Abstract
Stem cells are unspecialized cells that can self renew indefinitely and differentiate into several somatic cells given the correct environmental cues. In the stem cell niche, stem cell-extracellular matrix (ECM) interactions are crucial for different cellular functions, such as adhesion, proliferation, and differentiation. Recently, in addition to chemical surface modifications, the importance of nanometric scale surface topography and roughness of biomaterials has increasingly becoming recognized as a crucial factor for cell survival and host tissue acceptance in synthetic ECMs. This review describes the influence of nanotopography on stem cell phenotypes.
Keywords: Stem cells, Nanofibers, Nanotopography, Biomaterials, Extracellular matrix, Differentiation
INTRODUCTION
Stem cells are a natural choice for cell therapy due to their pluripotent nature and self-renewal capacity. In humans, stem cells have been identified in the inner cell mass of the early embryo, in some tissues of the fetus, the umbilical cord and placenta, and in several adult organs. The microenvironment in which the stem cells exist is called the stem cell niche. There are several factors which regulate the stem cell niche in vivo, such as extracellular matrix (ECM) molecules, growth factors, cytokines, and cell secreted metabolites. Molecular signals are exchanged between the stem cells and other neighbouring cells within the stem cell niche. The niche saves stem cells from depletion, while still protecting the host from excessive stem-cell proliferation. In short, the stem niche encompasses all of the elements immediately surrounding the stem cells when they are in their naive state, including the non-stem cells that might be in direct contact with them, as well as the ECM and proximal soluble molecules[1]. Typically, a niche contains a few stem cells with high potential of differentiation into different kinds of mature cells. These stem cells are supported by, or incorporated into, the niche walls formed by the neighbouring cells. After asymmetrical division, a stem cell remains in the same position, while a daughter cell with a narrower potential for differentiation migrates, divides symmetrically or asymmetrically, and eventually leaves the niche[2].
Stem cells can be broadly classified, based on their origin, into two types - embryonic stem cells (ESCs) and adult stem cells (ASCs). Their potency may be classified into three types - totipotent, pluripotent and multipotent stem cells (Table 1)[3].
Table 1.
Different types of stem cells, their properties, and functions
| Stem cell type | Properties | Functions |
| MSCs | Multipotent and pluripotent. Bone marrow is the major source of MSC | MSCs are capable of differentiating into bone, cartilage, fat, muscle, marrow stroma, and other tissue types |
| ESCs | Derived from an early stage embryo and can differentiate into derivatives of all three primary germ layers. ESCs are multipotent and pluripotent | Can differentiate into brain and nervous system cells, insulin producing cells of the pancreas, bone cells, hematopoietic cells, endothelial cells, cardiomyocytes |
| ASCs | Multipotent, oligopotent, or unipotent progenitor cells. Derived from a more mature tissue, such as the umbilical cord, bone marrow, or skin | To treat leukemia and related bone/blood cancers through bone marrow transplants |
| HSCs | Found in the bone marrow. Multipotent | All types of blood cells |
| iPS | Derived from epithelial cells. Pluripotent | The iPS cell lines could be differentiated into heart muscle and neuronal cells, in addition to basic cell types (ectoderm, mesoderm, and endoderm) |
| Mammary stem cells | Isolated from human and mouse tissue | Growth of mammary glands |
| Endothelial stem cells | Multipotent cells found in the bone marrow | Can differentiate into endothelial cells, the cells that make up the lining of blood vessels |
ASCs can be employed for various tissue regeneration applications for the following reasons: (1) They are naturally poised to generate a particular tissue, which might consist of several cell types; (2) They are able to migrate to injured tissue or other discrete sites in the body; and (3) Some cells secrete growth factors that mobilize or protect other cells residing in the tissue. However they are rare, difficult to identify and purify, and, when grown in culture, are difficult to maintain in the undifferentiated state.
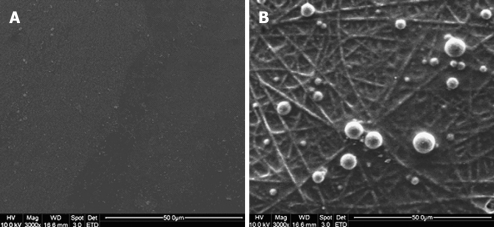
Hematopoietic stem cells (HSCs) are capable of self-renewing continuously. HSCs reside in two different niches-the endosteal niche and the perivascular niche. In the endosteal niche, HSCs are associated with a subset of osteoblasts that line the inner surface of the cavities of trabecular bone. It supports quiescence and self-renewal of the HSCs[4]. HSCs that are found in the vicinity of sinusoidal endothelial cells are referred to as the vascular niche. The vascular niche forms a milieu that supports the proliferation, differentiation, and trans-endothelial migration of HSCs[5]. Ma et al[6] demonstrated that topographical and biological cues are responsible for early adhesion of bone marrow derived HSCs. They showed that the adhesion of the HSCs was faster onto the collagen blended poly-lactic-co-glycolic acid (PLGA) nanofibrous scaffold compared to the tissue culture polystyrene (TCP) (Figure 1)[6].
Figure 1.
Capture of BM-HSCs by different substrates after 30 min of incubation. A: No BM-HSCs captured on tissue culture polystyrene (TCP); B: Rounded morphology of BM-HSCs captured on E-selectin-coated collagen-blended polylactide-co-glycolide (PLGA) nanofiber[6].
Human bone marrow-derived mesenchymal stem cells (hBM-MSCs) have attracted substantial attention in the field of tissue engineering and regenerative medicine due to the following advantages: firstly, the techniques for collecting and purifying MSCs from bone marrow are relatively convenient[7]; secondly, they are naturally poised to generate a particular tissue, which might consist of several cell types such as adipocytes, chondrocytes, osteoblasts, tenocytes, myoblasts, or neurocyte[8-11]; thirdly, MSCs can escape the immune system[12]; and fourthly some cells secrete growth factors that mobilize or protect other cells residing in the tissue[13]. There is little ethical controversy in the application of MSCs. Besides the perivascular areas in the bone marrow, where the MSCs could be in close association with HSCs, the MSCs can also be isolated from other tissues, such as the periosteum, synovial membrane, and synovial fluid[12]. Muguruma et al[13] demonstrated that upon insertion of human MSCs into the bone marrow of immunodeficient mice, the human MSCs differentiated into stromal cells, bone-lining osteoblasts, and endothelial cells, all functional constituents of the marrow hematopoietic microenvironment. Thus, understanding how niche cells and the ECM control stem cell fate will provide new tools to stimulate the differentiation of stem cells into desired cell types.
Factors influencing stem cell behaviors
The influence of the substratum on cell migration was first reported by Harrison in 1911 when he grew cells on a spider web and found that the embryonic cells followed the fibers of the web. This phenomenon was called stereotropism or physical guidance[14]. The role of topography on cells such as endothelia, fibroblasts, epithelia and epitena, was first explained by Curtis et al[15,16]. A very wide range of cell types, such as fibroblasts, osteoblast, nerve cells, and mesenchymal stem cells respond profoundly to nanotopography[17,18]. Cells seeded onto artificially produced micro- and nano-grooves aligned their shape and elongated in the direction of the groove. However, it was reported by Wilkinson et al[19] that cells do not respond to groove width other than depth of size greater than 2 micrometer. Cells adhere well onto surfaces having structures on the nanoscale range of 58 nm, but do not adhere that well on structures with diameter of more than 73 nm[20]. It was also reported that cells can recognize symmetries in the nanorange[21].
