Abstract
The possibility of treating degenerative diseases by stem cell-based approaches is a promising therapeutical option. Among major concerns for the clinical application of stem cells, some derive from the possibility that stem cells may be rejected by the immune system as a consequence of histoincompatibility and that stem cells themselves may interfere with the normal functions of host immune response. Therefore, the immunogenicity and the immunomodulatory properties of stem cells must be carefully addressed. Although these properties are common features of different stem cell types, some peculiarities can be recognized and characterized for their proper clinical use.
Keywords: Immune suppression, Embryonic stem cells, Mesenchymal stem cells, Immunogenicity, Regenerative medicine, Neural stem cells
INTRODUCTION
In the case of tissue injury, activation of the immune system and cell regeneration from precursor cells normally occur. In mammals, these defensive mechanisms may differ depending on the organ considered: some organs such as the skin are highly regenerating; others such as the central nervous system (CNS) apparently are not. Embryonic and adult stem cells, due to their ability to self-renew and differentiate into many cell types, have been recently considered as promising tools for regenerative and cell-based therapies in a number of degenerative diseases[1]. Especially, those resulting from the destruction and/or dysfunction of a limited number of cell types such as diabetes mellitus, Parkinson’s disease[2], spinal cord injury[3], liver[4] and heart failure[5], Duchenne’s muscular dystrophy[6] and osteogenesis imperfecta[7]. Embryonic stem cells (ES) have remarkable long-term proliferative potential, providing the possibility of unlimited expansion in culture[8] and a broad differentiation potential[9]. However, important ethical and safety issues still need to be addressed[10], i.e. the risk of teratoma formation after transplantation[1,11]. On the other hand, ES cells are highly prone to be killed by effector cells in immunocompetent allogeneic recipients[12]. Strategies to provide HLA-matched human ES are focused on the establishment of HLA-typed ES bank[13].
Adult, tissue-specific, somatic stem cells have more restricted proliferation and differentiation potential but less ethical and safety implications. Many adult tissues host a stem cell compartment that could be ex-vivo expanded and used as a therapeutic tool for tissue regeneration. Theoretically, tissue-specific, adult stem cell-based therapy could be designed in the autologous setting. However, many of the clinical and preclinical studies with tissue specific adult stem cells require the allogeneic setting[14]. Thus, the immunological properties of these stem cells as well as the interaction with host immune effector cells are very important.
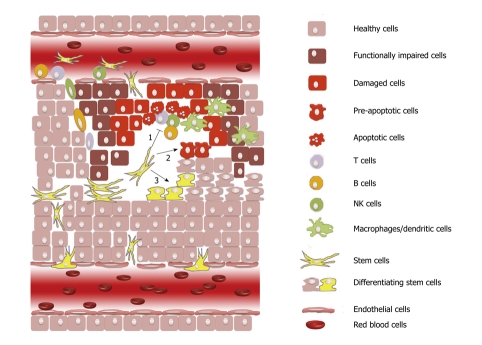
Some of the benefits obtained with stem cell therapy are not due to cell replacement but rather to the protective effect of trophic and anti-apoptotic factors released in the damaged tissues by either the grafted stem cells themselves or by endogenous cells following the interaction with the grafted stem cells[15-18]. Many of these factors are mediators of inflammation that allow stem cells to survive and specifically migrate to the damaged area[19] such as cell-adhesion molecules and chemokine receptors[20,21]. The ability of different stem cell types, especially mesenchymal stem cells, to modulate the immune response has been described in many in vitro and in vivo studies. Immunomodulatory mechanisms seem to play an important role not only in the autologous and allogeneic therapeutic approaches but also for the normal endogeneous tissue regeneration[22]. Considering the pathological processes occurring upon degeneration, cell loss and immune activation/inflammation are indeed strictly related. Therefore, it is not surprising that stem cells and the immune system may play a finely tuned cross-talk aimed to confine tissue loss and to promote regeneration (Figure 1).
Figure 1.
Different mechanisms may have a role in the positive effects following the recruitment of stem cells. (1) modulation of the immune effector cells involved in the onset and extension of tissue damage; (2) release of trophic and anti-apoptotic factors that may either hamper tissue degeneration or favor spontaneous cell recovery; and (3) direct replacement of dead cells through tissue-specific differentiation.
EMBRYONIC STEM CELLS
ES express low levels of HLA class I molecules[23] which are up-regulated by IFN-γ stimulation, after teratoma formation[11,23,24] or differentiation[24-28] and almost undetectable expression of HLA class II and costimulatory molecules[25]. Although the immune stimulation induced by ES is lower than that by allogeneic adult cells, HLA class I molecule expression in ES is sufficient for rejection mediated by cytotoxic T cells[25,29]. Data regarding immunogenicity of ES are not concordant. Mouse ES have been shown to survive in immunocompetent mice[24,30] as well as in rats[31] and sheep[32] for many weeks after transplantation. Similarly, rat ES permanently engraft in allogeneic recipients leading to allo-specific down-regulation of the host immune response[33]. On the contrary, murine ES transplantion into injured myocardium determined tissue infiltration by T cells, B cells and macrophages, followed by the disappearance of ES cells and their progeny over a period of weeks[28,34]. When transplanted in an immunocompetent xenogeneic host, human ES triggered robust cellular and humoral immune responses leading to intragraft infiltration of inflammatory cells and subsequent ES rejection[35]. In this setting, CD4+ T cells seem to play an important modulatory role in ES immune-mediated rejection. Notably, repeated transplantation of ES into immunocompetent hosts results in accelerated human ES death, suggesting an adaptive donor-specific immune response[28]. Transplantation in immunodeficient mice or together with the administration of immunosuppressive drug regimens can mitigate the anti-ES immune response and significantly prolongs xeno-transplantation survival. Beside the low immunogenicity, ES have also shown evidence of immunomodulatory properties both in vitro and in vivo. Human ES are not recognized in vitro by NK cells and inhibit T-cell activation by third party antigen presenting cells[25]. However, ES cells injected in vivo into immunocompetent recipients resulted in being highly susceptible to killing by NK cells due to their expression of ligands of the activating NK receptor NKG2D[11]. For this reason and as a consequence of the increasing tissue transplantation demand, some countries are making efforts to establish HLA-typed human ES banks to collect HLA-matched human ES to overcome the current immunological problem[13].
