Abstract
Filamin, a large cytoskeletal adaptor, connects plasma membrane to cytoskeleton by binding to transmembrane receptor integrin and actin. Seven of twenty-four filamin immunoglobulin repeats have conserved integrin binding sites, of which 19 and 21 were shown to be auto-inhibited by their adjacent repeats 18 and 20 respectively. Here we show using NMR spectroscopy that the auto-inhibition can be relieved by integrin or an integrin regulator migfilin. We further demonstrate that repeats 19 and 21 can simultaneously engage ligands. The data suggest that filamin is mechanically stretched by integrin or migfilin via a multi-site binding mechanism for regulating cytoskeleton and integrin-mediated cell adhesion.
Many transmembrane receptors are tethered to the actin cytoskeleton and this interaction determines the residence time of the membrane protein on the plasma membrane of animal cells. The tethering causes a variety of phenomena like receptor endocytosis, ligand affinity, receptor signaling, and cytoskeletal remodeling. Integrins are a class of conserved heterodimeric transmembrane glycoproteins in multicellular animals that attach cells to the extracellular matrix and to other cells (1). Upon activation (2), the extracellular side of integrins bind to collagen, laminin, fibronectin, fibrinogen and other matrix proteins, whereas their generally short cytoplasmic tails (CTs) can link to actin cytoskeleton by binding a variety of cytoplasmic proteins, thereby modulating cell adhesion and signaling outcomes (3, 4). One of the major integrin CT binding proteins is filamin, a large actin binding cytoskeletal protein. The filamin-integrin interaction was found to compete with the integrin activator talin (5, 6, 7), which has led to a proposal that filamin is a negative regulator of integrin activation (8, 9). Interestingly, a LIM domain containing protein migfilin was found to bind to filamin (10) in the same manner as integrin (8, 11), thereby competing with filamin for binding to integrin CTs and facilitating integrin activation and cell-matrix adhesion (8).
Filamin monomer consists of an N-terminal actin binding domain followed by 24 immunoglobulin (Ig) repeat domains of ~95 amino acids. Filamin dimerises through the 24th Ig repeat (12). 7 of these 24 repeats share a common interface where the ligands dock (13). This interface is made up of the β-strands C and D of the Ig structure (5, 8, 11, 14). Filamin ligand themselves extend one of the β-sheets of the Ig fold. The C-terminus of filamin has four alternate repeats (17, 19, 21 and 23) whose conserved binding sites to integrin, migfilin, and GP1bα have been confirmed (13). Interestingly, recent structural studies have shown that even numbered repeats 18 and 20 mask the binding pocket of 19 and 21 respectively, resulting in “auto-inhibited” filamin (15, 16, 17). This implies that filamin can function in a “closed” and an “active/stretched” mode during the regulation of integrin-actin dynamics. In the auto-inhibited form filamin repeats are globular and not in a “string of beads” alignment. It was thought that the mechanical tug of the actin cytoskeleton stretches filamin and exposes the masked CD groove (17). A splice variant of filamin, filamin-Avar-1 that lacks amino acids 2127-2167 is a naturally occurring auto-inhibition deficient filamin (18, 19). This mutant lacks 14 amino acids of repeat 19 and 27 amino acids of repeat 20. For Ig repeats 17 and 23 no auto-inhibition phenomena is reported. The segments of repeats 18 and 20 that cause auto-inhibition weakly resemble integrin/migfilin (15, 16 and figure S1 of supporting information). Despite being auto-inhibited, Ig repeat 21 is frequently identified in biological screens as the principal docking site for filamin ligands. We previously showed that Ig repeat 21 binds the ligand tightest and postulated that the presence of seven binding sites in a single filamin molecule could lead to membrane receptor clustering mediated by filamin. A key issue is how the auto-inhibition of filamin is relieved. Moreover, can multiple Ig repeats truly engage with ligands simultaneously to mediate integrin clustering (13)? Using E. coli expressed and purified multi-repeat filamin constructs we show here that auto-inhibition can be potently relieved by integrin or migfilin, suggesting that filamin is in a stretched mode when bound to integrin or migfilin. Further, we demonstrate that filamin can indeed simultaneously engage more than one integrin/migfilin, thus providing the first experimental evidence of the multi-site ligand binding by filamin.