There are five key design parameters that influence cell behaviour in a biomaterial, depending on the surface molecules present in the biomaterial, including: ligand identity, presentation, and density; material architecture; and material mechanical properties. Together, these material properties coordinate the interplay between intrinsic and extrinsic determinants of stem cell fate to produce a desired phenotype[22]. In addition, the properties of the scaffold surface that must be taken into careful consideration include the rate of degradation of the scaffold, optimal fluid transport, and delivery of bioactive molecules, cell-recognizable surface chemistries, mechanical integrity, and the ability to induce signal transduction. The ultimate success of a scaffold is dependent on these properties because they influence cell adherence, nutrient/waste transport, matrix organization and cell differentiation[23].
The nanostructured surfaces of nanometallic and nanoceramic materials have several advantages compared to conventional surfaces. These include: (1) they possess greater surface roughness resulting from both decreased grain size and possibly decreased diameter of surface pores; (2) enhanced surface moisture retention due to greater surface roughness; and (3) greater numbers of grain boundaries. For example, nanoceramics are commercially available as new bone grafts or as implant coating materials (i.e. nano-HA paste-Ostim® from Obernburg, Germany; nano-beta-tricalcium phosphate-Vitoss from Orthovita, USA)[24].
The types of biomaterials used commonly in stem cell cultures ranges from polymers [polystyrene, polysulfone, polytetrafluoroethylene, cellulose acetate, PLGA, Collagen, and PCL (polycaprolactone)] to metals (titanium, alumina, and stainless-steel) and glasses. Many polymers do not have the desired surface properties to be used as biomaterials in tissue engineering; therefore, surface modification is used to improve surface characteristics, such as hydrophilicity, cell attachment, expansion, proliferation, and differentiation[25]. Cell response is affected by the physicochemical parameters of the biomaterial surface, such as surface energy, surface charges or chemical composition. Topography is one of the most crucial physical cues for stem cells and recently it has been proven that nanotopography is the main influencing factor, rather than microtopography[26].
Nanofibrous scaffolds present a 3D nanostructured topology that resembles the fibrillar ECM proteins in vivo. Polyglycolic acid (PGA), polylactic acid (PLA) and the copolymer PLGA have been extensively used as nanofibrous scaffolds. These materials are hydrolytically degradable and their by-products are physiologically removed via metabolic pathways[27,28]. The mechanics of the nanofibrous scaffold are determined primarily by its composition, water content, and structure, which affect intermolecular and intramolecular forces and stress distributions[29-31]. Common methods of altering the mechanical properties of biomaterials include modulating the molecular composition and connectivity, thermal processing, and creating reinforced and porous composites. The mechanical properties of a material affect cell behaviors such as proliferation and migration[31-35].
Fabrication of scaffolds with various nanotopographies
There are several techniques for the fabrication of nano- and microsurfaces suitable for the growth of cells, as depicted in Table 2. These include laser deposition and etching, soft lithography, electrospinning, and colloidal lithography[36-39].
Table 2.
Various fabrication techniques to achieve nanotopography
| Fabrication technique | Advantages | Drawbacks |
| Laser deposition | Uniform distribution of pore size, simple and fast | Reduced resolution and poor surface finish |
| Self assembly | Can generate fibrous networks capable of supporting cells in three dimensions. Cell-seeding problems associated with using prefabricated nanofibrous scaffolds eliminated owing to spontaneous assembly | Lack mechanical strength, Limited amphiphilic materials, random and very short nanofibers |
| Lithography | Relatively good resolution | Time consuming and expensive. |
| Electrospinning | The properties of electrospun nanofibers, such as fiber diameter, can be controlled readily via manipulation of spinning parameters. Capable of mimicking the stem cell niche | Electrospinning yields a flat mat that has limited three dimensionality and suffers from cell infiltration problems because of the small pore size of the mats |
| Phase separation | A nanofibrous (fibers with diameters of 50-500 nm) three-dimensional scaffold can be constructed. Has controllable high porosity, surface-to- volume ratios, and well as defined mechanical properties | Nanofiber distribution and uniformity is subject to the controllability of the processing |
Electrospinning is the most widely used technique to create fibrous structures with favourable mechanical and biological properties. Electrospun nanofibers have been incorporated in stem cell cultures, to provide the desired microenvironment for their growth and differentiation, and to ultimately mimic the stem cell niche. Electrospun nanofibrous matrices provide integrated networks of nanoscale fibers with a specified pattern, high porosity, high spatial interconnectivity, and a high surface area to volume ratio[40].
There are a number of electrospinning parameters that affect both the fibers and the scaffold. These include solvent type, material concentration and viscosity, distance of the collecting target from the spinning nozzle, the gauge of the needle, and the voltage. The above parameters should be optimized depending on the desired application, as cell proliferation and differentiation are influenced by the fiber diameter[41,42]. HFP (1,1,1,3,3,3-hexafluoro-2-propanol) is a commonly used solvent for electrospinning. It is an organic solvent allowing full extension of the polymer, without leaving any residue on the electrospun fibers. However, some proteins, such as collagen, tend to lose their 3D molecular structure when using HFP as the solvent. Hence cross-linking agents like glutaraldehyde or stabilizers are proposed to be applicable[43]. Recently, it has been found that adding PCL not only reduced the potential cytotoxicity that a chemical cross-linking reagent such as glutaraldehyde can cause, but also produced a new composite with improved mechanical and biological properties[44-47]. Heydarkhan-Hagvall et al[48] demonstrated that electrospinning of natural proteins like collagen/gelatin with synthetic polymers like PCL/PLGA can be used to produce tissue-engineered scaffolds that better recapitulate key features of the native ECM, including its mechanical and biochemical properties.
The biocompatible scaffold materials can be synthetic or natural. Collagen, fibrinogen, hyaluronic acid, glycosaminoglycans (GAGs), hydroxyapatite (HA), cellulose, chitosan, and silk fibroin are the most commonly used biomaterials. Although the natural biomaterials have the advantage of being biocompatible and bioactive, they have certain disadvantages compared to synthetic biomaterials such as the difficulty in modifying degradation rates, difficulty in sterilization and purification. Grafting of polymers with collagen is said to increase the surface hydrophilicity and thereby facilitates cell attachment and proliferation on the modified surface[49-52]. In addition, plasma surface treatment of scaffolds with N2, O2, and NH3 makes the polymer surface more hydrophilic, more polar, and more bio-adhesive[53,54].
Surface modification of implants with nanotopographies
Using bone/dental implants as an example, once an implant is placed into the body, the adjoining bone will interact with the surface of the load bearing implant. This process is called osseointegration. The success of an implant depends on how early osseointegration is achieved[55]. Hence, the surface of the implants ought to be modified, to create a nanostructured surface matching native bone ECM and enhancing osteoblast incorporation, to improve early osseointegration. Various techniques have been attempted to improve the surface roughness of the implant, such as plasma treatment, acid-etching, and heat treatment. For example, the TPS (titanium plasma sprayed) surfaces used by the Straumann Company, recommended a healing period of 12 wk[56] and this was reduced to six to eight weeks with the introduction of the SLA (sand blasted, acid etched) surface[57]. Alternatively, nano-hydroxyapatite (n-HA) has been widely used as a bioceramic in orthopaedics and dentistry due its osteoconductive properties[58], which makes the combination of a load bearing biomaterial like titanium with the osteoconductive properties of n-HA very attractive.
The current time required for osseointegration ranges from three to six months. This delay might be because the osteoprogenitor cells and/or stem cells need a long time to recognize the implant surface, attach onto it, followed by proliferation and differentiation. The surface creation of nanotopography such as a nanofiber offers the possibility to optimize cell capture as well as other cell functions, because both the substrate topography and the biological cues enhance the initial attachment of MSCs, which might be very helpful for osseointegration.