NEURAL STEM CELLS
Neural stem/progenitor cells (NSCs) are tissue precursor cells that have been found in the main neurogenic regions of the adult brain, i.e. hippocampus, subventricular zone (SVZ), olfactory bulb[36,37] and in some non-neurogenic regions, i.e. spinal cord[38]. Despite their self-renewal capability, NSC neuro-glial differentiation potential and the possible use in autologous setting are still debated. Among technical problems, of relevance is that NSCs are not easily accessible, they are difficult to expand in vitro as homogeneous stem cell population and show a low rate of in vivo neuronal differentiation efficiency[39]. Moreover, adult NSCs can be easily expanded only from rodent adult brain; by contrast, it is difficult to obtain adult NSCs from human tissues. For this reason, most of the studies on human NSCs are carried out with NSCs of fetal origin[40-42].
For a long time the central nervous system has been considered an immune privileged organ as it does not contain either lymphoid or dendritic cells and it is partially isolated from circulating immune cells by the blood-brain barrier (BBB)[43]. However, this privilege is not absolute as neural grafts placed in CNS may be rejected although less quickly than in other organs. However, the rejection can be enhanced by brain trauma leading to BBB interruption and infiltration by immune effector cells[44]. Primary neural cell culture has been reported to up-regulate major histocompatibility complex (MHC) proteins in cell populations normally displaying low expression profiles in vivo[45]. In vitro cultured NSCs before differentiating exhibit low MHC molecule expression[46,47] that then increases especially in differentiated astrocytes[47]. Isolated NSCs express also the costimulatory molecules CD80 (B7.1) and CD86 (B7.2). The exposure to pro-inflammatory cytokine (i.e. IFN-γ and TNF-α) enhances the expression of CD80, CD86 and MHC class I (but not class II) molecules[48,49]. Similarly, in vitro expansion of human forebrain and spinal cord neural cells results in the induction of HLA class I and II molecules[50]. However, human NSC lines can be recognized by allogeneic PBLs regardless low levels of MHC expression[51]. In a model of brain trauma allogeneic NSC grafts may be immunogenic as shown by the evidence of lymphocyte infiltration after transplantation[52]. In addition, human neural progenitor cells express many adhesion molecules involved in inflammation such as α2, α6 and β1 integrins[53], CD44 and chemokine receptors (CCR3, CCR6, CCR7, CCR9, CXCR3)[54]. Less than 25% of human NSCs express the inflammatory chemokine receptors CCR4, CCR5 and CXCR4[41]. Nevertheless, the in vivo trophic and immunomodulatory properties of NSCs have recently become as evident as their regenerative potential[20,55-57]. Rodent SVZ-derived and human ES-derived NSCs exhibit an inhibitory effect on T lymphocytes both in vitro and in vivo[55,56,58,59]. Rodent and human NSCs can suppress T cell proliferation in a dose-dependent fashion[41] and inhibit antigen (myelin)-specific immune responses. Interestingly, the suppression of T cell proliferation from NSCs does not require cell-to-cell contact[55,56,58,59]. Mouse and human NSCs may impair the activation of myeloid dendritic cells (DCs)[60] and the differentiation of CD14+ myeloid cells into CD1a+ immature and then functional (antigen-presenting) DCs. Additionally, NSCs prevent the up-regulation in DCs of the costimulatory molecules CD80, CD86 and of MHC class II molecules induced by LPS as well as the in vivo DC activation within draining lymph nodes[60]. The in vivo immunomodulatory properties of NSC have been tested in several neurological diseases in which the immune response plays a role[61].
MESENCHYMAL STEM CELLS
First described in bone marrow as multipotent non-hemapoietic progenitor cells[62], mesenchymal stem cells/multipotent marrow stromal cells (MSCs) are multipotent adult stem cells capable of differentiating both in vitro and in vivo into various tissues of mesodermal origin such as fibroblasts, osteocytes, adipocytes and chondrocytes[63]. Moreover, some studies have shown the MSCs potential to differentiate into tissues of endodermal and neuroectodermal lineages including hepatocyte[64], epithelia[65] and neurons[66,67]. Stromal cell precursors with the immunophenotype and multilineage differentiation potential of MSCs are present also in adult lymphoid tissues such as lymph nodes[68] spleen and thymus[69]. MSCs reside virtually in all the tissues as part of the pericyte population in the vasculature wall[70]. Besides their differentiation potential, MSCs can exert important trophic effects supporting hematopoiesis and angiogenesis[71,72].
MSCs exert a profound immune modulatory effect capable of suppressing lymphocyte proliferation in vitro and prolonging MHC-mismatched skin graft survival in vivo[73]. Subsequently, MSC regulatory activity has been characterized on a large number of effector cells of adaptive and innate immunity including CD4+ and CD8+ T cells[74-82], B cells[76,83-87], NK cells[76,88-90], monocyte-derived DCs[91-96] and neutrophils[96]. The interaction with MSCs leads to lymphocyte[78,97] and DC[97] anergy due to early proliferation arrest and inhibits apoptosis of resting and activated neutrophils[96]. MSCs may suppress immune reactions in vitro and in vivo in a MHC-independent manner[75,76]. Interestingly, the immune regulatory properties are expressed not only by bone marrow MSCs but also by MSCs derived from other tissues including fat[98], thymus, spleen[69], and others[99-101]. Moreover, MSCs differentiated into fibroblasts, adipocytes, and osteoblasts[102-104] retain similar functions. At present, there is no unique and hierarchically prevalent mechanism responsible for MSC immune regulation but there is a redundant panel of mechanisms which suggests the in vivo importance of the immune regulation by stromal cell compartment. Some contradictory results have been produced by different groups probably due to different experimental factors related to MSC origin, culture conditions, lymphocyte subset and cell activation state. Interactions between MSCs and cells of the adaptive immune system could vary depending on the microenvironment in which the reaction takes place. On the whole, both soluble factors and cell-cell contact are involved.
