Abstract
Embryonic stem (ES) cells have the ability to differentiate into all germ layers, holding great promise not only for a model of early embryonic development but also for a robust cell source for cell-replacement therapies and for drug screening. Embryoid body (EB) formation from ES cells is a common method for producing different cell lineages for further applications. However, conventional techniques such as hanging drop or static suspension culture are either inherently incapable of large scale production or exhibit limited control over cell aggregation during EB formation and subsequent EB aggregation. For standardized mass EB production, a well defined scale-up platform is necessary. Recently, novel scenario methods of EB formation in hydrodynamic conditions created by bioreactor culture systems using stirred suspension systems (spinner flasks), rotating cell culture system and rotary orbital culture have allowed large-scale EB formation. Their use allows for continuous monitoring and control of the physical and chemical environment which is difficult to achieve by traditional methods. This review summarizes the current state of production of EBs derived from pluripotent cells in various culture systems. Furthermore, an overview of high quality EB formation strategies coupled with systems for in vitro differentiation into various cell types to be applied in cell replacement therapy is provided in this review. Recently, new insights in induced pluripotent stem (iPS) cell technology showed that differentiation and lineage commitment are not irreversible processes and this has opened new avenues in stem cell research. These cells are equivalent to ES cells in terms of both self-renewal and differentiation capacity. Hence, culture systems for expansion and differentiation of iPS cells can also apply methodologies developed with ES cells, although direct evidence of their use for iPS cells is still limited.
Keywords: Embryoid body, Embryonic stem cells, Induced pluripotent stem cells, Bioreactors, Differentiation
INTRODUCTION
Embryonic stem (ES) cells are capable of unlimited self-renewal in vitro and differentiate into cells constituting all three somatic germ layers. ES cells were first isolated from the inner cell mass of mouse blastocyst stage embryos[1,2], subsequently, followed by the derivation of non-human primate and human ES cell lines[3,4]. Currently, an alternative method has derived pluripotent cells by retroviral transduction of a combination of four transcription factors, Oct4, Sox2, C-myc and Klf4 into somatic cells; known as “induced pluripotent stem (iPS) cells”[5,6]. These cells are equivalent with ES cells in terms of both self-renewal and differentiation capacity[7,8]. The unique ability of pluripotent cells to generate a vast range of different cells makes both ES and iPS cells suitable for various cell transplantation, tissue engineering and drug testing applications. Efficient and controlled means of directing ES or iPS cell differentiation is crucial for the development of cell replacement therapies[9,10].
To realise the therapeutic potential of ES cells, it is essential to regulate their differentiation in a reproducible manner. Differentiation of ES cells is performed in two main ways; either by direct differentiation from pluripotent cells or through the formation of cell aggregates in non-adherent spheroids, called embryoid bodies (EBs)[11,12]. The molecular and cellular morphogenic signals and events within EBs recapitulate numerous aspects of the embryo development and result in differentiation to cells of three embryonic germ layers (endoderm, mesoderm, and ectoderm lineages), similar to gastrulation of an epiblast-stage embryo in vivo[13]. The precise number and spatial coordination of the various cell-cell interactions involved in EB formation are considered to influence the course of ES cell differentiation and, as a result, the control of cell number, size of EBs and quality of EB formation are important step directed differentiation strategies[14,15].
Methods of inducing EB formation are based on preventing ES cells from attaching to the surfaces of culture vessels, thus allowing the suspended ES cells to aggregate and form EBs. Standard methods of achieving EBs are via hanging drop and in static suspension culture to allow small scale formation of aggregates. These culture systems maintain a balance between ES cell aggregation essential for EB formation and prevention of EB agglomeration[16]. Even though hanging drop method is commonly used to prepare uniform-sized EBs (see details below), this method has disadvantages in the mass preparation of EBs due to its labor-intensive procedure, which hinders the use of differentiated ES cells for therapeutic application[17]. Mass EB production is easier from static suspension culture in which ES cells are suspended in a static Petri-dish. One drawback of this method, however, is that the EBs often fuse together to form large aggregates. This has negative effects on cell proliferation and differentiation, as well as causing extensive cell death. Hence, these methods are restricted as far as industrial applications are concerned because of their complication and difficult manageability[18].
Recently, novel bioreactors for large-scale production of ES-derived cells have been developed. A bioreactor is often defined as a device in which biological processes (cell expansion, differentiation or tissue formation on biomaterial scaffolds) occur in a tightly controlled environment in vitro, including the exchange of oxygen, nutrients and metabolites[19]. There are several types of bioreactors. For example, stirred suspension cultures (spinner flasks) have been successfully employed in some studies of mass scale production of ES-derived cells[20,21]. Conventional stirrer vessels may have the disadvantage of generating shear forces and, although manageable, these forces still can damage the cells[22]. Another bioreactor that allows agglomeration-free EB formation is the rotating cell culture system (RCCS) developed by the US National Aeronautics and Space Administration (NASA). This system is characterized by EB immobility in space, due to an extremely low fluid shear stress and oxygenation by diffusion[23]. EBs produced by bioreactors were more uniform in size and had less necrotic centers in comparison to static suspension culture. Furthermore, bioreactors can be also used for culturing iPS cells, which is expected to become a main further application of mass EB production in the near future. This review is focused on EB production in different systems, provides data on a number of existing bioreactors in comparison to conventional methods (hanging drop and static suspension culture) and describes differentiation of end-product EBs towards specific lineages.
METHODS FOR CULTURING EMBRYOID BODIES
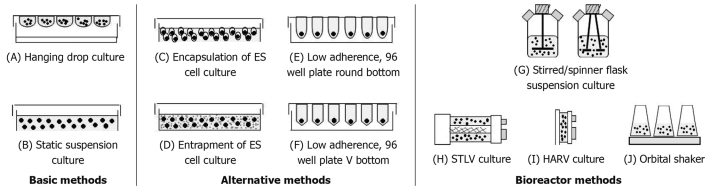
At the present time, no universally accepted standard exists for measurement of EB formation although characteristics such as EB size, shape, homogeneity and the quality of EB formation, ratio of apoptotic and viable cell are typically used as benchmarks for evaluation[24]. There are several methods to generate EB formation, as schematically shown in Figure 1. A summary of all of the important methods (described below) is presented in Table 1. Traditionally the most common EB culture methods, such as hanging drop method and static suspension culture, were used for inducing differentiation.
Figure 1.
Schematic representation for vessels used in methods to form EBs from ES cells.
Table 1.