EFFECTS OF NANOTOPOGRAPHY ON STEM CELLS
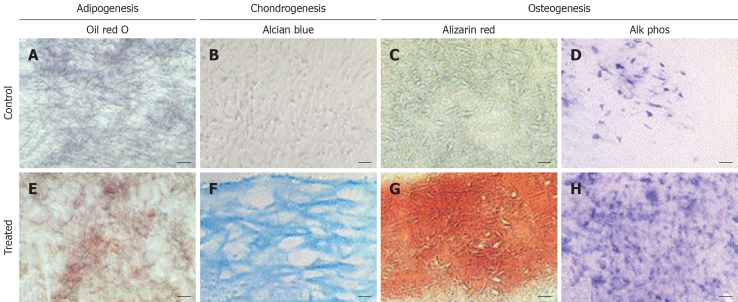
Nanotopography is of critical importance in various biomedical applications. The nanoscale surface morphology, along with mechanical and biochemical cues, determines stem cell attachment, proliferation, and differentiation. Nanotextured scaffolds, besides providing structural support to the cultured stem cells, can also provide the topographical signals to influence cell differentiation, particularly through the nanostructural architecture provided by the fibers. Li et al[59] showed that a 3D electrospun nanofibrous scaffold was capable of supporting multilineage differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages, as shown in Figure 2. Stem cells use transmembrane actin-integrin adhesion complexes as mechanosensors to probe the rigidity of the extracellular environment, mediate adhesion, trigger signaling, and remodel the ECM[60]. Culturing hESCs in the presence of actin disrupting agents proved that cytoskeleton remodelling through actin polymerization is critical for the morphological and proliferative behaviour of hESCs cultured on nanotopographic surfaces[61].
Figure 2.
Histological analysis of cell-polymer constructs maintained in adipogenic, chondrogenic, or osteogenic medium. Sections of the constructs were stained with (A,E) oil red O, (B,F) alcian blue, or (C,G) alizarin red, or histochemically stained for alkaline phosphatase (D,H). In adipogenic cultures (E), oil red O-positive lipid droplets were seen, compared to the lack of staining in the control culture (A). In chondrogenic cultures (F), intense alcian blue was seen, showing cells surrounded by a sulfated proteoglycan-rich ECM (F), whereas control cultures (B) showed little staining. In osteogenic cultures (G,H), mineralization (G) and alkaline phosphatase activity (H) were both significantly higher than in control cultures (C,D). Bar: 20 μm (B,F), 40 μm (A,C,E,G), or 80 μm (D,H)[59].
Effect of nanotopography on embryonic stem cells
Gerechta et al[61] recently reported the influence of surface topography on the morphology and proliferation of hESCs. They demonstrated that poly (di-methyl siloxane) substrates with nanoscale line-grating (in the range of 600 nm ridges with 600 nm spacing and 600 ± 150 nm feature height) induced more hESC alignment and elongation, compared to the flat surface[61]. These were characterized by the cytoskeletal proteins actin, vimentin, and tubulin.
The maintenance and differentiation of hESCs is mainly dependent on the use of feeder cells, which are obtained from animal sources. Hence, there is always a risk of immune recognition. The mechanism of how feeder cells maintain the proliferation of undifferentiated ESCs is unknown. Such in vitro culturing presents certain theoretical hazards to the use of stem cells for regenerative medicine, such as the spread of viruses and other infectious agents not normally found in humans. However, it is believed that the nanotopographical substrates can maintain the proliferation of undifferentiated rhesus ESCs without the use of feeder cells[38].
Effect of nanotopography on mesenchymal stem cells
Mesenchymal stem cell adhesion and migration: The initial adhesion of the cells to the surface determines its long term cell viability. Different aspects, such as surface moisture retention and free energy[62], surface roughness, material composition[63,64], and method of preparation[65] of various materials have been studied and were determined to be the major factors influencing the attachment of cells, including MSCs, in vitro. Oha et al[66] found that hMSCs cultured on TiO2 nanotubes of diameter less than 50 nm adhered more strongly compared to the cells cultured on TiO2 nanotubes of 100 nm diameter. This was because nanotubes of diameter less than 50 nm had more surface area and hence a higher amount of ECM proteins can be deposited. In addition, he also showed that hMSCs adhered more effectively onto the smallest nanotube diameter, which was 30 nm, within two hours[66]. Park et al[67] also demonstrated that the adhesion of MSCs was reduced when the diameter of the TiO2 nanotubes increased beyond 50 nm[67]. These directly indicate the influence of nanotopography on the biological processes. Larger adhesions are usually associated with increased indirect mechanotransductive signalling (adhesion and cytoskeleton-related) such as integrin-related signalling, extracellular receptor kinase (ERK), and focal adhesion kinase (FAK).
Cells will migrate along topographical features when plated onto a chemically uniform surface, a phenomenon known as contact guidance, which is crucial in embryonic morphogenesis and wound healing[68]. Emerging evidence indicates that the surface topography, stiffness, and electrical properties play important roles in neuron adhesion and neurite outgrowth[69]. Fan et al[70] studied the adhesion of neural cells of prenatal rats on silicon wafers with different nanotopographies in the range of 20 nm to 70 nm. Cell adhesion and viability were significantly improved on the nanofeatured surface. Moreover, 15% of the cells remained dopaminergic after five days of culture. Massia et al[71] analyzed the cell adhesion kinetics and demonstrated that the surface threshold spacings for focal contact and stress fiber formation occurs at 140 nm, indicating that cell adhesion can be enhanced on a nanostructured surface. Kommireddy et al[72] proved that MSC attachment and migration increased after the deposition of TiO2 nanoparticle layers.
The surface modification of implants for cell attachment and migration has been the focus of much research, as the initial cell adhesion is critical for the functionality and the lifetime of the implant.
Mesenchymal stem cell proliferation and differentiation: Nanotopography not only enhances adhesion, but also improves proliferation efficiency of MSCs. Nanotopography induces certain biochemical and structural cues, which cause the differentiation of the MSCs into certain desired phenotypes. Table 3 gives an outline of the influence of various substrates and their topographies on several cell lineages, which might give some hints on the differentiation of stem cells into those cell types.
Table 3.
Various cell types and the nanotopographies on which they are cultured
| Cell type | Nanotopography | Advantages | Ref. |
| Chondrocytes | (a) PCL nanofibrous scaffold (200-800 nm) in the presence of TGF-β1; (b) Collagen nanofibers of diameter 110 nm-1.8 μm | The differentiation of the stem cells into chondrocytes in the nanofibrous scaffold was comparable to an established cell pellet culture. Nanotopography supports chondrocyte growth and infiltration | [82,90] |
| Osteoblasts | (a) Ceramics like HA, alumina and titania having nanostructures of grain sizes less than 100 nm and nanophase zinc oxide (23 nm); (b) PLGA, PLLA and PCL nanofibers (diameter 200-800 nm); (c) Nanotubes of diameter less than 100 nm | Enhanced proliferation and differentiation of MSC to osteoblasts | [67,77-79,105-113] |
| Smooth muscle cells (SMC) | (a) PLGA and PCL, PLLA-CL nanofibers (diameter 200-800 nm); (b) Nanogratings of 350 nm in width, spacing, and depth imprinted on PMMA or PDMS | SMC adhesion was enhanced on the nanostructured substrates compared to the conventional submicron substrates | [114-118] |
| Fibroblasts | (a) PLGA (85:15 ratio) nanofibers of diameter 500-800 nm; (b) Nanocolumns | Increased endocytic activity. Nanotopography can be used to improve hemocompatibility of blood-contacting biomaterials | [82] |
| Nerve cells | (a) Silicon wafer in the range of 20-70 nm; (b) PLLA or PCL scaffolds via electrospinning and phase separation | The cell adhesion and viability significantly improved on the nanofeatured surface | [70,91] |
PCL: Polycaprolactone; TGF-β: Transforming growth factor-β; HA: Hydroxyapatite; PLGA: Poly-lactide-co-glycolide; PLLA: Poly-L-lactide acid; MSC: Mesenchymal stem cell; PMMA: Poly-methylmethacrylate; PDMS: Polydimethylsiloxane.