In vivo, MSC infusion can significantly lower the incidence and cure the refractoriness to treatment of graft-versus-host disease (GvHD) after allogeneic hematopoietic stem cell transplantation in humans[14,105] and improve experimental autoimmune encephalomyelitis (EAE) in mice[106,107].
MSCs are unable to induce significant alloreactivity[75]. Human MSCs express low-intermediate level of HLA-class I and LFA-3 and they do not express the co-stimulatory molecules CD80 (B7-1), CD86 (B7-2), CD40 or CD40L even after IFN-γ stimulation[76,102,108] which in turn may induce HLA-class II molecule up-regulation[76]. In addition, human MSCs express HLA-G, a non-classical MHC class I antigen that may prevent the immune response against MSCs, as shown by blocking experiments although the expression seems to decrease in culture[109]. MSCs may escape not only from the recognition by alloreactive T-cells but also the cell-specific lysis by CD8+ cytotoxic cells[110] and freshly isolated NK cells[80]. By contrast, activated NK cells are capable to lyse MSCs efficiently[88]. Moreover, MSCs exogenously loaded with the relevant MHC class I peptide epitopes still remain resistant to lysis[111]. Transplanted allogeneic MHC-mismatched MSCs fail to induce specific rejection, thus engrafting in adult rodent, porcine and baboon experimental models. Engraftment of allogeneic MSCs in immune-compromised hosts or inside immune privileged sites have been shown in animals and in humans[73,112]. Xenogenic transplantation (mouse MSCs into rats) may induce immunological tolerance[113]. By contrast, allogeneic MSC transplantation into hosts with intact immune system may determine MSC rejection[114,115]. The infusion of allogeneic MSCs can prime naïve T cells in immunocompetent mice[116]. Moreover, intra-coronary injection of adult human MSCs in rat myocardium is associated with rejection and macrophages infiltration[117]. Culture conditions may affect MSC immunogenicity[118]. However, patients treated with allogeneic human MSCs did not show anti-allogeneic MSC antibody production or T-cell priming[119].
MSCs and T lymphocytes
T cell proliferation, activation and effector functions may be affected by MSCs in vitro[74] and in vivo[73]. Inhibition of T-cell proliferation by MSCs occurs not only when T cells are triggered by non-specific stimuli such as allogeneic peripheral blood lymphocytes, dendritic cells or mitogens such as phytohaemagglutinin (PHA) or IL-2[74] but also when T cells are activated by their specific antigen[75]. Similarly, T cell-mediated IFN-γ production[75,76,78] and cytotoxic activity[75,110] may be inhibited. Proliferation of CD4+ and CD8+ T cells is equally inhibited by MSCs[74-76]. This effect does not seem to be related either to the lack of activation or the induction of apoptosis[78]. In fact, in T-cell/MSC co-culture the number of T cells expressing early activation markers i.e. CD25 and CD69 is not affected although some data are contradictory[102,108,120,121]. CD8+ T-cell mediated lysis is suppressed by MSCs if they are added at the beginning of the mixed lymphocyte culture[80] but not when T cells are already in the cytotoxic phase[122,123] thus suggesting that it is the generation of activated lytic effector cells affected rather than the lytic effector phase. MSCs interfere with naive CD4+ T cell differentiation into T helper (Th)-1 effector cells by decreasing the amount of IFN-γ produced. In addition, MSCs may induce a Th-2 shift, by increasing the production of IL-4[124]. Both naive and memory T cells can be inhibited by MSCs[75]. In a mouse model, IFN-γ production by T cells may be restored after MSC removal from culture[75]; by contrast, T cell proliferation is irreversibly abrogated by cyclin-D2 inhibition, thus suggesting a mechanism of T cell arrest anergy in the early G1 phase of the cell cycle[78]. This anergic state is only partially reverted by exogenous IL-2. Other studies with human MSCs show that T cell unresponsiveness is transient and may be restored by MSC removal[125].
The presence of CD4+/CD25+ T cells is not required for the anti-proliferative effect of MSC on T-cells[75]; however, MSCs may induce the expansion of these regulatory T cells[123,124] capable of inhibiting mixed lymphocyte reactions and T cell activation[126]. MSC-induced suppression of T cell proliferation does not require MHC restriction but it may be mediated also by allogeneic MSCs[29] in a dose-dependent and antigen-independent manner[75,103]. The optimal ratio between MSCs and responder T cells is quite variable from 1:100[75] to 1:1[93] depending on the MSC model (human or animal), the culture conditions and the origin and purity of MSCs but most studies show that at 1:10 ratio the maximum inhibitory effect normally occurs[75,76,124]. It is difficult to assess if these ratios are reached inside the tissues but they are not unlikely; in addition, the persistence of the immune regulatory properties in MSC-derived tissue stromal cells[104,127] would suggest that this phenomenon may have a physiological role also in vivo. MSC may inhibit the apoptosis of proliferating thymocytes cultured in the absence of trophic factors and resting T-cells[127,128]. Moreover, MSCs may rescue from activation-induced cell death (AICD) T cells over-stimulated by T cell receptor (TCR) engagement through a down-regulation of Fas receptor and Fas ligand[128].