Overview of current in vitro cell culture systems for production of EBs and other cell types
| Cell culture methods |
Benefit |
Detriment |
Propose | Yield | Note | Ref. | ||||||||||
| a | b | c | d | e | f | g | h | i | j | k | l | |||||
| 1 Hanging drop method | x | x | x | x | Differentiation into three germ layers | ND | Using mES cells | [26] | ||||||||
| 2 Static suspension culture | x | x | Differentiation into three germ layers and neural lineage | ND | Using mES cells | [30,31] | ||||||||||
| 3 Entrapment of ES cells (methylcellulose) | x | x | x | x | Differentiation into hematopoietic lineage | ND | Using mES cells | [26] | ||||||||
| 4 Multiwell/ microfabrication | ||||||||||||||||
| 4.1 Round bottomed, low attachment, 96 well plate | x | x | x | Differentiation into cardiac and neural lineage | 94% of wells have a single EB with diameter of 415 microns | Using polyvinyl carbonate PCR plate without coating reagents | [37] | |||||||||
| 4.2 Low adherence, 96 well plate coated with MPC or CS | x | x | x | Differentiation into cardiac lineage | EB formed MPC and CS was increased cardiac differentiation | Using mES cells | [38] | |||||||||
| 4.3 Round bottomed, low attachment, 96 well plate | x | x | x | Differentiation into hematopoietic lineage | Single EBs were achieved from PC surface but not from PS surface | Comparison of EB formation derived various type of 96 well plate; PS and PS coated with MPC | [39] | |||||||||
| 4.4 Round bottomed, low attachment, 96 well plate polyvinyl carbonate PCR plate | x | x | x | Differentiation into cardiac lineage | Single EB achieved from PS coated with MPC was near 100% | Comparison of EB formation derived various type of 96 well plate; PS and PS coated with MPC | [40] | |||||||||
| 4.5 Round bottomed, low attachment, 96 well plate | x | x | x | Differentiation into hematopoietic lineage | Differentiation was achieved with blood cells formed in 90% of EBs | Force aggregation by using centrifugation; Using hES cells | [41] | |||||||||
| 4.6 V bottomed, 96 well plate | x | x | x | Differentiation into cardiac lineage | > 90% EB formation was achieved from this method | Force aggregation by using centrifugation; Using hES cells | [43] | |||||||||
| 5 Bioreactor | ||||||||||||||||
| 5.1 A 2-L controlled spinner flask | x | x | x | x | x | x | x | Differentiation into cardiac lineage | 4.6 × 109 of cardiomyocytes were produced in a single run | Using MHC-neo ES cells | [53] | |||||
| 5.2 Stirred | x | x | x | x | x | x | x | x | Expansion and differentiation into three germ layers | ES cells went through 13 passages over the same 28 d exhibiting higher pluripotency | Comparison of stirred and static suspension culture | [48] | ||||
| 5.3 Stirred | x | x | x | x | x | x | x | Differentiation into vascular lineage | ND | ND | [28] | |||||
| 5.4 Stirred | x | x | x | x | x | x | x | x | Expansion and differentiation into neural lineage | 10 fold increase towards neural differentiation | Using hEC cells | [54] | ||||
| 5.5 Stirred | x | x | x | x | x | x | x | x | Expansion and differentiation into osteogenic lineage | 10 fold of calcium per total grams of protein increase over the control culture | Comparison of stirred and static suspension culture; Transplantation | [75] | ||||
| 5.6 Stirred | x | x | x | x | x | x | x | Differentiation into hepatic lineage | No significant difference in the specific albumin productivity of EB derived from different groups | Comparison of stirred suspension culture and hanging drop | [81] | |||||
| 5.7 Stirred + encapsulation (HA and dextran) | x | x | x | x | x | x | x | x | Expansion and differentiation into three germ layers | Dextran can induce EB formation from ES cells | Using mES cells | [31] | ||||
| 5.8 Stirred + encapsulation (agarose) + perfusion | x | x | x | x | x | x | x | Differentiation into cardiac lineage | The cardiomyocytes production in encapsulated culture was higher than without encapsulation | Using MHC-neo ES cells; Comparison of O2 tension | [72] | |||||
| 5.9 Two type of stirred, STLV and static suspension culture | x | x | x | x | x | x | Differentiation into cardiac lineage | EB formed GBI resulted in high EB yield with homogenous in size | Comparison of hydrodynamic condition (shear force) | [65] | ||||||
| 5.10 RCCS (STLV and HARV) | x | x | x | x | x | x | Differentiation into three germ layers | 3 fold enhancement in generation of EBs compared to static culture | Comparison of different type of bioreactors and suspension culture; Using hES cells | [23] | ||||||
| 5.11 STLV | x | x | x | x | x | x | Differentiation into cardiac lineage | > 90% of the NTEBs generated beating area | Comparison of STLV and static suspension | [62] | ||||||
| 5.12 HARV+ encapsulation (alginate) | x | x | x | x | x | x | Differentiation into osteogenic lineage | ND | Using mES cells | [70] | ||||||
| 5.13 HARV+ encapsulation (alginate) + biograss | x | x | x | x | x | x | Differentiation into osteogenic lineage | ND | Using 70s bioglass | [71] | ||||||
| 5.14 Rotary suspension culture using an orbital rotary shaker | x | x | x | x | x | x | Differentiation into three germ layers | 20-fold enhancement in the number of cells incorporated into primitive EBs in rotary vs static conditions was detected in the first 12 h | Comparison of rotation, static suspension and hanging drop | [64] | ||||||
| 5.15 Orbital shaker + microsphere fibrification | x | x | x | x | x | x | x | Differentiation into three germ layers | Degradable PLGA microspheres releasing RA were incorporated within EBs and induced cystic formation earlier than in non microspheres | Degradable PLGA microspheres releasing RA were incorporated within EBs and induced cystic formation | [68] | |||||
| 5.16 Perfused and dialyzed STLV | x | x | x | x | x | x | Differentiation into neural lineage | Perfused STLV can decrease in expression of markers of undifferentiated stage and increase in expression of markers of differentiation, specifically focusing on the neural lineage | Comparison of perfused and dialyzed STLV, perfused STLV, non-perfused STLV and suspension culture | [73] | ||||||
a: Homogeneity of EB; b: Scalable production of EB; c: Controlled monitoring; d: Integrated single step of culture (expansion and differentiation); e: Easy to manage; f: Flexible culture cells; g: Heterogeneity of EB; h: Small scale production of EB; i: Labor-intensive procedure; j: Difficult to manage; k: Requires a lot of medium; l: Shear force; ND: No available data; EB: Embryoid body; MPC: Methacryloyloxyethyl phosphorylcholine; ES: Embryonic stem; hES: Human embryonic stem cells; mES: Mouse embryonic stem cells; PCR: Polymerase chain reaction; MPC plate: 96-well polystyrene plate coated with 2-methacryloyloxyethyl phosphorylcholine; PS plate: Polystyrene plate; CS plate: A polystyrene plate coated with a type of glycosaminoglycan; HD: Hanging drop; hEC: Human embryonic carcinoma stem cells; MHC-neo: Myosin heavy chain-neomycin resistance; O2: Oxygen; RCCS: Rotating cell culture system; HARV: A high aspect rotating vessel; STLV: A slow turning lateral vessel; NTEB: EB derived from nuclear transfer ES; PLGA: Poly(lactic-co-glycolic acid)/poly (L-lactic acid).