The influence of nanotopography on the osteogenic, chondrogenic, and neural differentiation of MSCs will be discussed in detail in the following sections. MSCs develop into osteoblasts via a series of developmental stages - osteoprogenitor cell, preosteoblast, and finally osteoblast cells. Osteoblast adhesion on nanostructured surfaces was first reported in 1999 by Webster et al[73]. He reported that osteoblast adhesion was improved when they were cultured on nanostructured surfaces, compared to the conventional micro surfaces. Specifically, alumina with grain sizes between 49 nm and 67 nm and titanium with grain sizes between 32 nm and 56 nm enhanced osteoblast proliferation and differentiation compared to their respective micro-grained materials. This can be measured by monitoring ECM protein synthesis, such as collagen and alkaline phosphatase (ALP). Enhanced bone formation was reported on the nanophase HA coated tantalum compared to the microscale HA coated tantalum, and the non-coated tantalum[73]. Webster et al[74,75] demonstrated that osteoblast adhesion increased by 146% on nanophase zinc oxide (23 nm) compared to microphase zinc oxide (4.9 nm). Nanophase metals have been extensively investigated for orthopedic applications due to their higher surface roughness, energy, and the presence of more particle boundaries at the surface compared with conventional micron metals. Moreover, osteoblasts were even further increased on nanofiber structures compared to nanospherical structures of alumina; this was believed to occur because, compared to nanospherical geometries, nanofibers more closely approximate the shape of HA crystals and collagen fibers in the natural bone[76]. Woo et al[77] observed enhanced osteoblast attachment on nanofibrous scaffolds when compared to solid pore walls.
Yoshimoto et al[78] cultured rat MSCs on PCL nanofibrous scaffolds of diameter 400 nm. ECM production (Collagen) and the multiple cell layer formation occurred within a short span of one week. Hosseinkhani et al[79] investigated mesenchymal stem cell (MSC) behavior on self-assembled peptide-amphiphile (PA) nanofiber scaffolds. Significantly enhanced osteogenic differentiation of MSCs occurred in the 3D PA scaffold compared to 2D static tissue culture. It was characterized by enhanced collagen synthesis, alkaline phosphatase activity, and calcium mineral deposition. It was demonstrated that when hMSC loaded constructs made of PCL nanofibers were cultured in an osteogenic differentiation media comprising of β-glycerolphosphate, ascorbic acid, and dexamethasone, a dense, opaque bone-like tissue was observed, indicating the osteogenic differentiation of hMSCs. Polygonal-shaped osteoblast/osteocyte-like cells with upregulated expression of alkaline phosphatase, bone sialoprotein, and osteocalcin were observed[59].
Dalby et al [80] demonstrated the use of nanoporous topography to stimulate hMSCs to produce bone mineral in vitro, in the absence of osteogenic supplements. Their results demonstrated that highly ordered nanoporous topographies produce low to negligible cellular adhesion and osteoblastic differentiation. Cells on random nanoporous topographies however exhibited a more osteoblastic morphology. This enhanced differentiation was due to the nanodisorder. This work demonstrated that topographical strategies provide further orthopedic approaches to be exploited and harnessed. However, the intracellular events controlling the differentiation of hMSCs into osteoblasts have still not been clearly analyzed. Salasznyk et al[81] suggested that focal adhesion kinase signalling plays an important role in regulating ECM-induced differentiation of hMSCs into osteoblasts.
Li et al[82] investigated the chondrogenesis of MSCs on a PCL nanofibrous scaffold in the presence of TGF-β1 in vitro. The differentiation of the stem cells into chondrocytes in the nanofibrous scaffold was comparable to an established cell pellet culture. It was advantageous to use nanofibers rather than a cell pellet system, owing to their better mechanical properties, oxygen/nutrients exchange, and easy fabrication[83-87]. The findings reported suggested that the PLLA nanofibrous scaffold is a practical carrier for MSC transplantation, and represents a candidate scaffold for cell-based tissue engineering approaches to cartilage repair[88].
Cheng et al[89] reported that human cartilage cells attached and proliferated well on HA nanocrystals homogeneously dispersed in PLA, and collagen fibers of diameter 110 nm to 1.8 μm were proved to support chondrocyte growth and infiltration[90]. Such data shows the promise of nanomaterials for promoting cartilage regeneration.
Koh et al[91] fabricated various nanofibrous PLLA or PCL scaffolds via electrospinning, which demonstrated excellent cytocompatibility properties for neural tissue engineering applications. When laminin was incorporated into the nanofibrous scaffold, the neurite outgrowth improved on the laminin-PLLA scaffold produced by facile blended electrospinning. Yang et al[92] showed that the direction of Neural stem cell (NSC) elongation and its neurite outgrowth was parallel to the direction of aligned PLLA fiberous scaffolds. They also demonstrated that the differentiation rate of NSCs was higher for nanofibers than for micro fibers.
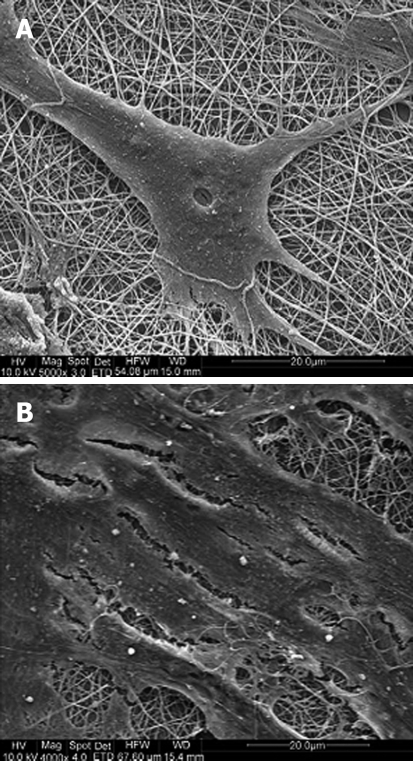
Most recently, Prabhakaran et al[93] demonstrated the potential of hMSCs for neuronal differentiation in vitro when cultured on poly (l-lactic acid)-co-poly-(3-caprolactone)/Collagen (PLA-CL/Col) nanofibrous scaffolds (Figure 3). The differentiation of MSCs into neuronal lineages was carried out using neuronal inducing factors, including β-mercaptoethanol, epidermal growth factor, nervegrowth factor, and brain derived growth factor, in DMEM/F12 media. These supplements, in addition to the nanoscaffold, induced the differentiation of the MSCs into neuronal cells.
Figure 3.
SEM images of (A) MSCs induced to neuronal cells grown using neuronal induction medium and (B) undifferentiated MSCs on electrospun PLA-CL/Collagen nanofibers grown using MSC growth medium[93].
Stem cells have the potential to differentiate and self-renew into the desired cell types. Therefore, many efforts have focused on impregnating multi-potential stem cells into the nanofibrous scaffolds, which can be directly transplanted into injury sites and assist neural tissue recovery. In addition, the development in nerve repair grafts for peripheral nerve injuries to bridge nerve gap has advanced to the next level where the nanofibers were been used as guidance channel[94]. However, a challenging problem has been to determine how to effectively deliver and selectively differentiate stem cells into nerve cells at injury sites to regenerate desirable tissue. Although the underlying mechanisms triggering differentiation of stem cells are not entirely clear, previous research has indicated that novel biomimetic nanomaterials might contribute to selective stem cell differentiation[95].