MSC-induced immunosuppression is due to both soluble factors and cell-cell contact but the latter mechanism is prevalent in rodent MSCs[74-82]. Most of the inhibitory soluble factors are not constitutively secreted by MSCs but they can be induced by the interaction between activated effector cells and MSCs. A broad panel of factors is involved in the immune regulation induced by MSCs including interferon-γ (IFN-γ)[69,75], IL-1β[120], transforming growth factor-β1 (TGF-β1)[74,82,103], indoleamine 2,3-dioxygenase (IDO)[75,76,79], IL-6[129,130], IL-10[91,92], prostaglandin E2 (PGE2)[124], hepatocyte growth factor (HGF)[74], tumor necrosis factor (TNF)-α[122,125,131], nitrix oxide (NO)[64], heme oxygenase-1 (OH-1)[132], HLA-G5[109,133] and other unknown factors. This probably reflects the redundancy of the MSCs immune regulatory mechanisms. It is interesting that cytokines flavoring the immune responses such as IFN- γ produced by activated T lymphocytes or NK cells may promote the immune modulation by MSCs which in turn suppress T- or NK-cell proliferation. This effect is related at least in part to the enhancement of the IDO activity[76,134]. However, human IFN-γ receptor 1(R1)-deficient MSCs do not elicit IDO transcription despite the preservation of immune regulation[135]. Following cell-cell contact with T cells, MSCs can secrete the soluble isoform of HLA-G5, CCL-1 and LIF that seem to mediate, at least in part, the expansion of functional CD4+CD25highFoxP3+ regulatory T cells[83,136,137]. MSCs recruit, regulate and maintain T-regulatory phenotype and function for a long period of time.
MSCs were found to express some Toll-like receptors such as TLR 1, TLR3, TLR4 and TLR5. The triggering of TLR3 and TLR4 by their natural ligands may suppress MSC immune regulatory activity thus suggesting that T-cell responses may arise efficiently during infections leading to pathogen elimination[138].
MSCs and NK cells
MSCs inhibit both IL-2- and IL-15-induced NK proliferation[75,88]. Soluble factors or cell-cell contact mediate different effects depending on the experimental settings. Thus, IFN-γ secretion following IL-2-mediated NK stimulation is responsible for the inhibition of NK proliferation by MSCs[75]; on the other hand, MSC-dependent inhibition of IL-15-activated NK cells requires both cell-cell contact and soluble factors such as TGFβ1 and PGE2 that are produced during MSC/NK co-culture[88]. The influence of MSCs on cytotoxicity of freshly isolated NK is still controversial. In some studies with freshly isolated NK cells, no MSC-mediated modulation of cytotoxicity has been observed towards HLA-class I negative targets (K562 cell line) whereas MSCs may impair the cytolytic activity against HLA-class I positive targets[88]. In other experiences, MSCs not only inhibit the cytokine-induced proliferation of freshly isolated NK cells but also prevent their effector functions and cytokine production against HLA- class I -positive as well as class I-negative target cells (SKNBE and HTLA-30 cell lines)[90]. Thus, MSC suppression of NK cytolytic activities may be stronger against HLA-class I negative targets expressing a limited number of ligands for different NK receptors. Instead, when considering IL-15-activated NK, the suppressive effect of MSCs on NK cytotoxicity depends on culture time. In fact, short-term co-culture of IL-15-stimulated NK cells and MSCs leads to the inhibition of NK cytolytic activity against both the HLA class I -negative and -positive cells[89]. This phenomenon is associated with the reduction of IL-15-induced cytokines such as IFN-γ, IL-10 and TNF-α and it requires cell-cell contact[89]. Similar results have been obtained with prolonged co-culture of IL-2-activated NK cells with MSCs, leading to the decrease of killing against the HLA class I-negative K562 cell line[75]. Taken together, these data show that MSCs may inhibit NK functions against HLA class I-negative and positive targets which, in turn become less susceptible to NK attack. The suppression of NK lytic activity and IFN-γ secretion have been related to the release by MSCs of HLA-G5[133], a soluble isoform of non classical HLA class I, usually expressed in a few healthy tissues such as cytotrophoblasts but also involved in tumor-driven immune escape and to IDO activity[90].
MSC susceptibility to NK-mediated killing by activated NK is due to the MSC expression of some ligands for NK receptors such as NKp30, NKG2D and DNAM-1 KK[89]. After IL-2 activation, NK may lyse MSCs in both autologous and allogeneic settings[89]. However, this phenomenon may be partially prevented by IFN-γ which up-regulates the expression of HLA I molecules by MSCs[95]; in addition, MSCs may inhibit the surface expression of NKp30 and NKG2D as well as NKp44 activating receptor, thus impairing NK effector functions[139].
MSCs and dendritic cells
MSCs strongly inhibit DC generation from peripheral blood monocytes[91,92] without interfering with LPS-induced maturation of immature DCs. Moreover, MSCs block monocytes by determining division arrest anergy[140]. Inhibition of DC differentiation by MSC seems to be reversible as MSCs do not affect the maturation process of DCs once they are already committed into immature DC. MSCs produce a shift from DCs type 1 to a more tolerogenic phenotype DC type 2 by increasing interleukin-10 (IL-10) production[93] and decreasing TNF-α secretion. This leads to a reduced number of IFN-γ-producing Th1 cells[124] and favors IL-4-producing Th2 cells and regulatory T cells[124]. The mechanisms leading to the inhibition of DC commitment by MSC imply both secretion of soluble factors and cell-to-cell contact. IL-6, macrophage-colony-stimulating factor and PGE2 are involved[116,130]. Interestingly, PGE2 seems to be a key inhibitory mediator acting independently of IL-6.
MSCs and B cells
B-cell development occurs in the bone marrow and is strictly dependent on the close interaction of B-cell progenitors with stromal cells that produce trophic factors both supporting B-cell survival and proliferation and maintaining long-living plasma cells. Depending on experimental conditions, particularly regarding the strength and the quality of B-cell stimulation, MSCs have been shown to either inhibit or support both proliferation and differentiation of B-cells. The proliferative stimuli used in MSC/B-cells interaction studies were either T-independent[75,141] or T-dependent[78,142], specific[83] or not specific[139]. When strong primary stimulus is used to activate B cells such as BCR triggering, CD40, Toll-like receptor 9 (TLR9), IL2R and IL4R, the inhibition of proliferation and immunoglobulin production occurs. The addition of blocking antibodies against the molecules of the programmed death pathway (PD-1, PD-L1 and PD-L2) may restore about 30% of B cell proliferation[139]. The arrest of B-lymphocytes cell cycle in G0/G1 phases rather than the induction of apoptosis[83], seems to be the MSC-dependent mechanism. Notably, adipose tissue-derived MSCs suppressed Ig production to a much greater extent than BM-MSCs. However, in some culture conditions, IgG secretion and B-cell proliferation can be induced[143] and B-cell survival sustained and this effect does not depend on the presence of IFN-γ in the culture. In the absence of B cell receptor triggering, naïve B cells stimulated with an agonist of TLR9 are promoted to proliferate and differentiate into immunoglobulin-secreting cells by MSC. The effects of MSCs on B cells are dose-dependent and the MSC/B-cell ratios at which effects have been observed may vary according to culture conditions. Most results have been observed MSC modulatory effect at 1:1 ratio[83] but other studies suggest that lower ratios such as 1:10[143,144] and 1:30[141] are still effective.