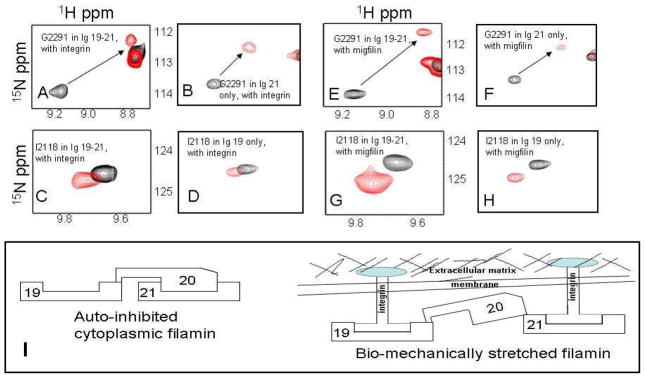
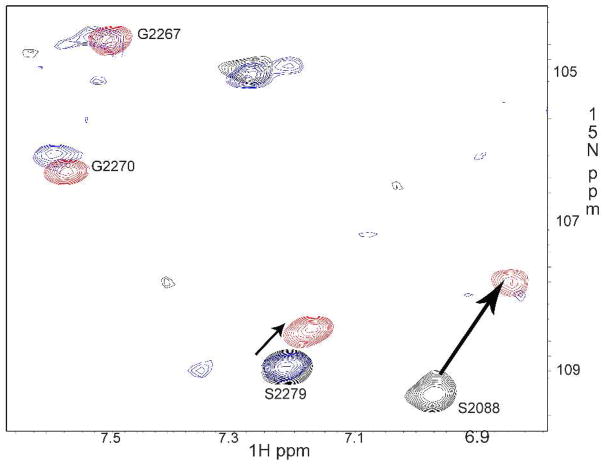
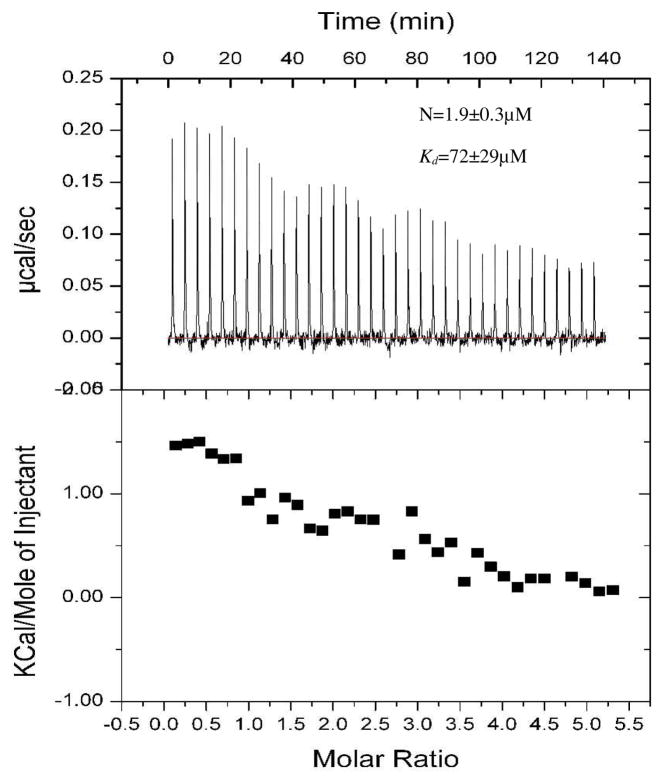
To address our questions, we used the construct encoding Ig19-21 repeats of human filamin A. The domain boundaries were 2045–2140 for Ig19, 2141–2235 for Ig20 and 2236–2329 for Ig21 (12). There are three reasons for using this construct: (i) it has a well-characterized auto-inhibitory unit (i.e., repeat 21 masked by repeat 20); (ii) it has two ligand binding repeats (Ig19 and Ig21) that can be used as a proof-of-the-principle to demonstrate the multi-ligand binding; (iii) it has well resolved NMR spectrum for unambiguous NMR analysis. We expressed repeats 19-21, and for comparison filamin-Avar-1 19-21 also in E. coli using standard molecular biology protocols. Constructs were confirmed by DNA sequencing. The GST fused protein was cleaved and purified to homogeneity using standard affinity and chromatography methods described earlier (13). 15N labeled proteins for heteronuclear single quantum coherence (HSQC) experiments were made in minimal medium supplemented with 15N NH4Cl. All proteins and peptides used in the present study were dissolved in 25mM sodium phosphate buffer, 5mM NaCl, 1mM DTT at pH 6.3. pH was matched to within 0.03 units when comparing spectra. HSQC comparison of Ig19-21 with filamin-Avar-1 Ig19-21 revealed that most peaks corresponding to Igs 19 and 20 were missing or congested to the unstructured regions for the filamin-Avar-1 19-21 protein (Figure S2 of supporting information). Filamin-Avar-1 Ig19-21 peaks matched mostly with those of the free form 21 indicating that in this splice variant only Ig21 is folded (Figure S3 of supporting information), whereas the deletion of 14 amino acids in repeat 19 and 27 amino acids in Ig20 impaired the structural integrity and unfolded the repeats. Note that this variant could bind migfilin robustly indicating that Ig21 binding site is intact and accessible to ligands (Figure S4 of supporting information). In contrast to filamin-Avar-1 Ig19-21, the WT Ig19-21 protein has peaks corresponding to Ig19 and Ig21 but a significant number of peaks did not overlap with those of free form Ig21, which is consistent with the auto-inhibition of Ig21 by Ig20 (Figure S5 of supporting information). Since Ig21 is auto-inhibited in filamin Ig19-21, we thought that a ligand should in principle occupy the free Ig19 and cause the chemical shift changes of the residues in Ig19. However, spectral analysis based on the published chemical shifts (20) demonstrates unequivocally that both Ig19 and Ig21 were perturbed by either β7 integrin (Fig 1, panel A, C) or migfilin (Fig 1, panel E, G). The shifts were strikingly similar to that induced in Ig21 (Fig 