Abstract
Stem cell therapy is not a new field, as indicated by the success of hematopoietic stem cell reconstitution for various hematological malignancies and immune-mediated disorders. In the case of tissue repair, the major issue is whether stem cells should be implanted, regardless of the type and degree of injury. Mesenchymal stem cells have thus far shown evidence of safety, based on numerous clinical trials, particularly for immune-mediated disorders. The premise behind these trials is to regulate the stimulatory immune responses negatively. To apply stem cells for other disorders, such as acute injuries caused by insults from surgical trauma and myocardial infarction, would require other scientific considerations. This does not imply that such injuries are not accompanied by immune responses. Indeed, acute injuries could accompany infiltration of immune cells to the sites of injuries. The implantation of stem cells within a milieu of inflammation will establish an immediate crosstalk among the stem cells, microenvironmental molecules, and resident and infiltrating immune cells. The responses at the microenvironment of tissue injury could affect distant and nearby organs. This editorial argues that the microenvironment of any tissue injury is a key consideration for effective stem cell therapy.
Keywords: Stem cell therapy, Microenvironment, Mesenchymal stem cells, Immune responses
INTRODUCTION
The delivery of stem cells, regardless of their source, is expected to be within, or surrounding regions of tissue injuries. This editorial discusses the mechanisms by which stem cells could interact with different molecules at and within areas of tissue injury. For the purpose of this review, the area of injury and molecules found within the zones of injuries are referred as microenvironments. The sources of molecules with regions of tissue damage are varied. For example, cytokines can be produced by cells within and around the damaged tissue; neurotransmitters can be released from damaged and/or intact nerve fibers as well as from infiltrating immune cells. While the sources of molecules are varied, the types of molecules belong to different families. These include, but are not limited to, peptides, cytokines, and extracellular matrix proteins. Interactions between cells and soluble molecules are two-way processes. While the stem cells respond by producing other factors, these factors stimulate the stem cells, through autocrine and paracrine mechanisms, to induce further changes in the stem cells. The mechanisms of these interactions could be positive and/or negative to the injuries. The questions that are pertinent for stem cells therapy include the method by which stem cells should be implanted at the region of injury. Intuitively, one should consider if the implanted stem cells should be delivered to allow for modulation of the damaged microenvironment or vice versa. The answer to this key question will depend on the goal of the therapy. Another question is whether the aim of stem cell therapy is to attain protection that prevents further damage to the tissue or to replace damaged cells. This would require the stem cells to generate differentiated or specialized cells. In any event, the stem cells or specialized cells will establish crosstalk with cells and molecules within the microenvironment.
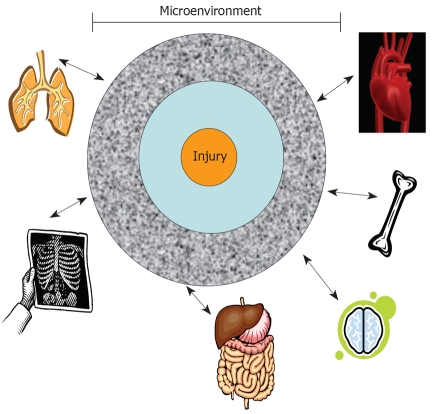
Future therapies will need to consider the degree of changes within the microenvironment because tissue injuries differ. Furthermore, the milieu would differ at zones away from the area of injury (Figure 1). If future research studies show that altered tissue microenvironments could attain effective therapy by stem cells, such information will be crucial to stem cell therapies. Adjuvant treatments will be required for drugs to achieve the desired changes. Drugs are available to target a variety of molecules and, when administered with stem cells, will enhance stem cell treatments. This editorial discusses general issues facing adult human mesenchymal stem cells (MSCs). These discussions could be extrapolated for any type of stem cell and for methods to repair damaged tissues.
Figure 1.
A representative focal point of tissue injury. This central point expands to zones of injury with each region showing varied degrees of tissue damage. The responses from the zones of injuries, through nerve fibers, soluble factors, or immune cells, could establish cross communications with other distant organs.
The immune properties of adult human MSCs have been well studied. A review of the list of clinical trials with these stem cells (clinicaltrials.gov) shows their global application for various diseases. Based on the current large numbers of trials with MSCs, it appears that these stem cells are safe for use in humans. However, a clear statement on their safety awaits longitudinal follow-up.