Pre-clinical and clinical trials based on the immunomodulation of MSCs have been attempted. Overall data are encouraging and confirm the profound immunomodulation of MSCs described in vitro[14,107,145-150].
OTHER STEM CELL TYPES
Other stem cell populations have been studied for their immunomodulatory properties. Amnion-derived multipotent progenitor cells express MHC class I molecules but they lack MHC class II antigens and the co-stimulatory molecules B7-1 and B7-2. Moreover, they express HLA–G that can be increased after IFNγ treatment. These stem cells may inhibit peripheral blood mononuclear cell proliferation in response to mitogens, alloantigens and recall antigens. This immunomodulatory effect was found to be dependent on cell-to-cell contact. Recently, a stem cell population with trophic and immunoregulatory functions from human intestinal tissues was characterized. Immunomodulatory activity was shown in co-cultures with normal heterologous phytohemagglutinin-stimulated peripheral blood mononuclear cells[151].
CONCLUSION
Tissue damage derives from both cell degeneration and development of inflammation, variably combined. The therapeutical potential of stem cell-based therapy is complex and related to different effects in vivo that may vary depending on the pathological microenvironments. Many stem cell types have both regenerative potential and immunomodulatory functions. As stem cells not only may be theoretically rejected by immune system but also interfere with the normal functions of host immune response, the understanding of their immunomodulatory properties in vitro and in vivo have great relevance for their proper clinical use.
Footnotes
Peer reviewers: James Alexander Ross, PhD, Professor, University of Edinburgh, Tissue Injury & Repair Group, Centre for Regenerative Medicine, The Chancellor’s Building, 49 Little France Crescent, Edinburgh EH16 4SB, United Kingdom; Alain Chapel, PhD, Departement of Man Radioprotection, Institute of Nuclear Safety and Radioprotection, Fontenay-aux-Roses Cedex 92262, France
S- Editor Li LF L- Editor Roemmele A E- Editor Yang C
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 3.Barnabé-Heider F, Frisén J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Fiegel HC, Lange C, Kneser U, Lambrecht W, Zander AR, Rogiers X, Kluth D. Fetal and adult liver stem cells for liver regeneration and tissue engineering. J Cell Mol Med. 2006;10:577–587. doi: 10.1111/j.1582-4934.2006.tb00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 6.Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthélémy I, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 9.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 10.Vogel G, Holden C. Stem cells. Ethics questions add to concerns about NIH lines. Science. 2008;321:756–757. doi: 10.1126/science.321.5890.756b. [DOI] [PubMed] [Google Scholar]
- 11.Dressel R, Schindehütte J, Kuhlmann T, Elsner L, Novota P, Baier PC, Schillert A, Bickeböller H, Herrmann T, Trenkwalder C, et al. The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients' immune response. PLoS One. 2008;3:e2622. doi: 10.1371/journal.pone.0002622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci USA. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borstlap J, Kurtz A. Integration of immunological aspects in the European Human Embryonic Stem Cell Registry. Eur J Immunol. 2008;38:1181–1185. doi: 10.1002/eji.200890016. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 15.Cho JS, Park HW, Park SK, Roh S, Kang SK, Paik KS, Chang MS. Transplantation of mesenchymal stem cells enhances axonal outgrowth and cell survival in an organotypic spinal cord slice culture. Neurosci Lett. 2009;454:43–48. doi: 10.1016/j.neulet.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Sarnowska A, Braun H, Sauerzweig S, Reymann KG. The neuroprotective effect of bone marrow stem cells is not dependent on direct cell contact with hypoxic injured tissue. Exp Neurol. 2009;215:317–327. doi: 10.1016/j.expneurol.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 18.Shear DA, Tate MC, Archer DR, Hoffman SW, Hulce VD, Laplaca MC, Stein DG. Neural progenitor cell transplants promote long-term functional recovery after traumatic brain injury. Brain Res. 2004;1026:11–22. doi: 10.1016/j.brainres.2004.07.087. [DOI] [PubMed] [Google Scholar]
- 19.Bulte JW, Ben-Hur T, Miller BR, Mizrachi-Kol R, Einstein O, Reinhartz E, Zywicke HA, Douglas T, Frank JA. MR microscopy of magnetically labeled neurospheres transplanted into the Lewis EAE rat brain. Magn Reson Med. 2003;50:201–205. doi: 10.1002/mrm.10511. [DOI] [PubMed] [Google Scholar]
- 20.Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 21.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, Kim HO, Lee PH. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- 23.Drukker M, Katz G, Urbach A, Schuldiner M, Markel G, Itskovitz-Eldor J, Reubinoff B, Mandelboim O, Benvenisty N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Baroja ML, Majumdar A, Chadwick K, Rouleau A, Gallacher L, Ferber I, Lebkowski J, Martin T, Madrenas J, et al. Human embryonic stem cells possess immune-privileged properties. Stem Cells. 2004;22:448–456. doi: 10.1634/stemcells.22-4-448. [DOI] [PubMed] [Google Scholar]
- 25.Drukker M, Katchman H, Katz G, Even-Tov Friedman S, Shezen E, Hornstein E, Mandelboim O, Reisner Y, Benvenisty N. Human embryonic stem cells and their differentiated derivatives are less susceptible to immune rejection than adult cells. Stem Cells. 2006;24:221–229. doi: 10.1634/stemcells.2005-0188. [DOI] [PubMed] [Google Scholar]
- 26.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–1893. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 27.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swijnenburg RJ, Tanaka M, Vogel H, Baker J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL, Fedoseyeva EV, et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–I172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- 29.Robertson NJ, Brook FA, Gardner RL, Cobbold SP, Waldmann H, Fairchild PJ. Embryonic stem cell-derived tissues are immunogenic but their inherent immune privilege promotes the induction of tolerance. Proc Natl Acad Sci USA. 2007;104:20920–20925. doi: 10.1073/pnas.0710265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch CA, Geraldes P, Platt JL. Immunosuppression by embryonic stem cells. Stem Cells. 2008;26:89–98. doi: 10.1634/stemcells.2007-0151. [DOI] [PubMed] [Google Scholar]