1, panel B, F) and Ig19 (Fig 1, panel D, H) solitary repeats. This finding not only demonstrates the two-site ligand binding on Ig19-21 but also indicates the relief of the auto-inhibition on Ig21. The latter has a significant implication in that filamin is likely to be in a stretched mode when engaged with integrin or migfilin (Fig 1, panel I). The integrin β7 which binds filamin weaker than migfilin leads to some line broadening (Figure S6A) and thus it is more notable to see the peak shifts of both Ig19 and Ig21 by migfilin (Figure S6B). More careful titration of migfilin into Ig19-21 from 4:1 to 0.05:1 peptide-protein ratios revealed definitive chemical shift changes to specific Ig19 and Ig21 residues (Fig 2). Importantly, at all concentrations Ig19 and Ig21 appeared to bind simultaneously (Fig 2). This is evident in the spectra where migfilin peptide was substantially less than Ig19-21, but both Ig19 and Ig21 experienced the chemical shift changes (Fig 2). Further confirmation that both Ig19 and Ig21 can engage ligands simultaneously was obtained using isothermal titration calorimetry (ITC). Using migfilin peptide as a ligand we were able to show the two site occupation of the Ig19-21 tri-repeat protein. The obtained data was fit to a two “identical site” binding model and the affinities of the two sites were 72.9±29.3μM (Fig 3). The data could also be fit to a 2 site sequential model with dissociation constants of 98±29μM and 112±25μM respectively. The close numbers make it clear that in this construct the occupation of Ig19 and Ig21 is almost simultaneous corroborating our NMR titration data (Fig 2). The combined facts argue for a dynamic equilibrium to toggle between the free and bound forms of Ig19-21 and the sequential binding (first to 19 followed by 21 or vice versa) model is not supported. Compared to the affinity of the isolated Ig21 to migfilin (2–8μM), the Ig19-21 repeat has weaker binding presumably due to the effect of auto-inhibition. Our attempts to measure affinity of isolated Ig19 with migfilin were unsuccessful though we see robust binding by NMR(13). The energetics of Ig19 and Ig21 binding are therefore very different and may be a result of their varying stabilities (21). Migfilin occupation of 21 in the Ig19-21 protein displaces Ig20 and the resulting solvent and protein rearrangements account for the differential affinities in the individual repeats compared to multiple repeats. The NMR and ITC thus demonstrate that Ig21 can be readily unmasked by integrin β7 CT or migfilin ligands. In a natural protein, Ig19 pocket if blocked by 18 will be refractory to binding. In conclusion our results provide strong evidence that the filamin auto-inhibition can be relieved by integrin or migfilin