Hanging drop method
The hanging drop method (Figure 1A) provides uniform sizes of EBs by dispensing equal numbers of ES cells in physically separated droplets of media suspended from the lid of a Petri-dish. This method offers a similar environment for forming individual EBs within each drop via gravity-induced aggregation of the cells. For this reason, this technique has been used to generate plentiful cell types such as neuronal cells[25], hematopoietic cells[26], cardiomyocytes[27], vascular cells[28] and chondrocytes[29]. The hanging drop method is tremendously useful for appraisal of molecular mechanisms occurring in early embryogenesis in any cell type. However, this technique is mainly used for research purposes and is not suitable for large scale of EB production because of its laborious nature; a typical 100-mm Petri dish can contain no more than 100 drops and each drop usually creates only one EB[21]. Further limitations of this method include major difficulties in exchanging or manipulating the small volume of medium (less than 50 μL which can evaporate easily) without disturbing the EBs. Usually the hanging drop method is composed of two steps; the aggregation of ES cells in drops and maturation of aggregates to EBs in suspension culture using low adherence bacterial Petri-dishes. Several elements of the method may be troublesome such as losses of EBs during picking up the formed EBs by pipette and attachment of premature EBs on Petri-dishes[17].
Static suspension culture
Static suspension culture (Figure 1B) is used to produce a large number of EBs by simply inoculating a suspension of ES cells onto a bacteriological grade Petri-dish, ultra-low adherence plate or a Petri-dish coated with cell adhesion inhibitor such as poly 2-hydroxyethyl methacrylate (poly 2-HEMA), allowing the cells to spontaneously aggregate into spheroids[30]. Although simple, this method allows little control over the size and shape of EBs. The result is frequent agglomeration of EBs into large, irregular masses because of the probability that ES cells encounter each other accidentally[26]. An additional limitation of this technique is that EBs may prematurely attach to the plate because of the surface chemistry of the culture vessel, leading to a greater heterogeneity and loss of EBs from the suspension culture. On another hand, this method is popular for some applications such as differentiation of ES cells into the neuronal lineage[31,32].
Encapsulation/entrapment
Encapsulation/entrapment of a single cell suspension or small clusters of ES cells in hydrogels (Figure 1C and D, respectively), such as methylcellulose[26], fibrin[33], hyaluronic acid, dextran[34], alginate[35], or agarose[36] represents a transition between hanging drop and static suspension approaches by generating individually separated EBs in a semi-solid suspension media. Entrapment of ES cells in methylcellulose, a temperature sensitive hydrogel, improves the overall synchrony and reproducibility of EB differentiation as it produces EBs of clonal origin. However, the efficiency of EB formation from individual ES cells can be rather low. In addition, soluble factor treatments and retrieval of differentiated cells may be complicated by the presence of the hydrogel material[26]. Interestingly, this method showed the possibility of designing a single cell culture system that would mimic the early developmental milieu and allow ES cells to switch between differentiation states within the same culture setting. When human ES (hES) cells are encapsulated in a 3D hyaluronic acid hydrogel, the hES cells can be maintained in an undifferentiated state. On the other hand, when hES cells are encapsulated in a dextran hydrogel, the hES cells are induced to differentiate and form EBs. Different types of hydrogels, therefore, act as a unique microenviroment for maintaining ES cells in either undifferentiated or differentiating state[31].
Multiwell and microfabrication
As an alternative approach for EB formation and culture, multiwell (Figure 1E and F) and microfabrication technologies have also been developed recently. Round-bottomed 96-well plates coated with or without reagents[37]; 2-methacryloyloxyethyl phosphorylcholine (MPC)[38-40], glycosaminoglycan (CS)[24] and poly 2-hydroxyethyl methacrylate (poly 2-HEMA), have been utilized to prevent cell adhesion to the plastic surfaces. This technique is among the tools for forming EBs with high uniformity similar to the hanging drop method as a defined number of ES cells is seeded in the separated wells. In contrast to the hanging drop method, this technique has no requirement to exchange or manipulate the medium (approximately 200 μL) and it is easier to observe directly the EB formation with a microscope during cultivation. Because of these advantages, this technique may be used instead of hanging drop method for laboratory research. The forced aggregation system, involving centrifugation of ES cells within round-bottomed (U-shaped)[41,42] and triangle-bottomed (V-shaped) 96-well plates[43], can induce aggregation more rapidly than hanging drops. This procedure improves the reproducibility of EB production. On the other hand, it still requires individual processing and manipulation of the resulting EBs due to the requirement of one more additional plating step. Microwells fabricated by lithographic methods yield EBs in an equivalent or at a much higher density than other methods and allow preparation of size-controlled EBs in a scalable manner for reproducible of EB formation[44]. Likewise, batches of EBs can be formed in microfluidic chambers and separated from the flowing culture medium by a semi-permeable membrane, allowing for temporal control of the molecular makeup of the medium. The cell patterning method is also useful for high-throughput screening assays, such as the exploration of biochemical agents to direct aggregate-induced differentiation into a specific lineage without plating EBs[45].
Bioreactors
Stem cell-based technologies and tissue engineering possibly permit a wide span of clinical and biotechnology applications in future. Nevertheless, realization of the potential of stem cells will require their large-scale generation in a robust system without any limitation[46]. This highlights the requirement for the in vitro expansion of stem cells used for therapy prior to their commitment into tissue-specific applications. The potential of bioreactors to address this is demonstrated by their capacity to support a robust and well defined scale-up platform for expansion of ES cells[47], EB formation[48,49] as well as differentiation[50]. The scaling up of the design, given mass transfer limitations, will depend on the type of bioreactor chosen[51]. The theory of selecting bioreactors for stem cell expansion and differentiation beyond bench scale is largely reliant on whether the cells are adherent, suspension grown as single cells or aggregates for EB formation[52]. Therefore, bioreactor culture systems must be designed according to the application. In addition, bioreactors have a significant advantage over static suspension culture which are as follows: (1) scale up of expansion and differentiation of ES cells; (2) no labor-intensive requirements; (3) no space requirement for available area of ES cell growth; and (4) the ability to monitor and control critical culture parameters (i.e. pH, dissolved oxygen, glucose consumption, and lactic acid production)[53]. At the present time, EB formation in hydrodynamic conditions has been achieved by using bioreactors. They comprise (1) spinner flasks; (2) RCCS; (3) rotary orbital culture; and (4) complex methods combining these techniques. All of these techniques generally improve ES cell aggregation and form EB faster and more homogeneously in size compared to typical static suspension cultures.