- 31.Min JY, Yang Y, Sullivan MF, Ke Q, Converso KL, Chen Y, Morgan JP, Xiao YF. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003;125:361–369. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 32.Ménard C, Hagège AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, Bel A, Messas E, Bissery A, Bruneval P, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 33.Fändrich F, Lin X, Chai GX, Schulze M, Ganten D, Bader M, Holle J, Huang DS, Parwaresch R, Zavazava N, et al. Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nat Med. 2002;8:171–178. doi: 10.1038/nm0202-171. [DOI] [PubMed] [Google Scholar]
- 34.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, Muskheli V, Pabon L, Reinecke H, Murry CE. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–1357. doi: 10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 35.Grinnemo KH, Kumagai-Braesch M, Månsson-Broberg A, Skottman H, Hao X, Siddiqui A, Andersson A, Strömberg AM, Lahesmaa R, Hovatta O, et al. Human embryonic stem cells are immunogenic in allogeneic and xenogeneic settings. Reprod Biomed Online. 2006;13:712–724. doi: 10.1016/s1472-6483(10)60663-3. [DOI] [PubMed] [Google Scholar]
- 36.Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisén J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- 37.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 38.Kehl LJ, Fairbanks CA, Laughlin TM, Wilcox GL. Neurogenesis in postnatal rat spinal cord: a study in primary culture. Science. 1997;276:586–589. doi: 10.1126/science.276.5312.586. [DOI] [PubMed] [Google Scholar]
- 39.Rosser AE, Zietlow R, Dunnett SB. Stem cell transplantation for neurodegenerative diseases. Curr Opin Neurol. 2007;20:688–692. doi: 10.1097/WCO.0b013e3282f132fc. [DOI] [PubMed] [Google Scholar]
- 40.Einstein O, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Polyzoidou E, Lavon I, Milonas I, Karussis D, Abramsky O, Ben-Hur T. Transplanted neural precursor cells reduce brain inflammation to attenuate chronic experimental autoimmune encephalomyelitis. Exp Neurol. 2006;198:275–284. doi: 10.1016/j.expneurol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Pluchino S, Gritti A, Blezer E, Amadio S, Brambilla E, Borsellino G, Cossetti C, Del Carro U, Comi G, 't Hart B, et al. Human neural stem cells ameliorate autoimmune encephalomyelitis in non-human primates. Ann Neurol. 2009;66:343–354. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008;265:102–104. doi: 10.1016/j.jns.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- 44.Holmin S, Söderlund J, Biberfeld P, Mathiesen T. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291–298; discussion 298-299. doi: 10.1097/00006123-199802000-00047. [DOI] [PubMed] [Google Scholar]
- 45.Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Modo M, Mellodew K, Rezaie P. In vitro expression of major histocompatibility class I and class II antigens by conditionally immortalized murine neural stem cells. Neurosci Lett. 2003;337:85–88. doi: 10.1016/s0304-3940(02)01301-0. [DOI] [PubMed] [Google Scholar]
- 47.McLaren FH, Svendsen CN, Van der Meide P, Joly E. Analysis of neural stem cells by flow cytometry: cellular differentiation modifies patterns of MHC expression. J Neuroimmunol. 2001;112:35–46. doi: 10.1016/s0165-5728(00)00410-0. [DOI] [PubMed] [Google Scholar]
- 48.Imitola J, Comabella M, Chandraker AK, Dangond F, Sayegh MH, Snyder EY, Khoury SJ. Neural stem/progenitor cells express costimulatory molecules that are differentially regulated by inflammatory and apoptotic stimuli. Am J Pathol. 2004;164:1615–1625. doi: 10.1016/S0002-9440(10)63720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26:2444–2454. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- 50.Odeberg J, Piao JH, Samuelsson EB, Falci S, Akesson E. Low immunogenicity of in vitro-expanded human neural cells despite high MHC expression. J Neuroimmunol. 2005;161:1–11. doi: 10.1016/j.jneuroim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 51.Ubiali F, Nava S, Nessi V, Frigerio S, Parati E, Bernasconi P, Mantegazza R, Baggi F. Allorecognition of human neural stem cells by peripheral blood lymphocytes despite low expression of MHC molecules: role of TGF-beta in modulating proliferation. Int Immunol. 2007;19:1063–1074. doi: 10.1093/intimm/dxm079. [DOI] [PubMed] [Google Scholar]
- 52.Zheng XS, Yang XF, Liu WG, Pan DS, Hu WW, Li G. Transplantation of neural stem cells into the traumatized brain induces lymphocyte infiltration. Brain Inj. 2007;21:275–278. doi: 10.1080/02699050701225754. [DOI] [PubMed] [Google Scholar]
- 53.Hall PE, Lathia JD, Miller NG, Caldwell MA, ffrench-Constant C. Integrins are markers of human neural stem cells. Stem Cells. 2006;24:2078–2084. doi: 10.1634/stemcells.2005-0595. [DOI] [PubMed] [Google Scholar]
- 54.Rampon C, Weiss N, Deboux C, Chaverot N, Miller F, Buchet D, Tricoire-Leignel H, Cazaubon S, Baron-Van Evercooren A, Couraud PO. Molecular mechanism of systemic delivery of neural precursor cells to the brain: assembly of brain endothelial apical cups and control of transmigration by CD44. Stem Cells. 2008;26:1673–1682. doi: 10.1634/stemcells.2008-0122. [DOI] [PubMed] [Google Scholar]
- 55.Einstein O, Fainstein N, Vaknin I, Mizrachi-Kol R, Reihartz E, Grigoriadis N, Lavon I, Baniyash M, Lassmann H, Ben-Hur T. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol. 2007;61:209–218. doi: 10.1002/ana.21033. [DOI] [PubMed] [Google Scholar]
- 56.Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–271. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- 57.Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265:73–77. doi: 10.1016/j.jns.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Einstein O, Karussis D, Grigoriadis N, Mizrachi-Kol R, Reinhartz E, Abramsky O, Ben-Hur T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol Cell Neurosci. 2003;24:1074–1082. doi: 10.1016/j.mcn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS One. 2008;3:e3145. doi: 10.1371/journal.pone.0003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pluchino S, Zanotti L, Brambilla E, Rovere-Querini P, Capobianco A, Alfaro-Cervello C, Salani G, Cossetti C, Borsellino G, Battistini L, et al. Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function. PLoS One. 2009;4:e5959. doi: 10.1371/journal.pone.0005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bacigaluppi M, Pluchino S, Peruzzotti Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain. 2009;132:2239–2251. doi: 10.1093/brain/awp174. [DOI] [PubMed] [Google Scholar]
