Abstract
As Th17 cell developmental requirements continue to be studied, Gutcher et al (2011) demonstrate in this issue of Immunity that autocrine TGF-β cytokine promotes Th17 cell development and maintenance.
The initial description of T helper 17 (Th17) cells depicted a CD4+ effector T cell that produced primarily IL-17 and whose development was inhibited by the cytokines IFN-γ and IL-4 (Harrington et al., 2005; Park et al., 2005). Delving further into defining the requirements for Th17 cell development, the cytokine TGF-β was logically considered, having been shown previously to repress IFN-γ expression and to potently inhibit Th1 and Th2 cell differentiation through the suppression of the transcription factors T-bet and GATA3. Not surprisingly, reports demonstrating the role of TGF-β in Th17 cell development, specifically in the presence of IL-6, followed shortly thereafter. However, in a rare case where the mouse immune system appeared not to mirror its more evolved counterpart, human Th17 cells were initially shown to develop in the absence of TGF-β, requiring only IL-6 and IL-1β or IL-23 and IL-β (Acosta-Rodriguez et al., 2007; Wilson et al., 2007). Later studies refuted these observations, demonstrating a requirement for TGF-β in the development of Th17 cells from painstakingly isolated naïve CD4+ T cells in medium lacking serum, an oft and heretofore ignored source of varying amounts of TGF-β (Korn et al., 2009). Although the role of TGF-β in “induced regulatory T (Treg) cell generation is widely accepted, considerable controversy regarding the requirement of TGF-β in directing Th17 cell development remains, and two recent works published in Nature (Ghoreschi et al., 2010) and here, in the current issue of Immunity (Gutcher et al. 2011), continue to add fuel to the fire.
Building on their previous study (Li et al., 2007) that showed T cells are the main source of TGF-β responsible for driving Th17 cell development, Gutcher et al. further dissects the T cell populations responsible for TGF-β driven Th17 cell differentiation. The current study begins by demonstrating that all progeny of naïve CD4+ T cells have the potential to express TGF-β, but which of these cells are the primary source of TGF-β involved in Th17 cell development? To address this question, Gutcher et al. took advantage of the preferential expression of OX-40 in activated CD4+ T cells and Treg cells and used an OX-40-driving Cre recombinase-expressing mouse strain to successfully delete Tgfb1 in the majority of these cells, designating the resultant mice, Tgfb1f/n Tnfrsf4-cre. In comparison to Tgfb1f/n Cd4-cre mice with deletion of TGF-β in all T cells(Li et al., 2007), Tgfb1f/n Tnfrsf4-cre animals developed a much less severe wasting disease and did not exhibit signs of spontaneous activation and differentiation in the periphery. Consequently, TGF-β deletion in the periphery was confined mainly to the Treg cell population, present in increased frequencies in these mice, thereby implicating Treg cell derived TGF-β as a critical requirement of Treg cell maintenance. In the gut though, there is a higher prevalence of activated T cells than in the periphery (Ivanov et al., 2008) and therefore the reduced percentages of gut-resident IL-17 expressing cells in Tgfb1f/n Tnfrsf4-cre mice along with their resistance to developing experimental autoimmune encephalomyelitis (EAE), due to decreased IL-17+ cells in the central nervous system (CNS) hint that activated T cells supply TGF-β needed for optimal Th17 cell development.
Utilizing a Foxp3-driving Cre recombinase to delete Tgfb1 in regulatory cells (designated Tgfb1f/n Foxp3-cre) Gutcher et al. demonstrated that these animals remained healthy, without signs of inflammatory disease, or spontaneous T cell activation, however increases in frequencies and numbers of Foxp3+ cells in peripheral and mesenteric lymph nodes were observed thereby confirming Treg cells as the source of TGF-β responsible for limiting their own proliferation. Induction of EAE in Tgfb1f/n Foxp3-cre mice resulted in the development of IL-17+ cells in the CNS with associated disease symptoms similar to that of wild type mice, demonstrating that Treg cell-derived TGF-β is insufficient to direct Th17 cell development, yet a role for Treg cells in promoting Th17 cell differentiation remains plausible. Studies by Chen et al and Pandiyan et al in this issue of Immunity report that Treg cells promote Th17 cell development by consuming the Th17 cell-prohibitive cytokine, IL-2, early in Th17 cell differentiation, which simultaneously promotes their own growth and proliferation, thereby allowing for a suppressive role later in the response.
The elimination of Treg cells as the source of Th17 cell-inducing TGF-β left activated T cells as likely suspects but which members of this heterogeneous population are key? Using a TGF-β reporter mouse line, Gutcher et al. determined that in vitro polarized Th1, Th2 and Th17 cells all can express TGF-β but it is most highly expressed in Th17 cells. Further, induction of EAE in Rag1−/− reconstituted with an equal mix of wild-type and Tgfb1f/n Cd4-cre bone marrow resulted in wild-type derived Th17 and Th1 cells in the CNS whereas substantially fewer IL-17+ cells originating from the CD4+ Tgfb1 deficient bone marrow were detected, elegantly establishing that TGFβ acts in an autocrine manner to promote Th17 cell differentiation.
The studies of Gutcher et al confirm and extend previous observations that T cell derived TGF-β is required for Th17 differentiation, adding the unforeseen finding that Th17 cell produced TGF-β is essential for reinforcing their own maintenance. Recently however, Ghoreschi et al demonstrated that Th17 cells can arise in the absence of TGF-β signaling,, a seemingly contradictory finding to that of Gutcher et al. Similar to the initial reports describing the generation of human Th17 cells, Ghoreschi et al. demonstrated that IL-17+ T cells can be generated from mouse naïve CD4+ T cells with solely IL-6, IL-23, and IL-1β, though significantly fewer IL-17+ cells arise in comparison to TGF-β plus IL-6 generated cells and those cells that do develop possess a more pathogenic phenotype than TGF-β derived cells.