ALLOGENEIC VARIATIONS IN KEY CONSIDERATIONS FOR CHOICE OF STEM CELL TYPE
A major consideration in stem cell therapy is the availability of “off the shelf” sources of stem cells. In theory, all stem cells could be readily available for transplantation. The issue is whether particular “off the shelf” stem cells would be rejected by immunological reactions. While such rejections are expected for allogeneic stem cells, thus far, allogeneic MSCs seem to behave contrary to this dogma. It is important to discern the differences between the immune suppressive property of a stem cell and its ability to elicit an immune response. In the case of immune suppression, stem cells negatively regulate a reaction of immune stimulation. If a stem cell initiates an immune response from a host, that stem cell is perceived as foreign to the host. The latter response will be initiated by differences in the major histocompatibility class II and/or class I (MHC II or MHC I) molecules on the stem cells. On the other hand, although less studied, a stem cell can also act as an immune cell and initiate inflammatory responses by autologous immune cells.
Autologous stem cells, or stem cells from fraternal twins, are ideal cases for avoiding rejection of the transplanted stem cells. However, probability of obtaining stem cells from fraternal twins would be low. Furthermore, in cases where the fraternal twin exists, if there is an injury that requires immune transplantation of stem cells, it would be problematic to wait for expansion of the stem cells. Immediate needs for stem cells include repair or protection of brain injuries and cardiac infarct. It could be argued that some forms of brain injuries could wait for the inflammation to subside, thereby providing healthcare workers a few days for the use of autologous stem cells. There are two problems with this scenario; firstly, the stem cells might require weeks for expansion and characterization in a good manufacturing facility. Secondly, a particular organ is not mutually exclusive of the other; thus, injury to one organ could cause damage to another where the stem cells might be located (Figure 1). Thus, during injury, stem cells from a distant organ could be defective, thereby leaving autologous stem cells as the preferred source for therapy.
If organ-specific autologous stem cells are transiently damaged during injury, the time before these stem cells are ready for therapy would be significantly delayed. Additional studies are needed on the integrity of autologous stem cells at sites distant from injuries. Several reports show a brain-bone marrow connection, based on functional studies and anatomical evidence by nerve tracing analyses[1-3]. Furthermore, studies with surgical trauma patients reported dysfunctional bone marrow-derived hematopoietic stem cells (HSCs)[4]. The next section discusses the immune properties of MSCs and argues for investigational studies on the bidirectional communication between these stem cells and inflammatory mediators within tissue microenvironments. It is imperative to dissect the responses of MSCs at areas of tissue injuries, especially because these stem cells are in various trials for immune-mediated disorders[5].
MSCs
MSCs are ubiquitously expressed, with the adult bone marrow as the major source[6-9]. MSCs differentiate into specialized cells, for example stroma, osteoblasts, adipocytes and chrondrocytes, via distinct lineages[10]. Stroma and osteoblasts are key supporting cells for hematopoietic stem cells functions, and form functional links between the two major bone marrow resident stem cells: hematopoietic and MSCs[6,11,12]. MSCs and HSCs are located at distinct regions of human bone marrow[13]. Hematopoietic stem cells prefer areas of low oxygen, close to the endosteum, whereas MSCs surround the blood vessels where oxygen levels are relatively high [6,14,15].
Blood vessels and nerve fibers generally follow each other into bone marrow. Therefore nerve endings would be in close contact with MSCs surrounding the abluminal blood vessels of bone marrow[6]. In fact, the anatomical literature shows nerve fibers forming synapse-like structures with reticular type cells of bone marrow[8]. MSCs have been referred to as reticular cells[6], indicating that MSCs are in contact with the nerve endings. In other reports, MSCs, are referred to as pericytes and form contacts with neurons[16]. The nerve contact with MSCs could be significant, based on the identification of neurotransmitter receptors on MSCs[17,18].
MSCs show potential for clinical application with evidence of tissue regeneration[19-23]. These stem cells could overcome the major obstacle associated with those of allogeneic sources. MSCs show unique immune properties, underscoring their clinical use for preventing graft vs host disease (GVHD)[24,25]. MSCs exhibit a veto property, indicating that they could thwart GVDH as third party cells[26,28]. Interestingly, the veto function of MSCs is specific for GVDH-type reactions because similar suppression has not been observed in responses to recall antigens[28].
MSCs express genes for different cytokines and their receptors and act as antigen presenting cells (APCs), underscoring the immune plasticity of MSCs as immune suppressor and immune enhancer cells[29-31]. The APC property occurs within a narrow window, followed by its reversion to an immune suppressor cell[29]. This bimodal property of MSCs is important to prevent exacerbated inflammation. It is suggested that this dual role of MSCs is responsible for homeostasis in bone marrow and prevents exacerbated hematopoietic suppression during inflammation[32-34].