- 62.Friedenstein AJ, Deriglasova UF, Kulagina NN, Panasuk AF, Rudakowa SF, Luriá EA, Ruadkow IA. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- 63.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 64.Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 65.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 66.Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 67.Krampera M, Marconi S, Pasini A, Galiè M, Rigotti G, Mosna F, Tinelli M, Lovato L, Anghileri E, Andreini A, et al. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone. 2007;40:382–390. doi: 10.1016/j.bone.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Amé-Thomas P, Maby-El Hajjami H, Monvoisin C, Jean R, Monnier D, Caulet-Maugendre S, Guillaudeux T, Lamy T, Fest T, Tarte K. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109:693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- 69.Krampera M, Sartoris S, Liotta F, Pasini A, Angeli R, Cosmi L, Andreini A, Mosna F, Bonetti B, Rebellato E, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Dev. 2007;16:797–810. doi: 10.1089/scd.2007.0024. [DOI] [PubMed] [Google Scholar]
- 70.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, Scarpa A, Dazzi F, Pizzolo G, Vinante F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106:59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- 73.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 74.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 75.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 76.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 77.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 78.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 79.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 80.Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–1213. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 81.Rasmusson I, Ringdén O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 82.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 83.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 84.Comoli P, Ginevri F, Maccario R, Avanzini MA, Marconi M, Groff A, Cometa A, Cioni M, Porretti L, Barberi W, et al. Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrol Dial Transplant. 2008;23:1196–1202. doi: 10.1093/ndt/gfm740. [DOI] [PubMed] [Google Scholar]
- 85.Tabera S, Pérez-Simón JA, Díez-Campelo M, Sánchez-Abarca LI, Blanco B, López A, Benito A, Ocio E, Sánchez-Guijo FM, Cañizo C, et al. The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica. 2008;93:1301–1309. doi: 10.3324/haematol.12857. [DOI] [PubMed] [Google Scholar]
- 86.Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2008;26:562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- 87.Bochev I, Elmadjian G, Kyurkchiev D, Tzvetanov L, Altankova I, Tivchev P, Kyurkchiev S. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol Int. 2008;32:384–393. doi: 10.1016/j.cellbi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 88.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 89.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 90.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 91.Zhang W, Ge W, Li C, You S, Liao L, Han Q, Deng W, Zhao RC. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 92.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 93.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 94.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 95.van den Berk LC, Roelofs H, Huijs T, Siebers-Vermeulen KG, Raymakers RA, Kögler G, Figdor CG, Torensma R. Cord blood mesenchymal stem cells propel human dendritic cells to an intermediate maturation state and boost interleukin-12 production by mature dendritic cells. Immunology. 2009;128:564–572. doi: 10.1111/j.1365-2567.2009.03142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, Ottonello L, Pistoia V. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151–162. doi: 10.1634/stemcells.2007-0416. [DOI] [PubMed] [Google Scholar]
- 97.Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 98.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 99.Jakob M, Hemeda H, Janeschik S, Bootz F, Rotter N, Lang S, Brandau S. Human nasal mucosa contains tissue-resident immunologically responsive mesenchymal stromal cells. Stem Cells Dev. 2010;19:635–644. doi: 10.1089/scd.2009.0245. [DOI] [PubMed] [Google Scholar]
- 100.Polisetty N, Fatima A, Madhira SL, Sangwan VS, Vemuganti GK. Mesenchymal cells from limbal stroma of human eye. Mol Vis. 2008;14:431–442. [PMC free article] [PubMed] [Google Scholar]
- 101.Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol. 2009;219:667–676. doi: 10.1002/jcp.21710. [DOI] [PubMed] [Google Scholar]
- 102.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 103.Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol. 2006;176:2864–2871. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- 104.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, Hilkens CM, Collin MP. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 105.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 106.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti F, Frassoni F, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 107.Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 108.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 109.Nasef A, Mathieu N, Chapel A, Frick J, François S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation. 2007;84:231–237. doi: 10.1097/01.tp.0000267918.07906.08. [DOI] [PubMed] [Google Scholar]
- 110.Angoulvant D, Clerc A, Benchalal S, Galambrun C, Farre A, Bertrand Y, Eljaafari A. Human mesenchymal stem cells suppress induction of cytotoxic response to alloantigens. Biorheology. 2004;41:469–476. [PubMed] [Google Scholar]
- 111.Rasmusson I, Uhlin M, Le Blanc K, Levitsky V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82:887–893. doi: 10.1189/jlb.0307140. [DOI] [PubMed] [Google Scholar]
- 112.Inoue S, Popp FC, Koehl GE, Piso P, Schlitt HJ, Geissler EK, Dahlke MH. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589–1595. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]
- 113.Saito T, Kuang JQ, Bittira B, Al-Khaldi A, Chiu RC. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2002;74:19–24; discussion 24. doi: 10.1016/s0003-4975(02)03591-9. [DOI] [PubMed] [Google Scholar]
- 114.Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057–4065. doi: 10.1182/blood-2005-03-1004. [DOI] [PubMed] [Google Scholar]