Combined effect of nanotopography and other factors
The fate of multipotent stem cells can be desirably controlled when they are cultured on nanopatterned substrates. It was recently demonstrated that FGF-2 could allow long-term self-renewal of hESCs and maintain their pluripotent status[96]. The addition of growth factors, such as retinoic acid and activin A, have demonstrated success in promoting in vitro differentiation of murine ESCs into cells of pancreatic lineage like α, β, γ, and δ cells. A commonly used cell adhesive ECM peptide is the RGD protein, which is an arginine-glycine-aspartic acid sequence. Holtorf et al[97], proved that when titanium fiber mesh scaffolds were coated with RGD peptides, MSCs attached more strongly to these RGD-coated scaffolds. However. no significant change was observed in ECM secretion. Murine MSCs seeded onto fibronectin(FN)-functionalized scaffolds created by an LbL (Layer by Layer) microfabrication system, adhered more strongly to the scaffold and readily differentiated into osteoblasts[98]. The addition of a phosphoester group to photo-polymerizable PEG-based hydrogels not only provides biodegradability but has also been shown to promote mineralization of encapsulated MSCs. The use of such phosphoester (Phosph-) groups is said to significantly increase the ALP and osteocalcin levels in differentiated cells[99]. Peter et al[100] demonstrated that MSC adhesion, proliferation, and differentiation into osteoblasts increased when TGF-β1 was encapsulated within polymer blends of PEG-PLGA particles (sized at an average of 158 μm). Thus we find that the cell-scaffold interactions increased in the presence of factors like RGD, FN, Phospho-group, and TGF-β1.
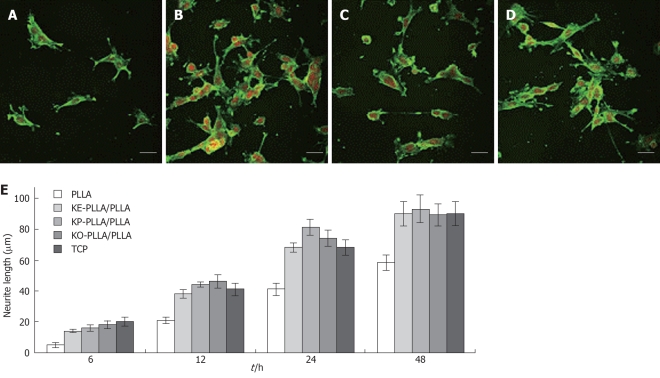
Chemical guiding cues were exploited to stimulate neuron adhesion and neurite outgrowth, using amino-functioned PLLA after phase separation with nanotopography. It was found that improved viability and neurite outgrowth were obtained on the peptide-grafted PLLA films compared to the ordinary PLLA films. Here, the neonatal mouse cerebellum C17.2 stem cells were cultured onto the K-(CH2)5-PLLA/PLLA peptide-grafted films and the PLLA films were used as controls. The enhanced neurite outgrowth of the C17.2 stem cells was shown to be due to the addition of laminin-derived peptide sequences (Figure 4)[101]. It is thus possible to further mimic the stem cell niche by covalently linking certain ligands or growth factors to the nanostructured scaffold.
Figure 4.
Laser scanning confocal microscopy (LSCM) micrographs of immunostained neurofilament 200 kDa in C17.2 after 24 h of culture on different films. A: Poly-L-lactide acid (PLLA); B: KE-PLLA/PLLA; C: KP-PLLA/PLLA; D: KO-PLLA/PLLA; E: The average length of the longest neurite per cell from 50 randomly selected cells on different films from PLLA and K-(CH2)n-PLLA/PLLA (10/90, w/w) over cultivation[101]. The neurite was stained by FITC and nuclei was stained by PI. Scale bar: 40 μm.
APPLICATIONS
The list of medical achievements of stem cells seems to be expanding at an incredible pace. ESCs have the advantage of multipotency and can be readily cultured in the laboratory. The degree of plasticity of adult stem cells is still unknown and there are difficulties in purifying and culturing them. The only proven stem cell-based medical therapies that are currently available rely on adult-derived stem cells from bone marrow and skin. The idea of employing adult stem cells for many applications is for the following reasons: (1) They are naturally poised to generate a particular tissue, which might consist of several cell types; (2) They are able to migrate to injured tissue or other discrete sites in the body; and (3) Some cells secrete growth factors that mobilize or protect other cells residing in the tissue[102]. Pluripotent stem cells could be used to create an unlimited supply of cells, tissues, or even organs that could be used to restore function without the requirement for immunosuppressive drugs. Such cells, when used in transplantation therapies, would in effect be suitable for “universal” donation.
Neural application
Nerve stem cells can be used to treat the neurodegenerative diseases such as Parkinson’s disease. Parkinson’s disease involves the loss of cells which produce the neurotransmitter dopamine. Recent clinical studies using fetal cell transplants reported survival and release of dopamine from the transplanted cells and a functional improvement of clinical symptoms[103]. Thus it opens yet another frontier for stem cell therapy.
Orthopaedic application
Bone marrow transplantation is a well known clinical application of stem cells in orthopedics and blood diseases. Nanostructured biocomposites provide alternatives that have not yet been fully explored for orthopedic applications such as implants. They may be fabricated to possess similar micro- and nanoarchitecture as that of healthy, physiological bone. The behavior of cells depends on their interactions with their environment. Consequently, the interactions between cells and implantable materials will determine the success or failure of a medical device. Thus, to achieve proper osseointegration, it is necessary that the implant has a nanostructured surface, ensuring early adhesion of stem cells. Biomaterials in the form of implants (sutures, bone plates, joint replacements, ligaments, vascular grafts, heart valves, and dental implants) and medical devices (for example pacemakers and biosensors) are widely used to replace and restore the functions of degenerated tissues or organs, to assist in healing, improve functionality, and thus improve quality of life[36]. Their improved mechanical and biocompatibility properties promise future greater orthopedic implant efficacy.
Cell based therapies
Perhaps the most important potential application of human stem cells is the generation of cells and tissues that could be used for cell-based therapies. Today, donated organs and tissues are often used to replace repaired or destroyed tissue, but the need for transplantable tissues and organs far outweighs the available supply. Hence stem cells, directed to differentiate into specific cell types using nanotopography, offer the possibility of a renewable source of replacement cells and tissues to treat a number of diseases. For example, it might become possible to generate healthy heart muscle cells in the laboratory and then transplant those cells into patients with chronic heart disease. Recent studies have demonstrated that stem cells that are injected into the circulation or directly into the injured heart tissue appear to improve cardiac function and/or induce the formation of new capillaries[104].
CONCLUSION
By carefully controlling the nanotopography and surface chemistry, in principle, one could design a device that enhances a selective cell population to grow in specific regions of the device. The literature presented in this review clearly indicates that cells respond to the topography of substrates in the nanometer range in terms of adhesion, proliferation, and migration. The substratum, besides providing mechanical support, acts as an intelligent surface, providing the necessary topographical cues and signals to guide cell adhesion, proliferation and differentiation. Although many challenges lie ahead, the nanofibrous scaffold having excellent cytocompatibility and controllable mechanical properties, can mimic properties of the natural ECM and thus, shows great potential for numerous tissue regeneration applications. Scaffolds with advanced technologies, by incorporating nanotopography, can be used to create complex guidance channels, which can be used to mimic the natural repair process of the human body. Recent advances made in the field of nanotopography mediated stem cell regeneration provide optimism for nerve tissue engineering and bone tissue engineering to create a permissive environment for nerve and bone regeneration. Of particular interest in tissue engineering is the creation of reproducible and nanotopographic scaffolds for stem cell migration and differentiation, resulting in bio-matrix composites for various tissue repair and replacement procedures. Though stem cell based-therapy seems to be very remarkable, there are many legal and social questions that must be addressed before stem cell-based therapies become clinically available.