The mechanisms by which MSCs exert immune suppression are complex. These functions involve reactions ranging from the production of cytokines with immune suppressor functions to the stimulation of regulatory T-cells and suppression of T cytotoxic activity[5,30,35]. The expression of MHC-II, as well as the involvement of interferon-gamma (IFN-γ) in the immune function of MSCs, needs special attention[29,30]. A subset of MSCs expresses MHC-II, which is decreased as the stem cells become specialized cells. This has been demonstrated in studies where the MSCs, transdifferentiated to neurons, show a gradual decrease in MHC-II expression[36]. The addition of IFNγ to the MSC-derived neurons resulted in re-expression of MHC-II. This indicates that in future stem cell therapy, the repair of organs with replaced cells could re-express MHC-II. These patients will need to be followed for long-term tolerance. However, these findings underscore the need further investigation to determine methods of inducing anergy to the “foreign” MHC molecules.
PERSPECTIVE
Issues of allogeneic vs autologous stem cell delivery are key points when considering stem cell therapies. Another consideration for effective stem cell therapy is changes to stem cells by microenvironmental-induced responses by the implanted stem cells. Another major consideration is the possibility of tumor formation by the stem cells, even though the incidence of such an occurrence would be greater in embryonic stem cells. However, one cannot be certain that tumors would not be a problem for adult stem cells.
The maintenance of stem cells involves several genes, in particular those that are linked to cancer biology. During tissue injury, such as in traumatic brain injury, spinal cord injury, or myocardial infarction, the immune system will migrate towards the regions of insult. Once in the area, the immune cells will produce several soluble mediators, such as cytokines and chemokines. These two families of mediators can act locally through specific receptors on MSCs[37]. The resulting functions will depend on the concentrations of cytokines and the responses by the MSCs. The responses of the stem cells could be beneficial and assist in the repair process, or can be deleterious. The latter could occur if the reaction attracts additional immune cells to exacerbate inflammation, activates genes in the stem cells that can cause tumor formation, or activates genes in the stem cells to produce factors prematurely, which would be produced by specialized cells. On the other hand, exacerbated immune reactions could be protective. Thus, the biology of stem cells and the microenvironment of the area of tissue injury will determine the methods by which the therapies are developed.
The following are relevant questions when considering stem cell delivery within the context of varying microenvironments: (1) Should one type of stem cell serve as effective therapy for a particular type of tissue injury over another type? It is possible that one type of stem cell would be effective for a particular repair and another for a distinct injury; (2) Should the particular type of stem cell depend on the extent of tissue injury? That is, one type of stem cell might be better for acute injury and another for chronic injury; (3) Would the microenvironment influence the developmental stage at which stem cells are delivered? It is possible that the factors present would determine if effective repair could be caused by undifferentiated vs partly vs completely differentiated stem cells; and (4) Would translational science be more effective by partnership between academia and pharmaceuticals? The latter would have drugs readily available to combine with stem cells for therapies.
In addition to variations among regions of tissue injuries, the method by which the stem cells are delivered is also an issue. While bioengineering has been an intense area of investigation, other methods are also available. These types of questions would entail inter- and multi-disciplinary teams to deliver stem cells to the clinic. There are numerous clinical trials of stem cells; thus, it is time for the scientific community to determine if stem cells can be referred to as drugs. This will be finally determined by the pharmaceutical companies as medicine moves to include stem cell therapies, perhaps for all types of disorders.
Footnotes
Supported by F. M. Kirby Foundation
Peer reviewer: Anna Chiara Piscaglia, MD, Specialist in Gastroenterology and Digestive Endoscopy, GI & Liver Stem Cell Research Group (GILSteR), Department of Internal Medicine - Digestive Endoscopy Unit, Gemelli Hospital - Catholic University of Rome, Largo A. Gemelli, 8 - 00168 Rome, Italy
S- Editor Li LF L- Editor Stewart GJ E- Editor Lin YP
References
- 1.Kang HS, Trzaska KA, Corcoran K, Chang VT, Rameshwar P. Neurokinin receptors: relevance to the emerging immune system. Arch Immunol Ther Exp (Warsz) 2004;52:338–347. [PubMed] [Google Scholar]