- 115.Trenado A, Sudres M, Tang Q, Maury S, Charlotte F, Grégoire S, Bonyhadi M, Klatzmann D, Salomon BL, Cohen JL. Ex vivo-expanded CD4+CD25+ immunoregulatory T cells prevent graft-versus-host-disease by inhibiting activation/differentiation of pathogenic T cells. J Immunol. 2006;176:1266–1273. doi: 10.4049/jimmunol.176.2.1266. [DOI] [PubMed] [Google Scholar]
- 116.Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grinnemo KH, Månsson A, Dellgren G, Klingberg D, Wardell E, Drvota V, Tammik C, Holgersson J, Ringdén O, Sylvén C, et al. Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 2004;127:1293–1300. doi: 10.1016/j.jtcvs.2003.07.037. [DOI] [PubMed] [Google Scholar]
- 118.Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu SC, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 2004;9:747–756. doi: 10.1016/j.ymthe.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 119.Sundin M, Ringdén O, Sundberg B, Nava S, Götherström C, Le Blanc K. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica. 2007;92:1208–1215. doi: 10.3324/haematol.11446. [DOI] [PubMed] [Google Scholar]
- 120.Groh ME, Maitra B, Szekely E, Koç ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33:928–934. doi: 10.1016/j.exphem.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 121.Le Blanc K, Rasmusson I, Götherström C, Seidel C, Sundberg B, Sundin M, Rosendahl K, Tammik C, Ringdén O. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scand J Immunol. 2004;60:307–315. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 122.Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- 123.Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 124.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 125.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 126.Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 127.Jones S, Horwood N, Cope A, Dazzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179:2824–2831. doi: 10.4049/jimmunol.179.5.2824. [DOI] [PubMed] [Google Scholar]
- 128.Benvenuto F, Ferrari S, Gerdoni E, Gualandi F, Frassoni F, Pistoia V, Mancardi G, Uccelli A. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25:1753–1760. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- 129.Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, Shi Y. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17:240–248. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 130.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noël D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 131.Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C, Noël D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–1603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 132.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, Anegon I, Cuturi MC. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 133.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 134.Chang CJ, Yen ML, Chen YC, Chien CC, Huang HI, Bai CH, Yen BL. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–2477. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 135.Gieseke F, Schütt B, Viebahn S, Koscielniak E, Friedrich W, Handgretinger R, Müller I. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNgammaR1 signaling and IDO expression. Blood. 2007;110:2197–2200. doi: 10.1182/blood-2007-04-083162. [DOI] [PubMed] [Google Scholar]
- 136.Gonzalez-Rey E, Gonzalez MA, Varela N, O'Valle F, Hernandez-Cortes P, Rico L, Büscher D, Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241–248. doi: 10.1136/ard.2008.101881. [DOI] [PubMed] [Google Scholar]
- 137.Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, Sportoletti P, Falzetti F, Tabilio A. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–318. doi: 10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 138.Liotta F, Angeli R, Cosmi L, Filì L, Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- 139.Poggi A, Prevosto C, Zancolli M, Canevali P, Musso A, Zocchi MR. NKG2D and natural cytotoxicity receptors are involved in natural killer cell interaction with self-antigen presenting cells and stromal cells. Ann N Y Acad Sci. 2007;1109:47–57. doi: 10.1196/annals.1398.007. [DOI] [PubMed] [Google Scholar]
- 140.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 141.Xu G, Zhang Y, Zhang L, Ren G, Shi Y. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;361:745–750. doi: 10.1016/j.bbrc.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 143.Rasmusson I, Le Blanc K, Sundberg B, Ringdén O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65:336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 144.Deng W, Han Q, Liao L, You S, Deng H, Zhao RC. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol. 2005;24:458–463. doi: 10.1089/dna.2005.24.458. [DOI] [PubMed] [Google Scholar]
- 145.Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P, Müller-Ehmsen J, Schenk K, Fries JW, Baldamus CA, et al. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2010;114:e107–e116. doi: 10.1159/000262318. [DOI] [PubMed] [Google Scholar]
- 146.Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone Marrow Mesenchymal Stem Cells Reduce Intestinal Ischemia/Reperfusion Injuries in Rats. J Surg Res. 2009:Epub ahead of print. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 147.Schäfer R, Dominici M, Müller I, Horwitz E, Asahara T, Bulte JW, Bieback K, Le Blanc K, Bühring HJ, Capogrossi MC, et al. Basic research and clinical applications of non-hematopoietic stem cells, 4-5 April 2008, Tubingen, Germany. Cytotherapy. 2009;11:245–255. doi: 10.1080/14653240802582117. [DOI] [PubMed] [Google Scholar]
- 148.Dahlke MH, Hoogduijn M, Eggenhofer E, Popp FC, Renner P, Slowik P, Rosenauer A, Piso P, Geissler EK, Lange C, et al. Toward MSC in solid organ transplantation: 2008 position paper of the MISOT study group. Transplantation. 2009;88:614–619. doi: 10.1097/TP.0b013e3181b4425a. [DOI] [PubMed] [Google Scholar]
- 149.Lange C, Tögel F, Ittrich H, Clayton F, Nolte-Ernsting C, Zander AR, Westenfelder C. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 150.Popp FC, Renner P, Eggenhofer E, Slowik P, Geissler EK, Piso P, Schlitt HJ, Dahlke MH. Mesenchymal stem cells as immunomodulators after liver transplantation. Liver Transpl. 2009;15:1192–1198. doi: 10.1002/lt.21862. [DOI] [PubMed] [Google Scholar]
- 151.Lanzoni G, Alviano F, Marchionni C, Bonsi L, Costa R, Foroni L, Roda G, Belluzzi A, Caponi A, Ricci F, et al. Isolation of stem cell populations with trophic and immunoregulatory functions from human intestinal tissues: potential for cell therapy in inflammatory bowel disease. Cytotherapy. 2009;11:1020–1031. doi: 10.3109/14653240903253840. [DOI] [PubMed] [Google Scholar]