Spinner flasks: Spinner flasks (Figure 1G) have been pioneered, as promising in vitro systems for stem cell expansion, EB cultivation and differentiation of ES/iPS cells into specific cell types[54]. Spinner flasks provide attractive benefits due to their simple design, scalable configuration, the flexible culture of cells as aggregates on microcarriers[55] or scaffolds[56], and ease of continuous monitoring for tight regulation of the culture environment (e.g. O2 tension, pH, shear forces, medium exchange rate)[57]. The simpler process in spinner flasks equipped with paddle-impellers results in the formation of large ES cells agglomerates within a few days[58]. The scaling-up is generally straightforward because of improved mass transport achieved by stirring. However, the flow environment created by the impeller renders them inappropriate, due to the shear stress[59]. Numerous culture parameters for this system have been optimized, including the agitation rate, cell initial concentration, medium compositions, and different culturing approaches have been developed. In addition, a low rate of paddle-impeller stirring results in cell clumping in aggregation supporting EB cultures (leading to lower mass transport to the cells), while high rates of paddle-impeller stirring can be harmful for the cells. Consequently, an optimal fluid velocity promoting the suitable shear stress for the cell type being cultured is critical[60].
RCCS: Cells in conventional stirrer vessels are exposed to hydrodynamic shear stress resulting in damage to the cells. Another approach for controlling EB agglomeration employs RCCS which is comprised of a slow turning lateral vessel (STLV) (Figure 1H) and a high aspect rotating vessel (HARV) (Figure 1I), as a milder bioreactor. The advantages of these bioreactors are as follows: (1) horizontal rotation is characterized by extremely low fluid shear stress; (2) fluid-filled culture vessels are equipped with membrane diffusion gas exchange to optimize oxygen levels; and (3) membrane area to volume of medium ratio is high, thus enabling efficient gas exchange[61]. The type of rotating vessel had significant impacted on the process of hEB formation and agglomeration; hEBs formed small aggregates with no necrotic centers in STLV. Conversely, hEBs of extensive cell aggregation with large necrotic centers are formed in HARV[23]. STLV rotating bioreactors were used for cultivating mouse ES (mES) and hES cells to produce EBs and to compare both the quality and quantity of EBs with those from static suspension culture. ES cells grown in a STLV bioreactor were of higher quality and yielded a nearly 4-fold increase in the number of EB particles. EBs derived from a STLV bioreactor showed enhanced cardiac differentiation in comparison to static suspension culture[62].
Rotary orbital culture: Bioreactors may offer a more uniform differentiation environment capable of sustaining increased EB and differentiated cell yield. However, these methods may not be suitable solutions for assessing multiple experimental samples in parallel because of the requirement for larger-volume bioreactors. Orbital rotary shakers (Figure 1J) have been used to produce EBs as the constant circular motion provided by this simple system is good for improving the efficiency of EB formation[63]. The advantages of this technique include accommodation of cell culture dishes on the rotary platform, easily allowing production of numerous parallel samples and allowing comparison of different experimental parameters. EBs formed by using orbital rotary shakers appeared to differentiate more efficiently than those produced in static suspension culture on the basis of morphological appearance and gene expression profile patterns. A 20-fold enhancement in the number of cells incorporated into primitive EBs in rotary versus static conditions was detected after the first 12 h, and a fourfold increase in total cell yield was achieved by rotary culture after 7 d[64].
Complex methods combining these techniques: Recently, complex methods combining the above mentioned techniques have been adopted for solving the problems of these methods and keeping cells floating continuously in the culture medium. For example, the agglomeration of cells was avoided by keeping EBs in Petri-dishes for several days before transferring them into a different kind of environment; (1) spinner flasks; (2) a rotation culture system of Petri-dishes which were rotated on a horizontal rotation device; (3) rotary suspension culture in dishes on an orbital rotary shaker; (4) direct seeding ES cells into a spinner flask equipped with a glass ball bulb-shaped impeller or (5) two litres Stirred Tank bioreactor (STR) equipped with a newly developed pitched-blade turbine impeller[65].
In other cases, the encapsulation of ES cells was combined with transferring them into a bioreactor. For example, encapsulation of ES cells in defined conditions (i.e. number of cells per EB and capsule size); alginate[35], agarose[66], poly (lactic-co-glycolic acid)/poly (L-lactic acid) microsphere[67,68], hyaluronic acid[31] and Matrigel[69] was used to control agglomeration of cells. Then, after the initial period of EB formation, all encapsulated ES cells were transferred to a spinner flask. The encapsulation system allowed a 61-fold expansion in the number of cells, similar to the static control non-stirred culture but significantly higher than the stirred non-encapsulated system. Moreover, combination of the encapsulation of ES cells within alginate hydrogel, with or without 70s bioglass, followed by culturing cells in an HARV bioreactor directly enhanced both osteogenic differentiation in a functional test and generation of functional 3D mineralized constructs for further application of bone tissue engineering transplantation[70,71]. Finally, mES cells expanded as aggregates on microcarriers in stirred vessels retained expression of stem cell markers and could form EBs. Perfusion combined with frequent feeding has been shown to increase the expansion of ES cells and their differentiation into specific lineages, without compromising their stem cell performance[72]. Additionally, the effect of a rotary bioreactor promoted neural differentiation of hES cells in perfused and dialyzed STLV. The mean time delay for growing to so-called “neural rosette” formations was significantly shortened under STLV conditions compared to conventional static suspension culture. Likewise a perfused STLV bioreactor can decrease the expression of markers of undifferentiated stage and increase the expression of markers of differentiation, especially towards neural lineage commitments[73].