Footnotes
Supported by The National University of Singapore, Grant No. R-224-000-035-133 and NMRC/1151/2008, Singapore
Peer reviewer: Hong-Bo R Luo, Assistant Professor, Department of Pathology and Lab Medicine, Harvard Medical School and Children’s Hospital Boston, 10214 Karp Research Building, 1 Blackfan Circle, Boston, MA 02115, United States
S- Editor Li LF L- Editor Stewart GJ E- Editor Lin YP
References
- 1.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 2.Lemischka IR, Moore KA. Stem cells: interactive niches. Nature. 2003;425:778–779. doi: 10.1038/425778a. [DOI] [PubMed] [Google Scholar]
- 3.Can A. A concise review on the classification and nomenclature of stem cells. Turk J Hematol. 2008;25:57–59. [PubMed] [Google Scholar]
- 4.Yang L, Wang L, Geiger H, Cancelas JA, Mo J, Zheng Y. Rho GTPase Cdc42 coordinates hematopoietic stem cell quiescence and niche interaction in the bone marrow. Proc Natl Acad Sci USA. 2007;104:5091–5096. doi: 10.1073/pnas.0610819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Ma K, Chan CK, Liao S, Hwang WY, Feng Q, Ramakrishna S. Electrospun nanofiber scaffolds for rapid and rich capture of bone marrow-derived hematopoietic stem cells. Biomaterials. 2008;29:2096–2103. doi: 10.1016/j.biomaterials.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 7.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 8.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 9.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavassoli M, Minguell JJ. Homing of hemopoietic progenitor cells to the marrow. Proc Soc Exp Biol Med. 1991;196:367–373. doi: 10.3181/00379727-196-43201. [DOI] [PubMed] [Google Scholar]
- 11.Wilson JG. Adhesive interactions in hemopoiesis. Acta Haematol. 1997;97:6–12. doi: 10.1159/000203654. [DOI] [PubMed] [Google Scholar]
- 12.Mitsiadis TA, Barrandon O, Rochat A, Barrandon Y, De Bari C. Stem cell niches in mammals. Exp Cell Res. 2007;313:3377–3385. doi: 10.1016/j.yexcr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, Kato S, Ito M, Hotta T, Ando K. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- 14.Harrison RG. On the stereotropism of embryonic cells. Science. 1911;34:279–281. doi: 10.1126/science.34.870.279. [DOI] [PubMed] [Google Scholar]
- 15.Curtis AS, Varde M. Control of cell behavior: topological factors. J Natl Cancer Inst. 1964;33:15–26. [PubMed] [Google Scholar]
- 16.Curtis AS, Dalby M, Gadegaard N. Cell signaling arising from nanotopography: implications for nanomedical devices. Nanomed. 2006;1:67–72. doi: 10.2217/17435889.1.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Wagner V, Dullaart A, Bock AK, Zweck A. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 18.Wójciak-Stothard B, Madeja Z, Korohoda W, Curtis A, Wilkinson C. Activation of macrophage-like cells by multiple grooved substrata. Topographical control of cell behaviour. Cell Biol Int. 1995;19:485–490. doi: 10.1006/cbir.1995.1092. [DOI] [PubMed] [Google Scholar]
- 19.Wilkinson CDW, Riehle M, Wood M, Gallagher J, Curtis ASG. The use of materials patterned on a nano and micro-metric scale in cellular engineering. Mater Sci Eng C. 2002;19:263–269. [Google Scholar]
- 20.Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Topographical control of cell behaviour: II. Multiple grooved substrata. Development. 1990;108:635–644. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]
- 21.Curtis AS, Gadegaard N, Dalby MJ, Riehle MO, Wilkinson CD, Aitchison G. Cells react to nanoscale order and symmetry in their surroundings. IEEE Trans Nanobioscience. 2004;3:61–65. doi: 10.1109/tnb.2004.824276. [DOI] [PubMed] [Google Scholar]
- 22.Saha K, Pollock JF, Schaffer DV, Healy KE. Designing synthetic materials to control stem cell phenotype. Curr Opin Chem Biol. 2007;11:381–387. doi: 10.1016/j.cbpa.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60:215–228. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Webster TJ. Nanophase ceramics: The future orthopedic and dental implant material. Adv Chem Eng. 2001;27:125–166. [Google Scholar]
- 25.Mondrinos MJ, Koutzaki S, Jiwanmall E, Li M, Dechadarevian JP, Lelkes PI, Finck CM. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006;12:717–728. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 26.Martínez E, Engel E, Planell JA, Samitier J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann Anat. 2009;191:126–135. doi: 10.1016/j.aanat.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Leroueil PR, Hong S, Mecke A, Baker JR Jr, Orr BG, Banaszak Holl MM. Nanoparticle interaction with biological membranes: does nanotechnology present a Janus face? Acc Chem Res. 2007;40:335–342. doi: 10.1021/ar600012y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaIuppa JA, McAdams TA, Papoutsakis ET, Miller WM. Culture materials affect ex vivo expansion of hematopoietic progenitor cells. J Biomed Mater Res. 1997;36:347–359. doi: 10.1002/(sici)1097-4636(19970905)36:3<347::aid-jbm10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 30.Nur-E-Kamal A, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24:426–433. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Gao C, Shen J. Surface modification of polycaprolactone with poly(methacrylic acid) and gelatin covalent immobilization for promoting its cytocompatibility. Biomaterials. 2002;23:4889–4895. doi: 10.1016/s0142-9612(02)00247-8. [DOI] [PubMed] [Google Scholar]
- 32.Zhu Y, Gao C, Liu X, He T, Shen J. Immobilization of biomacromolecules onto aminolyzed poly(L-lactic acid) toward acceleration of endothelium regeneration. Tissue Eng. 2004;10:53–61. doi: 10.1089/107632704322791691. [DOI] [PubMed] [Google Scholar]
- 33.Kim TG, Park TG. Biomimicking extracellular matrix: cell adhesive RGD peptide modified electrospun poly(D,L-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 2006;12:221–233. doi: 10.1089/ten.2006.12.221. [DOI] [PubMed] [Google Scholar]
- 34.Ma Z, He W, Yong T, Ramakrishna S. Grafting of gelatin on electrospun poly(caprolactone) nanofibers to improve endothelial cell spreading and proliferation and to control cell Orientation. Tissue Eng. 2005;11:1149–1158. doi: 10.1089/ten.2005.11.1149. [DOI] [PubMed] [Google Scholar]
- 35.Ma Z, Kotaki M, Yong T, He W, Ramakrishna S. Surface engineering of electrospun polyethylene terephthalate (PET) nanofibers towards development of a new material for blood vessel engineering. Biomaterials. 2005;26:2527–2536. doi: 10.1016/j.biomaterials.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Ramakrishna S, Mayer J, Wintermantel E, Leong Kam W. Biomedical applications of polymer-composite materials: a review. Compos Sci Technol. 2001;61:1189–1224. [Google Scholar]
- 37.Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson L, Kahan B, Djamali A, Thomson J, Odorico JS. Differentiation of endoderm derivatives, pancreas and intestine, from rhesus embryonic stem cells. Transplant Proc. 2001;33:674. doi: 10.1016/s0041-1345(00)02196-5. [DOI] [PubMed] [Google Scholar]
- 39.Dalby MJ, Berry CC, Riehle MO, Sutherland DS, Agheli H, Curtis AS. Attempted endocytosis of nano-environment produced by colloidal lithography by human fibroblasts. Exp Cell Res. 2004;295:387–394. doi: 10.1016/j.yexcr.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Sill TJ, von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 42.von Recum AF, van Kooten TG. The influence of micro-topography on cellular response and the implications for silicone implants. J Biomater Sci Polym Ed. 1995;7:181–198. doi: 10.1163/156856295x00698. [DOI] [PubMed] [Google Scholar]