- 2.Serobyan N, Jagannathan S, Orlovskaya I, Schraufstatter I, Skok M, Loring J, Khaldoyanidi S. The cholinergic system is involved in regulation of the development of the hematopoietic system. Life Sci. 2007;80:2352–2360. doi: 10.1016/j.lfs.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepherd AJ, Downing JE, Miyan JA. Without nerves, immunology remains incomplete -in vivo veritas. Immunology. 2005;116:145–163. doi: 10.1111/j.1365-2567.2005.02223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238:748–753. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 2008;36:733–741. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 7.Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–634. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 8.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 10.Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 11.Moore KA, Ema H, Lemischka IR. In vitro maintenance of highly purified, transplantable hematopoietic stem cells. Blood. 1997;89:4337–4347. [PubMed] [Google Scholar]
- 12.Müller-Sieburg CE, Deryugina E. The stromal cells' guide to the stem cell universe. Stem Cells. 1995;13:477–486. doi: 10.1002/stem.5530130505. [DOI] [PubMed] [Google Scholar]
- 13.Castillo M, Liu K, Bonilla L, Rameshwar P. The immune properties of mesenchymal stem cells. Int J Biomed Sci. 2007;3:100–104. [PMC free article] [PubMed] [Google Scholar]
- 14.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J. 2001;81:685–696. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh's model. Biophys J. 2001;81:675–684. doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelletier L, Angonin R, Regnard J, Fellmann D, Charbord P. Human bone marrow angiogenesis: in vitro modulation by substance P and neurokinin A. Br J Haematol. 2002;119:1083–1089. doi: 10.1046/j.1365-2141.2002.03969.x. [DOI] [PubMed] [Google Scholar]
- 17.Greco SJ, Corcoran KE, Cho KJ, Rameshwar P. Tachykinins in the emerging immune system: relevance to bone marrow homeostasis and maintenance of hematopoietic stem cells. Front Biosci. 2004;9:1782–1793. doi: 10.2741/1373. [DOI] [PubMed] [Google Scholar]
- 18.Greco SJ, Rameshwar P. Enhancing effect of IL-1alpha on neurogenesis from adult human mesenchymal stem cells: implication for inflammatory mediators in regenerative medicine. J Immunol. 2007;179:3342–3350. doi: 10.4049/jimmunol.179.5.3342. [DOI] [PubMed] [Google Scholar]
- 19.Gojo S, Umezawa A. Plasticity of mesenchymal stem cells--regenerative medicine for diseased hearts. Hum Cell. 2003;16:23–30. doi: 10.1111/j.1749-0774.2003.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 20.Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003;8:467–474. doi: 10.1016/s1525-0016(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 21.Gerson SL. Mesenchymal stem cells: no longer second class marrow citizens. Nat Med. 1999;5:262–264. doi: 10.1038/6470. [DOI] [PubMed] [Google Scholar]
- 22.Heinrich AC, Patel SA, Reddy BY, Milton R, Rameshwar P. Multi- and inter-disciplinary science in personalized delivery of stem cells for tissue repair. Curr Stem Cell Res Ther. 2009;4:16–22. doi: 10.2174/157488809787169075. [DOI] [PubMed] [Google Scholar]
- 23.Huang NF, Li S. Mesenchymal stem cells for vascular regeneration. Regen Med. 2008;3:877–892. doi: 10.2217/17460751.3.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devine SM, Bartholomew AM, Mahmud N, Nelson M, Patil S, Hardy W, Sturgeon C, Hewett T, Chung T, Stock W, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244–255. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 25.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 26.von Bonin M, Stölzel F, Goedecke A, Richter K, Wuschek N, Hölig K, Platzbecker U, Illmer T, Schaich M, Schetelig J, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2009;43:245–251. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Sun K, Welniak LA, Murphy WJ. Immunomodulation and pharmacological strategies in the treatment of graft-versus-host disease. Expert Opin Pharmacother. 2008;9:2305–2316. doi: 10.1517/14656566.9.13.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 29.Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, Rameshwar P. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-gamma. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romieu-Mourez R, François M, Boivin MN, Stagg J, Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007;179:1549–1558. doi: 10.4049/jimmunol.179.3.1549. [DOI] [PubMed] [Google Scholar]
- 31.Silva WA Jr, Covas DT, Panepucci RA, Proto-Siqueira R, Siufi JL, Zanette DL, Santos AR, Zago MA. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells. 2003;21:661–669. doi: 10.1634/stemcells.21-6-661. [DOI] [PubMed] [Google Scholar]
- 32.Kan O, Heyworth CM, Dexter TM, Maudsley PJ, Cook N, Vallance SJ, Whetton AD. Interferon-gamma stimulates the survival and influences the development of bipotential granulocyte-macrophage colony-forming cells. Blood. 1991;78:2588–2594. [PubMed] [Google Scholar]
- 33.Young NS, Maciejewski JP, Sloand E, Chen G, Zeng W, Risitano A, Miyazato A. The relationship of aplastic anemia and PNH. Int J Hematol. 2002;76 Suppl 2:168–172. doi: 10.1007/BF03165111. [DOI] [PubMed] [Google Scholar]
- 34.Zoumbos NC, Djeu JY, Young NS. Interferon is the suppressor of hematopoiesis generated by stimulated lymphocytes in vitro. J Immunol. 1984;133:769–774. [PubMed] [Google Scholar]
- 35.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 36.Castillo MD, Trzaska KA, Greco SJ, Ponzio NM, Rameshwar P. Immunostimulatory effects of mesenchymal stem cell-derived neurons: implications for stem cell therapy in allogeneic transplantations. Clin Transl Sci. 2008;1:27–34. doi: 10.1111/j.1752-8062.2008.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]