Recently, researchers have sought to develop culture systems with integrated bioprocesses, controlling stem cell expansion and differentiation tightly in a fully controlled bioreactor environment. For example, ten fold increase in expansion of ES cells as well as consequent neural differentiation was reported while drastically reducing, by 30%, the time required for the differentiation process[54]. Moreover, microcarrier spinner flasks have been used for the culture of mES and hES cell expansion and directed differentiation. Mouse ES cells were allowed to proliferate on microporous collagen-coated dextran beads (Cytodex 3), glass microcarriers, and macroporous gelatin-based beads (Cultispher S) in spinner flasks[74]. Under different inoculated cell densities and microcarrier concentrations, mES cells on microcarriers showed increased yield of approximate 70-fold (8 d) to about 190-fold (15 d). These cultured cells also successfully expressed Oct4, Nanog, and SSEA-1, and when dissociated from the beads, they formed EBs yielding cells with differentiation markers such as Flk-1, CD34 and α-MHC (mesoderm), HNF-3b19 (endoderm), and b3-tubulin57 (ectoderm)[60].
Computer-controlled bioreactors
As mentioned before, the main advantage of computer-controlled bioreactors is process development by allowing online monitoring and control of specific culture parameters (temperature, pH, PO2, lactic acid production and glucose consumption), and ensuring a fully controlled environment for stem cell cultivation[18]. Oxygen-controlled bioreactors have been used for culturing mES and hES cell-derived cardiomyocytes. These experiments also assessed the effect of oxygen tension on cardiac differentiation which is a main concern[72]. Moreover, this system was recently applied to culturing cells not only for stem cell expansion but also for differentiation. Expansion of a variety of stem cell types in bioreactors under defined and controlled conditions remains to be addressed. Future challenges also include the combination of expansion and directed differentiation steps in an integrated bioprocess that will ultimately result in scale-up of well differentiated cells to clinically relevant numbers.
It is worth mentioning that although differentiating cells in bioreactors have numerous benefits, these cells have been assessed for functionality by transplantation, and did not always perform well. Ten and twenty days post-implantation ES cells derived chondrogenic and osteogenic bioreactor aggregates showed no obvious influence on the healing process. In these experiments, all of the bioreactor derived cells showed higher Oct4 expression in the aggregates, even after 30 d of induced differentiation in a medium without LIF[75]. This emphasizes the importance of proper condition set-up and timing during cultivation of cells in bioreactors.
EMBRYONIC STEM CELL DIFFERENTIATION TO CARDIOMYOCYTES USING BIOREACTOR
Regenerative medicine based on cell transplantation therapies has attracted increasing attention as a potential alternative to organ transplantation[76]. Pluripotent stem cells (ES/iPS cells), because of their pluripotency and unlimited self-renewal capacity are promising cell sources to provide sufficient number of cells for therapeutic applications. However, the expansion and differentiation of these cells is still limited as a result of their complexity and difficult manageability in scale-up production for industrial purposes[77,78]. To solve these problems, bioreactor culture systems offer attractive advantages of ready scalability and relative simplicity[79,80].
Recently, a single-step bioprocess for ES cell-derived cardiomyocyte production have been developed by combining methods to prevent ES cell aggregation (hydrogel encapsulation) and to purify for cardiomyocytes from the heterogeneous cell populations by using genetic selection (myosin heavy chain-neomycin resistance; MHC-neo), with medium perfusion in a controlled bioreactor environment. It has been shown that the cardiomyocyte yield per input ES cells achieved in encapsulated culture was much higher than without encapsulation (3.17 ± 0.90 vs 0.16 ± 0.07). Furthermore, higher cardiomyocyte yield was achieved under hypoxic conditions (4% oxygen tension) versus normoxia conditions (20% oxygen tension), when cultured in the stirred culture system[72]. In addition, a 2-L bioreactor process enabling the controlled generation of EBs, derived from MHC-neo ES cell line, has been adopted for enhancing yield of ES-derived cardiomyocyte production. The fill-and-draw feeding protocol was replaced in a 2-L bioreactor, which allowed constant medium supply and avoided daily fluctuations of medium components. An optimized protocol resulted in more than five times greater cardiomyocyte yield, whereas medium consumption was 40% less than that in the control system[53].
For the controlled large-scale generation for clinical and industrial applications in humans, the efficacy of the dynamic process [Erlenmeyer, STLV bioreactor, Glass Ball Impeller (GBI) spinner flask and Paddle-Impeller (PI) spinner flask] was compared to static suspension culture in Petri-dishes by analyzing the quality of EB formation and subsequent differentiation into cardiomyocytes. The EB prearrangement in the static system and EB cultivation in the GBI spinner flask resulted in high EB yield, a round homogenous shape, the fastest growth rate and high contracting EB percentages over all other systems[65].
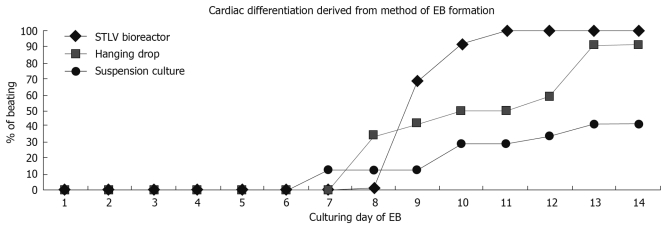
As noted above, cardiomyocytes derived from ES cells are anticipated to be valuable for cardiovascular drug testing and disease therapies. However, the overall efficiency and quantity of cardiomyocytes obtained by differentiation of ES cells is still low. Recently, to enable large-scale culture of ES-derived cells, we have tested a scalable bioprocess that allows direct EB formation in a well controlled STLV bioreactor system. Our laboratory has developed protocols of cardiomyocyte differentiation from mES cells by using STLV. We have optimized the initial ES cell seeding density into the bioreactor, the rotation speed and the day of transferring and plating of EBs on gelatin coated Petri-dishes. We have compared the quantity and quality of EB production, as well as the efficiency of cardiac differentiation of samples derived form STLV, static suspension culture and hanging drop method. We found that the optimized rotary suspension culture method can produce a highly uniform population of efficiently differentiating EBs in large quantities in a manner that can be easily implemented by basic research laboratories (Figure 2). Although EBs derived from STLV start rhythmically contracting later than static suspension culture and hanging drop method, they beat with nearly 100% efficacy (Figure 3). Furthermore, our results are similar to other reports of EBs formed in STLV which were more uniform in size, and contained mostly viable cells whilst lacking necrotic centers. Additionally, STLV-produced EBs differentiated into cardiomyocytes more efficiently than those from static suspension culture[62]. Hence, this method provides a technological platform for the controlled large-scale generation of ES-derived cells for clinical and industrial applications.
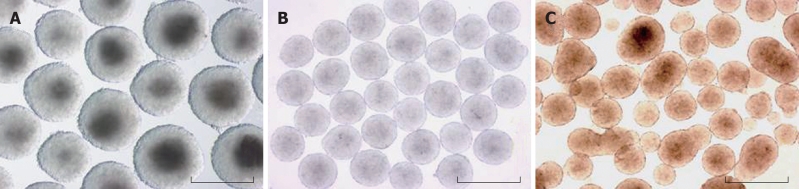
Figure 2.