- 43.Powell HM, Kniss DA, Lannutti JJ. Nanotopographic control of cytoskeletal organization. Langmuir. 2006;22:5087–5094. doi: 10.1021/la052993q. [DOI] [PubMed] [Google Scholar]
- 44.Smetana K Jr. Cell biology of hydrogels. Biomaterials. 1993;14:1046–1050. doi: 10.1016/0142-9612(93)90203-e. [DOI] [PubMed] [Google Scholar]
- 45.Boyan BD, Hummert TW, Dean DD, Schwartz Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17:137–146. doi: 10.1016/0142-9612(96)85758-9. [DOI] [PubMed] [Google Scholar]
- 46.Nikolovski J, Mooney DJ. Smooth muscle cell adhesion to tissue engineering scaffolds. Biomaterials. 2000;21:2025–2032. doi: 10.1016/s0142-9612(00)00079-x. [DOI] [PubMed] [Google Scholar]
- 47.Cao H, McHugh K, Chew SY, Anderson JM. The topographical effect of electrospun nanofibrous scaffolds on the in vivo and in vitro foreign body reaction. J Biomed Mater Res A. 2009:Epub ahead of print. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, Shemin R, Beygui RE, MacLellan WR. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008;29:2907–2914. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Bei J, Wang S. Enhanced cell affinity of poly (D,L-lactide) by combining plasma treatment with collagen anchorage. Biomaterials. 2002;23:2607–2614. doi: 10.1016/s0142-9612(01)00400-8. [DOI] [PubMed] [Google Scholar]
- 50.Duan Y, Wang Z, Yan W, Wang S, Zhang S, Jia J. Preparation of collagen-coated electrospun nanofibers by remote plasma treatment and their biological properties. J Biomater Sci Polym Ed. 2007;18:1153–1164. doi: 10.1163/156856207781554019. [DOI] [PubMed] [Google Scholar]
- 51.Bisson I, Kosinski M, Ruault S, Gupta B, Hilborn J, Wurm F, Frey P. Acrylic acid grafting and collagen immobilization on poly(ethylene terephthalate) surfaces for adherence and growth of human bladder smooth muscle cells. Biomaterials. 2002;23:3149–3158. doi: 10.1016/s0142-9612(02)00061-3. [DOI] [PubMed] [Google Scholar]
- 52.Ma Z, Gao C, Gong Y, Shen J. Cartilage tissue engineering PLLA scaffold with surface immobilized collagen and basic fibroblast growth factor. Biomaterials. 2005;26:1253–1259. doi: 10.1016/j.biomaterials.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 53.Yang J, Bei JZ, Wang SG. [Study on improvement of cell affinity of polymer materials--modified poly(D,L-lactide) by anhydrous ammonia gaseous plasma] Zhongguo Xiufu Chongjian Waike Zazhi. 2001;15:269–272. [PubMed] [Google Scholar]
- 54.Ryu GH, Yang WS, Roh W, Lee IS, Kim JK, Lee GH, Lee DH, Park BJ, Lee MS, Park JC. Plasma surface modification of poly (D, L-lactic-co-glycolic acid) (65/35) film for tissue engineering. Surf Coat Technol. 2005;193:60–64. [Google Scholar]
- 55.Kasemo B. Biocompatibility of titanium implants: surface science aspects. J Prosthet Dent. 1983;49:832–837. doi: 10.1016/0022-3913(83)90359-1. [DOI] [PubMed] [Google Scholar]
- 56.Cochran D, Oates T, Morton D, Jones A, Buser D, Peters F. Clinical field trial examining an implant with a sand-blasted, acid-etched surface. J Periodontol. 2007;78:974–982. doi: 10.1902/jop.2007.060294. [DOI] [PubMed] [Google Scholar]
- 57.Spoerke ED, Stupp SI. Synthesis of a poly(L-lysine)-calcium phosphate hybrid on titanium surfaces for enhanced bioactivity. Biomaterials. 2005;26:5120–5129. doi: 10.1016/j.biomaterials.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 58.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 59.Li WJ, Tuli R, Huang X, Laquerriere P, Tuan RS. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials. 2005;26:5158–5166. doi: 10.1016/j.biomaterials.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 61.Gerechta S, Bettingerb CJ, Zhang Z, Borensteind JT, Vunjak-Novakovic G, Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28:4068–4077. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ponsonnet L, Reybier K, Jaffrezic N, Comte V, Lagneau C, Lissac M, Martelet C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater Sci Eng C. 2003;23:551–560. [Google Scholar]
- 63.Zhu X, Chen J, Scheideler L, Reichl R, Geis-Gerstorfer J. Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials. 2004;25:4087–103. doi: 10.1016/j.biomaterials.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Anselme K, Bigerelle M, Noel B, Dufresne E, Judas D, Iost A, Hardouin P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 2000;49:155–166. doi: 10.1002/(sici)1097-4636(200002)49:2<155::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 65.Ahmad M, Gawronski D, Blum J, Goldberg J, Gronowicz G. Differential response of human osteoblast-like cells to commercially pure (cp) titanium grades 1 and 4. J Biomed Mater Res. 1999;46:121–131. doi: 10.1002/(sici)1097-4636(199907)46:1<121::aid-jbm14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 66.Oha S, Brammera KS, Li YSJ, Teng D, Adam J, Engler AJ, Chien S, Jin S. Stem cell fate dictated solely by altered nanotube dimension. Proc Natl Acad Sci USA. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park J, Bauer S, von der Mark K, Schmuki P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007;7:1686–1691. doi: 10.1021/nl070678d. [DOI] [PubMed] [Google Scholar]
- 68.Friedl P, Bröcker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu LMY, Leipzig ND, Shoichet MS. Promoting neuron adhesion and growth. Materials Today. 2008;11:36–43. [Google Scholar]
- 70.Fan YW, Cui FZ, Hou SP, Xu QY, Chen LN, Lee IS. Adhesion of neural cells on silicon wafer with nanotopographic surface. Appl Surf Sci. 2002;187:313–318. [Google Scholar]
- 71.Massia SP, Hubble JA. An rgd spacing of 440 nm is sufficient for integrin a-v-b-3-mediated fibroblast spreading and 140nm for focal contact and stress fiber formation. J Cell Biol. 1991;114:1089–1100. doi: 10.1083/jcb.114.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kommireddy DS, Sriram SM, Lvov YM, Mills DK. Stem cell attachment to layer-by-layer assembled TiO2 nanoparticle thin films. Biomaterials. 2006;27:4296–4303. doi: 10.1016/j.biomaterials.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 73.Webster TJ, Siegel RW, Bizios R. Osteoblast adhesion on nanophase ceramics. Biomaterials. 1999;20:1221–1227. doi: 10.1016/s0142-9612(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 74.Seil JT, Webster TJ. Decreased astroglial cell adhesion and proliferation on zinc oxide nanoparticle polyurethane composites. Int J Nanomedicine. 2008;3:523–531. doi: 10.2147/ijn.s4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Webster TJ, Hellenmeyer EL, Price RL. Increased osteoblast functions on theta + delta nanofiber alumina. Biomaterials. 2005;26:953–960. doi: 10.1016/j.biomaterials.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 76.Gutwein LG, Tepper F, Webster TJ. Increased osteoblast function on nanofibered alumina. 26th Annual American Ceramic Society Meeting. FL: Cocoa Beach; 2004. [Google Scholar]
- 77.Woo KM, Chen VJ, Ma PX. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J Biomed Mater Res A. 2003;67:531–537. doi: 10.1002/jbm.a.10098. [DOI] [PubMed] [Google Scholar]
