon et al., 2009). While there was also a significant group difference in sequence learning performance, this cognitive feature cannot be explained by genotype-related changes in dopamine receptor binding. Moreover, given the large number of mutation carriers who also participated in the 11C-raclopride PET studies, these negative findings are unlikely to have been limited by insufficient sample size. Needless to say, caudate/putamen D2 receptor measurements reflect only one feature of overall striatal neurotransmission. Although the observed striatal D2 receptor changes are unlikely to be the major determinant of sequence learning in DYT1 carriers, the current data do not preclude a contribution from the dopamine system in mediating these functional abnormalities.
Interestingly, the sequence learning deficit observed in DYT1 carriers proved to be isolated and was not correlated with deficits in neuropsychological test performance, particularly those with sequencing elements and visuospatial processing requirements. Fiorio and colleagues (2007) found a reduced ability to perceive visual, tactile or visuo-tactile stimuli as temporally separated in DYT1 mutation carriers. Unfortunately, our study was not designed to include the assessment of these functions. While the DYT1 mutation carriers included in our study potentially exhibited such perceptual deficits, these were not likely to have been the cause of the observed sequence learning deficits. Compared with the visual, point-like stimuli in Fiorio's experiments (Fiorio et al., 2007), the targets displayed in our tasks are quite large (diameter of 2 cm; Ghilardi et al., 2000) and appear at a frequency of 1 Hz/1000 ms, which exceeds the visual temporal discrimination threshold determined for DYT1 carriers (Fiorio et al., 2007). That said, the possibility exists that both the sequence learning and temporal discrimination deficits seen in the mutation carriers are linked to an underlying information processing deficit as a single DYT1 endophenotype. A genotype-related abnormality of temporal information processing can be understood as a manifestation of impaired connectivity in the pathways linking the cerebellar cortex to the supplementary motor area—both of which are involved in perceptual and motor timing (O'Reilly et al., 2008; Aso et al., 2010). An analogous mechanism may underlie the deficits in vibration-induced movement illusion described in adult onset dystonia (Frima et al., 2003). This psychophysical phenomenon has been attributed to posterior parietal and cerebellar activation in healthy subjects (Thyrion and Roll, 2009). In this regard, deficits in visuospatial integration can be construed as involving abnormalities in the pathways that connect the cerebellum to the parietal cortex (Clower et al., 2001).
We acknowledge that the small number of subjects included in this study may limit its overall generalization. Nevertheless, despite its rarity, DYT1 dystonia can provide relevant information regarding the mechanisms underlying the more common sporadic forms of primary dystonia.
The lack of brain activation data in DYT6 mutation carriers poses an additional shortcoming. Abnormal reductions in cerebellar connectivity are present in DYT6 as well as DYT1 carriers (Argyelan et al., 2009). However, sequence learning deficits were evident in the former but not in the latter dystonia genotype. It is therefore likely that genotype-specific compensatory factors exist to support normal learning performance in DYT6 carriers. The identification of these mechanisms may help explain the performance differences that were found between these genotypes. This information will also provide knowledge of the compensatory strategies that the brain can employ in response to neurodevelopmental alterations in pathway connectivity.
Another shortcoming is the choice of a relatively complicated baseline task. The counter-clockwise (motor execution task) reaching task involves the combination of several moves into a predictable sequence, whereas the learning tasks involve the explicit acquisition of a novel sequence. It is possible that brain activation abnormalities, in particular the increases observed in the supplementary motor area, derive from this sequencing feature of motor execution task performance. By subtracting these effects as part of the baseline (non-learning) condition, activation abnormalities such as those found in the supplementary motor area may have been underestimated.
In summary, the current study corroborates the role of cerebellar outflow pathways in DYT1 dystonia. In view of the strong evidence for a role of the basal ganglia in dystonia, and recent reports of a disynaptic connection of the subthalamic nucleus and cerebellar cortex (Bostan et al., 2010), future studies will need to assess the interaction of cerebellar and striatal functions in healthy subjects and in dystonia gene carriers.
Funding
The National Institutes of Health (R01 NS 047668 to D.E.); the General Clinical Research Centre of The Feinstein Institute for Medical Research (M01 RR 018535).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors wish to thank the participants and their families. We thank Dr Thomas Chaly (radiochemistry) and Mr Claude Margouleff (technical support). In particular, we are most thankful to Ms Toni Fitzpatrick for study coordination and article preparation. We are also indebted to Ms Deborah Raymond for participant recruitment.