ligand itself and that filamin is engaged with the ligands via a multi-site binding mechanism. At biological level, the data implies that filamin is already stretched when engaged with integrin and migfilin and that extracellular matrix/actin traction is not necessary for relief of auto-inhibition. The lack of any overt phenotype in the migfilin knock out mice is surprising given its strong affinity to filamin (22). The function of migfilin may be developmentally compensated by other important filamin binding proteins that are yet to be discovered. The balancing between the stretched and auto-inhibited forms may allow the dynamic regulation of the actin-extracellular matrix linkage, thereby promoting dynamic cell adhesion processes such as cell shape change, cell migration and survival.
Figure 1.
HSQC spectral changes of selected Ig19-21 residues (A, C, E and G), free (Black) and bound (Red) to integrin β7 peptide and migfilin peptide (protein:peptide is 1:4). Panels B, D, F and H show the chemical shift changes of the same residues in Ig21 and Ig19 only proteins (protein:peptide ratio is 1:2). All the spectra were recorded at 600MHz at 30°C. Full spectra are shown in figure 6A and B of the supporting information.
Figure 2.
HSQC titration of migfilin with 15N labeled Ig19-21 shows that even at low peptide concentrations both Ig19 and Ig21 resonances are perturbed. The diagnostic G2267, G2270, S2279 and S2088 peak changes are highlighted. The protein concentration in all the experiments was 0.1mM and the migfilin peptide was 0.005mM (black, protein: peptide=20:1), 0.025mM (blue, protein: peptide=4:1) and 0.4mM (red, protein: peptide=1:4) respectively. Notice that the signal for Ig19 S2088 at protein:peptide=4:1 is broadened but appears at the protein:peptide=1:4 (red). Also note that the signals for G2267 and G2270 for protein-peptide=20:1 are located elsewhere whereas the assignment of G2267 and G2270 at protein:peptide=4:1 (blue) and 1:4 (red) in Ig19-21 are unambiguous because they overlay exactly with those in the Ig21 bound to migfilin.
Figure 3.
Isothermal calorimetric profile of migfilin binding to filamin A 19-21. Note that the energetics was endothermic while the isolated Ig21 binding was exothermic. One possible explanation for this is the large scale displacement of repeat 20 when Ig21 is occupied. The data fit to a model having 2 identical sites is shown. The data fitted well even with a 2 sites sequential model of binding with Kds of 98±29μM and 112±25μM.
Supplementary Material
Acknowledgments
We thank Jianmin Liu and Jun Yang for assistance in resonance assignments and technical insights into protein-protein interactions by nuclear magnetic resonance. The study was supported by NIH grants to J.Q.