- 78.Yoshimoto H, Shin YM, Terai H, Vacanti JP. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 79.Hosseinkhani H, Hosseinkhani M, Tian F, Kobayashi H, Tabata Y. Osteogenic differentiation of mesenchymal stem cells in self-assembled peptide-amphiphile nanofibers. Biomaterials. 2006;27:4079–4086. doi: 10.1016/j.biomaterials.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 80.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 81.Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 83.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 84.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Muraglia A, Corsi A, Riminucci M, Mastrogiacomo M, Cancedda R, Bianco P, Quarto R. Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J Cell Sci. 2003;116:2949–2955. doi: 10.1242/jcs.00527. [DOI] [PubMed] [Google Scholar]
- 86.Osyczka AM, Nöth U, O'Connor J, Caterson EJ, Yoon K, Danielson KG, Tuan RS. Multilineage differentiation of adult human bone marrow progenitor cells transduced with human papilloma virus type 16 E6/E7 genes. Calcif Tissue Int. 2002;71:447–458. doi: 10.1007/s00223-001-1090-2. [DOI] [PubMed] [Google Scholar]
- 87.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 88.Hu J, Feng K, Liu X, Ma PX. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials. 2009;30:5061–5067. doi: 10.1016/j.biomaterials.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng L, Zhang SM, Chen PP. Fabrication and characterization of nano-hydroxyapatite/poly (D,L-lactide) composite porous scaffolds for human cartilage tissue engineering. Key Engineering Materials. 2006;18:943–946. [Google Scholar]
- 90.Matthews JA, Boland ED, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen type II: A feasibility study. J Bioactive Compatible Polym. 2003;18:125–134. [Google Scholar]
- 91.Koh HS, Yong T, Chan CK, Ramakrishna S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials. 2008;29:3574–3582. doi: 10.1016/j.biomaterials.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 92.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 93.Prabhakaran MP, Venugopal JR, Ramakrishna S. Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials. 2009;30:4996–5003. doi: 10.1016/j.biomaterials.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 94.Teo WE, Ramakrishna S. Electrospun nanofibers as a platform for multifunctional, hierarchically organized nanocomposite. Compos Sci Technol. 2009;69:1804–1817. [Google Scholar]
- 95.Yang F, Murugan R, Ramakrishna S, Wang X, Ma YX, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 96.Levenstein ME, Ludwig TE, Xu RH, Llanas RA, VanDenHeuvel-Kramer K, Manning D, Thomson JA. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holtorf HL, Jansen JA, Mikos AG. Ectopic bone formation in rat marrow stromal cell/titanium fiber mesh scaffold constructs: effect of initial cell phenotype. Biomaterials. 2005;26:6208–6216. doi: 10.1016/j.biomaterials.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 98.Lu Y, Mapili G, Suhali G, Chen S, Roy K. A digital micro-mirror device-based system for the microfabrication of complex, spatially patterned tissue engineering scaffolds. J Biomed Mater Res A. 2006;77:396–405. doi: 10.1002/jbm.a.30601. [DOI] [PubMed] [Google Scholar]
- 99.Taqvi S, Dixit L, Roy K. Biomaterial-based notch signaling for the differentiation of hematopoietic stem cells into T cells. J Biomed Mater Res A. 2006;79:689–697. doi: 10.1002/jbm.a.30916. [DOI] [PubMed] [Google Scholar]
- 100.Peter SJ, Lu L, Kim DJ, Stamatas GN, Miller MJ, Yaszemski MJ, Mikos AG. Effects of transforming growth factor beta1 released from biodegradable polymer microparticles on marrow stromal osteoblasts cultured on poly(propylene fumarate) substrates. J Biomed Mater Res. 2000;50:452–462. doi: 10.1002/(sici)1097-4636(20000605)50:3<452::aid-jbm20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 101.He L, Liao S, Quan D, Ngiam M, Chan CK, Ramakrishna S, Lu J. The influence of laminin-derived peptides conjugated to Lys-capped PLLA on neonatal mouse cerebellum C17.2 stem cells. Biomaterials. 2009;30:1578–1586. doi: 10.1016/j.biomaterials.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 102.Gupta D, Venugopal J, Prabhakaran MP, Dev VR, Low S, Choon AT, Ramakrishna S. Aligned and random nanofibrous substrate for the in vitro culture of Schwann cells for neural tissue engineering. Acta Biomater. 2009;5:2560–2569. doi: 10.1016/j.actbio.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 103.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 104.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 105.Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20:92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 106.Webster TJ, Ejiofor JU. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials. 2004;25:4731–4739. doi: 10.1016/j.biomaterials.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Vance RJ, Miller DC, Thapa A, Haberstroh KM, Webster TJ. Decreased fibroblast cell density on chemically degraded poly-lactic-co-glycolic acid, polyurethane, and polycaprolactone. Biomaterials. 2004;25:2095–2103. doi: 10.1016/j.biomaterials.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 108.Price RL, Waid MC, Haberstroh KM, Webster TJ. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials. 2003;24:1877–1887. doi: 10.1016/s0142-9612(02)00609-9. [DOI] [PubMed] [Google Scholar]
- 109.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 110.Kay S, Thapa A, Haberstroh KM, Webster TJ. Nanostructured polymer/nanophase ceramic composites enhance osteoblast and chondrocyte adhesion. Tissue Eng. 2002;8:753–761. doi: 10.1089/10763270260424114. [DOI] [PubMed] [Google Scholar]
- 111.Webster TJ, Schadler LS, Siegel RW, Bizios R. Mechanisms of enhanced osteoblast adhesion on nanophase alumina involve vitronectin. Tissue Eng. 2001;7:291–301. doi: 10.1089/10763270152044152. [DOI] [PubMed] [Google Scholar]
- 112.Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Specific proteins mediate enhanced osteoblast adhesion on nanophase ceramics. J Biomed Mater Res. 2000;51:475–483. doi: 10.1002/1097-4636(20000905)51:3<475::aid-jbm23>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 113.Gutwein LG, Webster TJ. Increased viable osteoblast density in the presence of nanophase compared to conventional alumina and titania particles. Biomaterials. 2004;25:4175–4183. doi: 10.1016/j.biomaterials.2003.10.090. [DOI] [PubMed] [Google Scholar]
- 114.Thapa A, Miller DC, Webster TJ, Haberstroh KM. Nano-structured polymers enhance bladder smooth muscle cell function. Biomaterials. 2003;24:2915–2926. doi: 10.1016/s0142-9612(03)00123-6. [DOI] [PubMed] [Google Scholar]
- 115.Thapa A, Webster TJ, Haberstroh KM. Polymers with nano-dimensional surface features enhance bladder smooth muscle cell adhesion. J Biomed Mater Res A. 2003;67:1374–1383. doi: 10.1002/jbm.a.20037. [DOI] [PubMed] [Google Scholar]
- 116.Mo XM, Xu CY, Kotaki M, Ramakrishna S. Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25:1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 117.Yim EKF, Pang SW, Yee AF, Chen CS, Leong KW. Alignment and reduced proliferation of smooth muscle cells on nanopattern [abstract] Proceedings of the annual meeting of the Biomedical Engineering Society; 2004. [Google Scholar]
- 118.Xu C, Inai R, Kotaki M, Ramakrishna S. Electrospun nanofiber fabrication as synthetic extracellular matrix and its potential for vascular tissue engineering. Tissue Eng. 2004;10:1160–1168. doi: 10.1089/ten.2004.10.1160. [DOI] [PubMed] [Google Scholar]