Formation of EBs in various methods. Gross morphology of EBs derived from slow turning lateral vessel (STLV) (A), hanging drop (B) and suspension culture (C). Scale bars correspond to 500 μm.
Figure 3.
Illustration of the cumulative percentage of EBs containing contracting area derived from STLV, hanging drop and suspension culture.
CONCLUSION
Bioprocessing and commercialization of ES or iPS cells and tissue engineering products in cell replacement therapy have the potential to facilitate and transform breakthroughs from the research bench to the patient bedside. This is expected to be a long process, however, as there are many key practical issues to be addressed before moving ahead from the laboratory-scale fundamental research level. Laboratory-scale suspension cultures in hanging drops or Petri-dishes are useful tools for process development and initial optimization, and encapsulation/entrapment of ES cells, multiwell and microfabrication methods can improve high-throughput EB production. However, these approaches are not suitable for further therapeutic application because of their labor intensive, time consuming nature, culture-to-culture variability and lack of monitoring. Bioreactor culture systems address many of these problems and offer several advantages over the conventional use of basic culture methods for expanding and differentiating ES cells into specific lineages, without compromising their stem cell performance. Future challenges in bioreactor development will include the design of advanced and sophisticated monitoring platforms that allow monitoring at the cellular level of parameters including temperature, pH and oxygen levels. With respect to ES or iPS cells, we envision a scenario, where a complete bioprocess would exist in the bioreactor for the expansion and subsequent differentiation of the ES or iPS cells to generate the specialized cell type of interest. For example, the current achievements with cardiomyocytes derived from ES cells would be developed into cardiovascular grafts tissue engineering, with an emphasis on its possible clinical use in cardiovascular surgery. The engineering of a human cardiac tissue patch would be used to illustrate the biological requirements and engineering approaches for human applications. For future therapeutic application, the specialized cells differentiated from ES or iPS cells could then be used for cell therapies or combined with scaffolds to produce tissue construct and transplants for patients.
Footnotes
Supported by Grants from EU FP6 (“MEDRAT”- LSHG-CT-2005-518240; “CLONET”, MRTN-CT-2006-035468), EU FP7 (“PartnErS”, PIAP-GA-2008-218205; “InduHeart”, EU FP7-PEOPLE-IRG-2008-234390; “InduStem”, PIAP-GA-2008-230675; “Plurisys”, HEALTH-F4-2009-223485); NKFP_07_1-ES2HEART-HU, No. OM-00202-2007 and CHE-TRF senior scholarship, No. RTA 5080010. Rungarunlert S was supported by grant under the program Strategic Scholarships for Frontier Research Network for the Joint Ph.D., and Program Thai Doctoral degree from the Office of the Higher Education Commission, Thailand, No. CHE-PhD-SW-2005-100
Peer reviewer: Takashi Tada, Professor, Stem Cell Engineering, Institute for Frontier Medical Sciences, Kyoto University, 53 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan
S- Editor Li LF L- Editor Hughes D E- Editor Lin YP
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55:254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 8.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 9.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 10.Ulloa-Montoya F, Verfaillie CM, Hu WS. Culture systems for pluripotent stem cells. J Biosci Bioeng. 2005;100:12–27. doi: 10.1263/jbb.100.12. [DOI] [PubMed] [Google Scholar]
- 11.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 12.Höpfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mol Biol. 2004;254:79–98. doi: 10.1385/1-59259-741-6:079. [DOI] [PubMed] [Google Scholar]
- 13.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 14.Mohr JC, Zhang J, Azarin SM, Soerens AG, de Pablo JJ, Thomson JA, Lyons GE, Palecek SP, Kamp TJ. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials. 2009:Epub ahead of print. doi: 10.1016/j.biomaterials.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messana JM, Hwang NS, Coburn J, Elisseeff JH, Zhang Z. Size of the embryoid body influences chondrogenesis of mouse embryonic stem cells. J Tissue Eng Regen Med. 2008;2:499–506. doi: 10.1002/term.125. [DOI] [PubMed] [Google Scholar]
- 16.Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 17.Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- 18.Dang SM, Zandstra PW. Scalable production of embryonic stem cell-derived cells. Methods Mol Biol. 2005;290:353–364. doi: 10.1385/1-59259-838-2:353. [DOI] [PubMed] [Google Scholar]
- 19.Barron V, Lyons E, Stenson-Cox C, McHugh PE, Pandit A. Bioreactors for cardiovascular cell and tissue growth: a review. Ann Biomed Eng. 2003;31:1017–1030. doi: 10.1114/1.1603260. [DOI] [PubMed] [Google Scholar]
- 20.Kehoe DE, Lock LT, Parikh A, Tzanakakis ES. Propagation of embryonic stem cells in stirred suspension without serum. Biotechnol Prog. 2008;24:1342–1352. doi: 10.1002/btpr.57. [DOI] [PubMed] [Google Scholar]
- 21.Kehoe DE, Jing D, Lock LT, Tzanakakis EM. Scalable Stirred-suspension Bioreactor Culture of Human Pluripotent Stem Cells. Tissue Eng Part A. 2009:Epub ahead of print. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron CM, Hu WS, Kaufman DS. Improved development of human embryonic stem cell-derived embryoid bodies by stirred vessel cultivation. Biotechnol Bioeng. 2006;94:938–948. doi: 10.1002/bit.20919. [DOI] [PubMed] [Google Scholar]
- 23.Gerecht-Nir S, Cohen S, Itskovitz-Eldor J. Bioreactor cultivation enhances the efficiency of human embryoid body (hEB) formation and differentiation. Biotechnol Bioeng. 2004;86:493–502. doi: 10.1002/bit.20045. [DOI] [PubMed] [Google Scholar]
- 24.Koike M, Sakaki S, Amano Y, Kurosawa H. Characterization of embryoid bodies of mouse embryonic stem cells formed under various culture conditions and estimation of differentiation status of such bodies. J Biosci Bioeng. 2007;104:294–299. doi: 10.1263/jbb.104.294. [DOI] [PubMed] [Google Scholar]