Footnotes
Supporting Information Available
Details of the materials and methods, sequence comparison and additional spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hynes RO. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 2.Shattil SJ, Kim C, Ginsberg MH. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moser M, Legate KR, Zent R, Fässler R. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 4.Qin J, Vinogradova O, Plow EF. PLoS Biol. 2004;2:726–729. doi: 10.1371/journal.pbio.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiema T, Lad Y, Jiang P, Oxley CL, Baldassarre M, Wegener KL, Campbell ID, Ylänne J, Calderwood DA. Mol Cell. 2006;21:337–347. doi: 10.1016/j.molcel.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Calderwood DA, Huttenlocher A, Kiosses WB, Rose DM, Woodside DG, Schwartz MA, Ginsberg MH. Nat Cell Biol. 2001;3:1060–1068. doi: 10.1038/ncb1201-1060. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura F, Stossel TP, Hartwig JH. Cell Adh Migr. 2011;5:160–169. doi: 10.4161/cam.5.2.14401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ithychanda SS, Das M, Ma YQ, Ding K, Wang X, Gupta S, Wu C, Plow EF, Qin J. J Biol Chem. 2009;284:4713–4722. doi: 10.1074/jbc.M807719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nieves B, Jones CW, Ward R, Ohta Y, Reverte CG, LaFlamme SE. J Cell Sci. 2010;123:1216–1226. doi: 10.1242/jcs.056549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu Y, Wu S, Shi X, Chen K, Wu C. Cell. 2003;113:37–47. doi: 10.1016/s0092-8674(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 11.Lad Y, Jiang P, Ruskamo S, Harburger DS, Ylänne J, Campbell ID, Calderwood DA. J Biol Chem. 2008;283:35154–163. doi: 10.1074/jbc.M802592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Flier A, Sonnenberg A. Biochim Biophys Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- 13.Ithychanda SS, Hsu D, Li H, Yan L, Liu D, Das M, Plow EF, Qin J. J Biol Chem. 2009;284:35113–35121. doi: 10.1074/jbc.M109.060954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura F, Pudas R, Heikkinen O, Permi P, Kilpeläinen I, Munday AD, Hartwig JH, Stossel TP, Ylänne J. Blood. 2006;107:1925–1932. doi: 10.1182/blood-2005-10-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lad Y, Kiema T, Jiang P, Pentikäinen OT, Coles CH, Campbell ID, Calderwood DA, Ylänne J. EMBO J. 2007;26:3993–4004. doi: 10.1038/sj.emboj.7601827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heikkinen OK, Ruskamo S, Konarev PV, Svergun DI, Iivanainen T, Heikkinen SM, Permi P, Koskela H, Kilpeläinen I, Ylänne J. J Biol Chem. 2009;284:25450–25458. doi: 10.1074/jbc.M109.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pentikäinen U, Ylänne J. J Mol Biol. 2009;393:644–657. doi: 10.1016/j.jmb.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 18.van der Flier A, Kuikman I, Kramer D, Geerts D, Kreft M, Takafuta T, Shapiro SS, Sonnenberg A. J Cell Biol. 2002;156:361–376. doi: 10.1083/jcb.200103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis MA, van der Flier A, Kammerer RA, Mould AP, Sonnenberg A, Humphries MJ. FEBS Lett. 2004;569:185–190. doi: 10.1016/j.febslet.2004.04.099. [DOI] [PubMed] [Google Scholar]
- 20.Heikkinen O, Permi P, Koskela H, Ylänne J, Kilpeläinen I. Biomol NMR Assign. 2009;3:53–56. doi: 10.1007/s12104-008-9140-6. [DOI] [PubMed] [Google Scholar]
- 21.Jiang P, Campbell ID. Biochemistry. 2008;47:11055–11061. doi: 10.1021/bi8011466. [DOI] [PubMed] [Google Scholar]
- 22.Moik DV, Janbandhu VC, Fässler R. J Cell Sci. 2011;124:414–421. doi: 10.1242/jcs.075960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.