Ghoreschi et al. find that Th17 cells differentiated with TGF-β express the regulatory cytokines IL-9 and IL-10 whereas those derived without TGF-β express Th1-associated molecules including IFN-γ and migrate to inflammatory sites. Using a transfer model of EAE, Ghoreschi et al. demonstrate that Th17 cells polarized without TGF-β induce a more severe disease and have greater numbers of IL-17+IFN-γ+ cells in the CNS compared to mice receiving TGF-β directed Th17 cells. In normal mice, MOG peptide induced EAE results in substantial numbers of CD4+ IFN-γ+IL-17+ T cells in the CNS but whether these double positive cells are derived from a TGF-β-independent pathway is unclear.
In the continued presence of TGF-β, alone, Th17 cells can develop into cells expressing both IL-17 and IFN-γ, demonstrating an intrinsic property of Th17 cells to deviate to a cell that has Th1-like characteristics (Lee et al., 2009). That Th17 cells can rapidly respond to IL-12 signaling by upregulating IFN-γ further emphasizes this property. It is therefore not entirely unexpected that cells generated in the absence of TGF-β express IFN-γ. However, because TGF-β is widely expressed, it is difficult to imagine a scenario where a tissue would be completely devoid of its expression, yet it is conceivable that limiting amounts in a cell’s immediate vicinity would have an impact on its fate as low concentrations of TGF-β appear to favor Th17 cell development. Ghoreschi et al suggest that Th17 cells are derived from both TGFβ-dependent and - independent pathways but an alternative and not mutually exclusive explanation is that the inherent plasticity of Th17 cells is amplified in conditions where high concentrations of inflammatory cytokines and limiting TGF-β tip the balance to a more aggressive phenotype (figure 1). Nevertheless, reconciling Ghoreschi et al.’s observations with the fact that Th17 cells fail to develop in the absence of T cell derived TGF-β in the EAE model is somewhat problematic and further complicated by the revelation that Th17 cells themselves can express TGF-β but perhaps the ability to generate appropriate IL-1β, IL-6, and IL-23 signals in the specific contextual environment of a naïve CD4+ T cell is dysregulated in the Tgfb1f/n Cd4-cre mouse model though this remains to be determined.
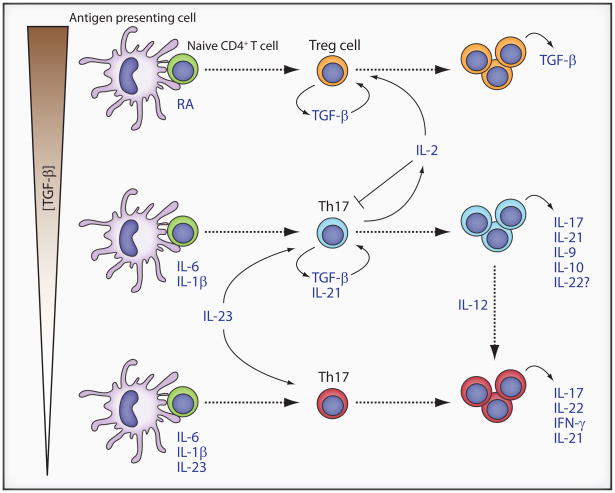
Figure 1. Proposed Th17 and Treg cell developmental pathways.
Naïve CD4+ T cells (green circles) in the presence of high concentrations of TGF-β and retinoic acid develop into regulatory T cells that produce autocrine TGF-β, instrumental in maintaining their development (orange circles). Lower concentration of TGF-β together with IL-6 and IL-1 β promote the development of Th17 cells (blue circles) expressing autocrine TGF–β and IL-21, further reinforcing their own development. In the presence of IL-12, these cells can be diverted to cells expressing both IFN–γ and IL-17 (red cells). The Th17-inhibiting cytokine, IL-2, is also expressed but is consumed by Treg cells, promoting both Th17 cell development and Treg cell survival. In the absence of TGF-β, IL-6, IL-1 β, and IL-23 together drive the development of Th17 cells with a pathogenic phenotype (red circles).
There appears to be little disagreement that Th17 cells can be generated from naïve CD4+ T cells in the presence of TGF-β and IL-6 and the studies of both Gutcher et al. and Ghoreschi et al. reaffirm this tenet. However, the role of autocrine TGF-β in promoting Th17 development at steady state requires further investigation particularly in regards to the signals that drive a Th17 cell to produce TGF-β. The combination of IL-6, IL-23, and IL-1β was largely inefficient in this respect as the addition of neutralizing TGF-β antibodies had little effect on IL-17 expression. Though considerable debate continues regarding the critical factors necessary for Th17 cell development, the varied environmental conditions in which they develop suggest a complex array of context specific signals rather than absolute requirements. Thus, future studies focusing on the contextual and environmental requirements for Th17 generation both at steady state and during inflammatory conditions are needed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang X, Tato C, Mcgeachy M, Konkel J, Ramos HL, Wei L, Davidson T, Bouladoux N, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L, Hatton RD, Mangan P, Turner H, Murphy T, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Ivanov I, Frutos RdeL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman D. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host & Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo V. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang X, Chang S, Nurieva R, Wang Y, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson J, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]