- 25.He Z, Li JJ, Zhen CH, Feng LY, Ding XY. Effect of leukemia inhibitory factor on embryonic stem cell differentiation: implications for supporting neuronal differentiation. Acta Pharmacol Sin. 2006;27:80–90. doi: 10.1111/j.1745-7254.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- 26.Dang SM, Kyba M, Perlingeiro R, Daley GQ, Zandstra PW. Efficiency of embryoid body formation and hematopoietic development from embryonic stem cells in different culture systems. Biotechnol Bioeng. 2002;78:442–453. doi: 10.1002/bit.10220. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–1916. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 28.Evans AL, Bryant J, Skepper J, Smith SK, Print CG, Charnock-Jones DS. Vascular development in embryoid bodies: quantification of transgenic intervention and antiangiogenic treatment. Angiogenesis. 2007;10:217–226. doi: 10.1007/s10456-007-9076-y. [DOI] [PubMed] [Google Scholar]
- 29.Kramer J, Hegert C, Guan K, Wobus AM, Müller PK, Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev. 2000;92:193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 30.Choi D, Lee HJ, Jee S, Jin S, Koo SK, Paik SS, Jung SC, Hwang SY, Lee KS, Oh B. In vitro differentiation of mouse embryonic stem cells: enrichment of endodermal cells in the embryoid body. Stem Cells. 2005;23:817–827. doi: 10.1634/stemcells.2004-0262. [DOI] [PubMed] [Google Scholar]
- 31.Nonaka J, Yoshikawa M, Ouji Y, Matsuda R, Nishimura F, Yamada S, Nakase H, Moriya K, Nishiofuku M, Ishizaka S, et al. CoCl(2) inhibits neural differentiation of retinoic acid-treated embryoid bodies. J Biosci Bioeng. 2008;106:141–147. doi: 10.1263/jbb.106.141. [DOI] [PubMed] [Google Scholar]
- 32.Suslov ON, Kukekov VG, Ignatova TN, Steindler DA. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci USA. 2002;99:14506–14511. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Collins SF, Suggs LJ. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6004–6014. doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magyar JP, Nemir M, Ehler E, Suter N, Perriard JC, Eppenberger HM. Mass production of embryoid bodies in microbeads. Ann N Y Acad Sci. 2001;944:135–143. doi: 10.1111/j.1749-6632.2001.tb03828.x. [DOI] [PubMed] [Google Scholar]
- 36.Kurosawa H, Imamura T, Koike M, Sasaki K, Amano Y. A simple method for forming embryoid body from mouse embryonic stem cells. J Biosci Bioeng. 2003;96:409–411. doi: 10.1016/S1389-1723(03)90148-4. [DOI] [PubMed] [Google Scholar]
- 37.Ezekiel UR. Single embryoid body formation in a multi-well plate. Electron J Biotechnol. 2007;10:328–335. [Google Scholar]
- 38.Koike M, Kurosawa H, Amano Y. A Round-bottom 96-well Polystyrene Plate Coated with 2-methacryloyloxyethyl Phosphorylcholine as an Effective Tool for Embryoid Body Formation. Cytotechnology. 2005;47:3–10. doi: 10.1007/s10616-005-3743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konno T, Akita K, Kurita K, Ito Y. Formation of embryoid bodies by mouse embryonic stem cells on plastic surfaces. J Biosci Bioeng. 2005;100:88–93. doi: 10.1263/jbb.100.88. [DOI] [PubMed] [Google Scholar]
- 40.Koike M, Sakaki S, Amano Y, Kurosawa H. Characterization of embryoid bodies of mouse embryonic stem cells formed under various culture conditions and estimation of differentiation status of such bodies. J Biosci Bioeng. 2007;104:294–299. doi: 10.1263/jbb.104.294. [DOI] [PubMed] [Google Scholar]
- 41.Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 42.Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One. 2008;3:e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burridge PW, Anderson D, Priddle H, Barbadillo Muñoz MD, Chamberlain S, Allegrucci C, Young LE, Denning C. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25:929–938. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- 44.Moeller HC, Mian MK, Shrivastava S, Chung BG, Khademhosseini A. A microwell array system for stem cell culture. Biomaterials. 2008;29:752–763. doi: 10.1016/j.biomaterials.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sasaki D, Shimizu T, Masuda S, Kobayashi J, Itoga K, Tsuda Y, Yamashita JK, Yamato M, Okano T. Mass preparation of size-controlled mouse embryonic stem cell aggregates and induction of cardiac differentiation by cell patterning method. Biomaterials. 2009;30:4384–4389. doi: 10.1016/j.biomaterials.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Placzek MR, Chung IM, Macedo HM, Ismail S, Mortera Blanco T, Lim M, Cha JM, Fauzi I, Kang Y, Yeo DC, et al. Stem cell bioprocessing: fundamentals and principles. J R Soc Interface. 2009;6:209–232. doi: 10.1098/rsif.2008.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krawetz R, Taiani JT, Liu S, Meng G, Li X, Kallos MS, Rancourt D. Large-Scale Expansion of Pluripotent Human Embryonic Stem Cells in Stirred Suspension Bioreactors. Tissue Eng Part C Methods. 2009:Epub ahead of print. doi: 10.1089/ten.TEC.2009.0228. [DOI] [PubMed] [Google Scholar]
- 48.zur Nieden NI, Cormier JT, Rancourt DE, Kallos MS. Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors. J Biotechnol. 2007;129:421–432. doi: 10.1016/j.jbiotec.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 49.Cormier JT, zur Nieden NI, Rancourt DE, Kallos MS. Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 2006;12:3233–3245. doi: 10.1089/ten.2006.12.3233. [DOI] [PubMed] [Google Scholar]
- 50.Lock LT, Tzanakakis ES. Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng Part A. 2009;15:2051–2063. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pörtner R, Nagel-Heyer S, Goepfert C, Adamietz P, Meenen NM. Bioreactor design for tissue engineering. J Biosci Bioeng. 2005;100:235–245. doi: 10.1263/jbb.100.235. [DOI] [PubMed] [Google Scholar]
- 52.King JA, Miller WM. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol. 2007;11:394–398. doi: 10.1016/j.cbpa.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E, et al. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493–507. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 54.Serra M, Brito C, Costa EM, Sousa MF, Alves PM. Integrating human stem cell expansion and neuronal differentiation in bioreactors. BMC Biotechnol. 2009;9:82. doi: 10.1186/1472-6750-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abranches E, Bekman E, Henrique D, Cabral JM. Expansion of mouse embryonic stem cells on microcarriers. Biotechnol Bioeng. 2007;96:1211–1221. doi: 10.1002/bit.21191. [DOI] [PubMed] [Google Scholar]