Glossary
Abbreviations
- CCW
motor execution task
- MSEQ
motor sequence learning
- VSEQ
visual sequence learning
References
- Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27:10659–73. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Ghilardi MF, Feigin A, Mattis P, Tang C, et al. Dopaminergic suppression of brain deactivation responses during sequence learning. J Neurosci. 2008;28:10687–95. doi: 10.1523/JNEUROSCI.2933-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–7. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, et al. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64:347–9. doi: 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- Aso K, Hanakawa T, Aso T, Fukuyama H. Cerebro-cerebellar interactions underlying temporal information processing. J Cogn Neurosci. 2010;22:2913–25. doi: 10.1162/jocn.2010.21429. [DOI] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ Alpert non-manifesting. Striatal dopamine release in sequential learning. Neuroimage. 2007;38:549–56. doi: 10.1016/j.neuroimage.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapi RS, Miyapuram KP, Graydon FX, Doya K. fMRI investigation of cortical and subcortical networks in the learning of abstract and effector-specific representations of motor sequences. Neuroimage. 2006;32:714–27. doi: 10.1016/j.neuroimage.2006.04.205. [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL. The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA. 2010;107:8452–6. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–34. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Bressman S. Genetics of dystonia. J Neural Transm Suppl. 2006:489–95. doi: 10.1007/978-3-211-45295-0_73. [DOI] [PubMed] [Google Scholar]
- Carbon M, Argyelan M, Habeck C, Ghilardi MF, Fitzpatrick T, Dhawan V, et al. Increased sensorimotor network activity in DYT1 dystonia: a functional imaging study. Brain. 2010;133:690–700. doi: 10.1093/brain/awq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Eidelberg D. Modulation of regional brain function by deep brain stimulation: studies with positron emission tomography. Curr Opin Neurol. 2002;15:451–5. doi: 10.1097/00019052-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Carbon M, Eidelberg D. Abnormal structure-function relationships in hereditary dystonia. Neuroscience. 2009;164:220–9. doi: 10.1016/j.neuroscience.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Ghilardi MF, Argyelan M, Dhawan V, Bressman SB, Eidelberg D. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131:146–54. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Ghilardi MF, Feigin A, Fukuda M, Silvestri G, Mentis MJ, et al. Learning networks in health and Parkinson's disease: reproducibility and treatment effects. Hum Brain Mapp. 2003;19:197–211. doi: 10.1002/hbm.10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Ma Y, Barnes A, Dhawan V, Chaly T, Ghilardi MF, et al. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage. 2004a;21:1497–507. doi: 10.1016/j.neuroimage.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Carbon M, Niethammer M, Peng S, Raymond D, Dhawan V, Chaly T, et al. Abnormal striatal and thalamic dopamine neurotransmission: Genotype-related features of dystonia. Neurology. 2009;72:2097–103. doi: 10.1212/WNL.0b013e3181aa538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Su S, Dhawan V, Raymond D, Bressman S, Eidelberg D. Regional metabolism in primary torsion dystonia: effects of penetrance and genotype. Neurology. 2004b;62:1384–90. doi: 10.1212/01.wnl.0000120541.97467.fe. [DOI] [PubMed] [Google Scholar]
- Carbon M, Trost M, Ghilardi MF, Eidelberg D. Abnormal brain networks in primary torsion dystonia. Adv Neurol. 2004c;94:155–61. [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–91. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol. 2008;21:478–83. doi: 10.1097/WCO.0b013e328304b6a3. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–7. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC. Functional anatomy of visuomotor skill learning in human subjects examined with positron emission tomography. Eur J Neurosci. 1996;8:637–48. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA. 2002;99:1017–22. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckart MT, Huelse-Matia MC, McDonald RS, Schwarting RK. 6-Hydroxydopamine lesions in the rat neostriatum impair sequential learning in a serial reaction time task. Neurotoxicity Research. 2010;17:287–98. doi: 10.1007/s12640-009-9103-4. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Antonini A, Kazumata K, Nakamura T, Dhawan V, et al. Functional brain networks in DYT1 dystonia. Ann Neurol. 1998;44:303–12. doi: 10.1002/ana.410440304. [DOI] [PubMed] [Google Scholar]
- Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, et al. Thalamic metabolism and symptom onset in preclinical Huntington's disease. Brain. 2007;130:2858–67. doi: 10.1093/brain/awm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrieri RA, Alexoff DL, Schlyer DJ, Wolf AP. Remote processing, delivery and injection of H2[15O] produced from a N2/H2 gas target using a simple and compact apparatus. Appl Radiat Isot. 1994;45:1149–54. doi: 10.1016/0969-8043(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Fiorio M, Gambarin M, Valente EM, Liberini P, Loi M, Cossu G, et al. Defective temporal processing of sensory stimuli in DYT1 mutation carriers: a new endophenotype of dystonia? Brain. 2007;130:134–42. doi: 10.1093/brain/awl283. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Dutilh G, Brown S, Neumann J, von Cramon DY, Ridderinkhof KR, et al. Striatum and pre-supplementary motor area facilitate decision-making under time pressure. Proc Natl Acad Sci USA. 2008;105:17538–42. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frima N, Rome SM, Grunewald RA. The effect of fatigue on abnormal vibration induced illusion of movement in idiopathic focal dystonia. J Neurol Neurosurg Psychiatry. 2003;74:1154–6. doi: 10.1136/jnnp.74.8.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Stephan KE, Lund TE, Morcom A, Kiebel S. Mixed-effects and fMRI studies. Neuroimage. 2005;24:244–52. doi: 10.1016/j.neuroimage.2004.08.055. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Gavarini S, Saunders-Pullman R, Raymond D, Ehrlich ME, Bressman SB, et al. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–8. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- Ghilardi MF, Carbon M, Silvestri G, Dhawan V, Tagliati M, Bressman S, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann Neurol. 2003;54:102–9. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–45. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- Hallett M. Physiology of dystonia. Adv Neurol. 1998;78:11–8. [PubMed] [Google Scholar]
- Ikeda A, Yazawa S, Kunieda T, Ohara S, Terada K, Mikuni N, et al. Cognitive motor control in human pre-supplementary motor area studied by subdural recording of discrimination/selection-related potentials. Brain. 1999;122:915–31. doi: 10.1093/brain/122.5.915. [DOI] [PubMed] [Google Scholar]
- Jung J, Jerbi K, Ossandon T, Ryvlin P, Isnard J, Bertrand O, et al. Brain responses to success and failure: Direct recordings from human cerebral cortex. Hum Brain Mapp. 2010;31:1217–32. doi: 10.1002/hbm.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov A, Cervenka S, de Manzano O, Forssberg H, Farde L, Ullen F. Dopamine D2 receptor density in the limbic striatum is related to implicit but not explicit movement sequence learning. Proc Natl Acad Sci USA. 2010;107:7574–9. doi: 10.1073/pnas.0911805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Lindquist MA, Nichols TE, Poldrack RA, Vul E. Everything you never wanted to know about circular analysis, but were afraid to ask. J Cereb Blood Flow Metab. 2010;30:1551–7. doi: 10.1038/jcbfm.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Muller ML, Bohnen NI, Dayalu P, Seidler RD. Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson's disease. J Neurophysiol. 2010;103:942–9. doi: 10.1152/jn.00197.2009. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Molinari M, Neri P, Graziano A, Mandolesi L, Petrosini L. Representation of actions in rats: the role of cerebellum in learning spatial performances by observation. Proc Natl Acad Sci USA. 2000;97:2320–5. doi: 10.1073/pnas.040554297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (supplementary motor area-proper) and area F6 (pre-supplementary motor area) in the macaque monkey. J Comp Neurol. 1993;338:114–40. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Hanakawa T, Maki S, Graybiel AM, Kimura M. Role of [corrected] nigrostriatal dopamine system in learning to perform sequential motor tasks in a predictive manner. J Neurophysiol. 1999;82:978–98. doi: 10.1152/jn.1999.82.2.978. [DOI] [PubMed] [Google Scholar]
- Molinari M, Filippini V, Leggio MG. Neuronal plasticity of interrelated cerebellar and cortical networks. Neuroscience. 2002;111:863–70. doi: 10.1016/s0306-4522(02)00024-6. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ghilardi MF, Mentis M, Dhawan V, Fukuda M, Hacking A, et al. Functional networks in motor sequence learning: abnormal topographies in Parkinson's disease. Hum Brain Mapp. 2001;12:42–60. doi: 10.1002/1097-0193(200101)12:1<42::AID-HBM40>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci. 2008;28:2252–60. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, Doyon J. Cerebellum and M1 interaction during early learning of timed motor sequences. Neuroimage. 2005;26:801–12. doi: 10.1016/j.neuroimage.2005.02.041. [DOI] [PubMed] [Google Scholar]
- Saunders-Pullman R, Raymond D, Senthil G, Kramer P, Ohmann E, Deligtisch A, et al. Narrowing the DYT6 dystonia region and evidence for locus heterogeneity in the Amish-Mennonites. Am J Med Genet A. 2007;143A:2098–105. doi: 10.1002/ajmg.a.31887. [DOI] [PubMed] [Google Scholar]
- Steele CJ, Penhune VB. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J Neurosci. 2010;30:8332–41. doi: 10.1523/JNEUROSCI.5569-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyrion C, Roll JP. Perceptual integration of illusory and imagined kinesthetic images. J Neurosci. 2009;29:8483–92. doi: 10.1523/JNEUROSCI.0683-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Ferlazzo F, et al. Changes in Cerebello-motor Connectivity during Procedural Learning by Actual Execution and Observation. J Cogn Neurosci. 2010;23:338–48. doi: 10.1162/jocn.2010.21471. [DOI] [PubMed] [Google Scholar]
- Trost M, Carbon M, Edwards C, Ma Y, Raymond D, Mentis MJ, et al. Primary dystonia: is abnormal functional brain architecture linked to genotype? Ann Neurol. 2002;52:853–6. doi: 10.1002/ana.10418. [DOI] [PubMed] [Google Scholar]
- van Gaal S, Ridderinkhof KR, Scholte HS, Lamme VA. Unconscious activation of the prefrontal no-go network. J Neurosci. 2010;30:4143–50. doi: 10.1523/JNEUROSCI.2992-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–90. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.