- 56.Vunjak-Novakovic G, Radisic M. Cell seeding of polymer scaffolds. Methods Mol Biol. 2004;238:131–146. doi: 10.1385/1-59259-428-x:131. [DOI] [PubMed] [Google Scholar]
- 57.Zandstra PW, Bauwens C, Yin T, Liu Q, Schiller H, Zweigerdt R, Pasumarthi KB, Field LJ. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9:767–778. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]
- 58.Schroeder M, Niebruegge S, Werner A, Willbold E, Burg M, Ruediger M, Field LJ, Lehmann J, Zweigerdt R. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol Bioeng. 2005;92:920–933. doi: 10.1002/bit.20668. [DOI] [PubMed] [Google Scholar]
- 59.Chisti Y. Hydrodynamic damage to animal cells. Crit Rev Biotechnol. 2001;21:67–110. doi: 10.1080/20013891081692. [DOI] [PubMed] [Google Scholar]
- 60.Fok EY, Zandstra PW. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333–1342. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 61.Lelkes PI, Unsworth BR. Neuroectodermal cell culture: endocrine cells. In: Atala A, Lanza RP, editors. Methods of tissue engineering. London: Academic Press; 2002. pp. 371–382. [Google Scholar]
- 62.Lü S, Liu S, He W, Duan C, Li Y, Liu Z, Zhang Y, Hao T, Wang Y, Li D, et al. Bioreactor cultivation enhances NTEB formation and differentiation of NTES cells into cardiomyocytes. Cloning Stem Cells. 2008;10:363–370. doi: 10.1089/clo.2007.0093. [DOI] [PubMed] [Google Scholar]
- 63.Gerlach JC, Hout M, Edsbagge J, Björquist P, Lübberstedt M, Miki T, Stachelscheid H, Schmelzer E, Schatten G, Zeilinger K. Dynamic 3D culture promotes spontaneous embryonic stem cell differentiation in vitro. Tissue Eng Part C Methods. 2009:Epub ahead of print. doi: 10.1089/ten.tec.2008.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carpenedo RL, Sargent CY, McDevitt TC. Rotary suspension culture enhances the efficiency, yield, and homogeneity of embryoid body differentiation. Stem Cells. 2007;25:2224–2234. doi: 10.1634/stemcells.2006-0523. [DOI] [PubMed] [Google Scholar]
- 65.Yirme G, Amit M, Laevsky I, Osenberg S, Itskovitz-Eldor J. Establishing a dynamic process for the formation, propagation, and differentiation of human embryoid bodies. Stem Cells Dev. 2008;17:1227–1241. doi: 10.1089/scd.2007.0272. [DOI] [PubMed] [Google Scholar]
- 66.Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–282. doi: 10.1634/stemcells.22-3-275. [DOI] [PubMed] [Google Scholar]
- 67.Fernandes AM, Fernandes TG, Diogo MM, da Silva CL, Henrique D, Cabral JM. Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system. J Biotechnol. 2007;132:227–236. doi: 10.1016/j.jbiotec.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 68.Carpenedo RL, Bratt-Leal AM, Marklein RA, Seaman SA, Bowen NJ, McDonald JF, McDevitt TC. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials. 2009;30:2507–2515. doi: 10.1016/j.biomaterials.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang YS, Cho J, Tay F, Heng JY, Ho R, Kazarian SG, Williams DR, Boccaccini AR, Polak JM, Mantalaris A. The use of murine embryonic stem cells, alginate encapsulation, and rotary microgravity bioreactor in bone tissue engineering. Biomaterials. 2009;30:499–507. doi: 10.1016/j.biomaterials.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J, Wang M, Cha JM, Mantalaris A. The incorporation of 70s bioactive glass to the osteogenic differentiation of murine embryonic stem cells in 3D bioreactors. J Tissue Eng Regen Med. 2009;3:63–71. doi: 10.1002/term.135. [DOI] [PubMed] [Google Scholar]
- 72.Bauwens C, Yin T, Dang S, Peerani R, Zandstra PW. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: oxygen-mediated enhancement of cardiomyocyte output. Biotechnol Bioeng. 2005;90:452–461. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- 73.Côme J, Nissan X, Aubry L, Tournois J, Girard M, Perrier AL, Peschanski M, Cailleret M. Improvement of culture conditions of human embryoid bodies using a controlled perfused and dialyzed bioreactor system. Tissue Eng Part C Methods. 2008;14:289–298. doi: 10.1089/ten.tec.2008.0029. [DOI] [PubMed] [Google Scholar]
- 74.Akasha AA, Sotiriadou I, Doss MX, Halbach M, Winkler J, Baunach JJ, Katsen-Globa A, Zimmermann H, Choo Y, Hescheler J, et al. Entrapment of embryonic stem cells-derived cardiomyocytes in macroporous biodegradable microspheres: preparation and characterization. Cell Physiol Biochem. 2008;22:665–672. doi: 10.1159/000185550. [DOI] [PubMed] [Google Scholar]
- 75.Taiani J, Krawetz RJ, Nieden NZ, Wu YE, Kallos MS, Matyas JR, Rancourt DE. Reduced Differentiation Efficiency of Murine Embryonic Stem Cells in Stirred Suspension Bioreactors. Stem Cells Dev. 2009:Epub ahead of print. doi: 10.1089/scd.2009.0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu K, Liu YL, Cui B, Han Z. Application of stem cells for cardiovascular grafts tissue engineering. Transpl Immunol. 2006;16:1–7. doi: 10.1016/j.trim.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Zweigerdt R, Burg M, Willbold E, Abts H, Ruediger M. Generation of confluent cardiomyocyte monolayers derived from embryonic stem cells in suspension: a cell source for new therapies and screening strategies. Cytotherapy. 2003;5:399–413. doi: 10.1080/14653240310003062. [DOI] [PubMed] [Google Scholar]
- 78.Mummery C, Ward D, van den Brink CE, Bird SD, Doevendans PA, Opthof T, Brutel de la Riviere A, Tertoolen L, van der Heyden M, Pera M. Cardiomyocyte differentiation of mouse and human embryonic stem cells. J Anat. 2002;200:233–242. doi: 10.1046/j.1469-7580.2002.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fong WJ, Tan HL, Choo A, Oh SK. Perfusion cultures of human embryonic stem cells. Bioprocess Biosyst Eng. 2005;27:381–387. doi: 10.1007/s00449-005-0421-5. [DOI] [PubMed] [Google Scholar]
- 80.Bratt-Leal AM, Carpenedo RL, McDevitt TC. Engineering the embryoid body microenvironment to direct embryonic stem cell differentiation. Biotechnol Prog. 2009;25:43–51. doi: 10.1002/btpr.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin CH, Chen W, Hsiao CC, Kuo CY, Chen CL, Wu WT. Production of mouse embryoid bodies with hepatic differentiation potential by stirred tank bioreactor. Biosci Biotechnol Biochem. 2007;71:728–734. doi: 10.1271/bbb.60568. [DOI] [PubMed] [Google Scholar]