Abstract
Recruitment of stem cells and partially differentiated progenitor cells is a process which accompanies and facilitates the progression of cancer. One of the factors complicating the clinical course of cancer is obesity, a progressively widespread medical condition resulting from overgrowth of white adipose tissue (WAT), commonly known as white fat. The mechanisms by which obesity influences cancer risk and progression are not completely understood. Cells of WAT secret soluble molecules (adipokines) that could stimulate tumor growth, although there is no consensus on which cell populations and which adipokines are important. Recent reports suggest that WAT-derived mesenchymal stem (stromal) cells, termed adipose stem cells (ASC), may represent a cell population linking obesity and cancer. Studies in animal models demonstrate that adipokines secreted by ASC can promote tumor growth by assisting in formation of new blood vessels, a process necessary for expansion of tumor mass. Importantly, migration of ASC from WAT to tumors has been demonstrated, indicating that the tumor microenvironment in cancer may be modulated by ASC-derived trophic factors in a paracrine rather than in an endocrine manner. Here, we review possible positive and adverse implications of progenitor cell recruitment into the diseased sites with a particular emphasis on the role in cancer progression of progenitors that are expanded in obesity.
Keywords: Progenitor, Stromal, Cell mobilization, Obesity, Adipose tissue, Cancer, Tumor
INTRODUCTION
The course of various pathological conditions depends on the recruitment of stem cells and partially differentiated progenitor cells[1,2]. Elevated systemic circulation of hematopoietic and endothelial precursors has been reported and these populations are believed to be implicated in progression of various diseases. In the past few years, it has become increasingly appreciated that mobilization of stem/progenitor cells from the endogenous pool may have tremendous clinical importance[3,4]. The notion that endogenous stem cells may be activated to mend damaged tissues during injury repair and organ regeneration has prompted efforts to identify stem cell mobilization factors for their potential use in regenerative medicine. Initially, approaches to cell mobilization have been developed as a strategy to induce the egress of hematopoietic stem cells (HSC) and hematopoietic progenitor cells (HPC) as an alternative to the highly invasive whole bone marrow transplantation[5].
Endothelial progenitor cells (EPC), giving rise to mature endothelial cells (EC), represent another mobile clinically important cell population[6]. New vessel formation in pathology/tissue repair depends on the local proliferation of EC in supporting microvasculature (angiogenesis)[7-9]. It has been realized that, in addition to angiogenesis, recruitment of circulating progenitors from the bone marrow into blood vessels (vasculogenesis) takes place in adulthood[10-12] in response to hypoxia and inflammation factors[13-15]. This paradigm shift has changed our view of how vascular composition is maintained in pathological tissues including tumors. Increased levels of circulating EPC are associated with a reduced risk of death from cardiovascular causes[16], indicating the benefit of their mobilization to ischemic tissues.
While bone marrow-derived progenitor cells may be implicated in cancer pathology, the full spectrum of circulating vascular progenitors, their individual roles, and the identity of the underlying stem cells is yet to be fully understood[17,18]. Endothelial and pericyte abnormalities observed in cancer suggest that endothelial mimicry plays an important role in tumor vascularization[19]. Several populations of circulating cells, other than EC and EPC, have been recently implicated in supporting tumor vasculogenesis. Tumor-associated dendritic cells (TADCs), a new leukocyte population expressing both DC and endothelial markers were shown to participate in the assembly of ovarian carcinoma neovasculature[20]. Recruited bone marrow-derived circulating cells (RBCCs), which express VEGFR1 but not VEGFR2, are transported to various organs where they engage in neovasculogenesis[21]. Moreover, identical to RBCCs, a population of TIE2-expressing monocytes (TEMs) was shown to be recruited to tumor sites and to promote cancer angiogenesis in a paracrine manner after adhering to newly forming blood vessels[22]. Some or all of these CD45+ circulating proangiogenic cell populations are likely to be identical to cells previously described as fibrocytes[23]. Interestingly, while fibrocytes are of myeloid origin, they have been proposed as progenitors of mesenchymal cells based on their in vivo differentiation into adipocytes[24].
MESENCHYMAL PROGENITOR CELLS AND THEIR CLINICAL IMPORTANCE
In the past few years, stromal mesenchymal progenitors, commonly referred to as mesenchymal stromal cells (MSC), have been identified as potentially important players in a number of pathological conditions[3,25]. Originally, MSC were isolated from the bone marrow stroma and termed fibroblast colony-forming units (CFU-F) based on their morphology[26]. The ability of this cell population to differentiate into cells of mesenchymal lineages, such as osteoblasts, chondrocytes, and adipocytes, has resulted in the term mesenchymal stem cells[27-29]. Recent studies in various organs by us and others have revealed that MSC function as perivascular cells maintaining vascular integrity (pericytes)[30-33]. Preclinical studies and clinical trials with allografted MSC, indicate the intrinsic therapeutic potential of these cells and suggest that they are activated in disease to engage in tissue repair[34]. It has been proposed that, upon injury, pericytes are mobilized and function as MSC that support the tissue regeneration process. This support involves trophic activity and the immunoprotection provided by the MSC. The trophic activity of MSC results from powerful bioactive molecules that they secrete to suppress apoptosis and scarring as well as to promote cell proliferation and vascularization[35,36]. In addition, MSC have immunomodulatory properties[37], and their capacity to mute T-cells is beneficial to autoimmune disease patients as it favors the outcome of bone marrow transplantation through the suppression of graft-versus-host-disease.
Candidate molecular pathways responsible for migration of mesenchymal stromal cells
Bone marrow transplantation studies in mice have demonstrated that a number of progenitor cell populations can undergo mobilization and migration[14,38-40]. Eukaryotic cells migrate in the body along external chemotactic gradients[1,41,42]. Chemotactic stimuli are sensed by heterotrimeric G-protein coupled receptors, the main class of which is the chemokine receptors[43]. There are multiple CCR-type and CXCR-type chemokine receptors characterized by their activation by cytokines called chemokines. Upon chemokine binding, activated chemokine receptors trigger G-protein subunit rearrangement allowing the exchange of GDP for GTP and activation of phospholipase C (PLC). At the same time, G-protein rearrangement upon chemokine binding can activate the protein tyrosine kinase, which phosphorylates the chemokine receptor and causes its desensitization. As a result of this transient activation, membrane-associated PLC carries out phosphatidylinositol phosphate cleavage, which produces diacylglycerol and leads to activation of protein kinase C, a flux in intracellular Ca2+ and downstream intracellular signaling transduced through the MAP kinase pathway[44]. Consistent with the role of chemokine gradients in MSC migration, there is evidence for the involvement of CXCR4 in mobilization of bone marrow MSC[45]. A number of chemokines and chemokine receptors have been identified as potential effector molecules upregulated in MSC that are activated to undergo migration. For example, expression of CCR1, CCR2, CCR3, CCR4, CCR7, CCR9, CXCR4, and CXCR5 was found to be upregulated on the surface of MSC in response to inflammation signals[46]. Further studies should be done to identify pathways governing migration of MSC in cancer.
The cell migration machinery triggered by chemotactic stimuli activates cell motility. This occurs via cell contraction coordinated with a cyclic gradient of reassembly of focal adhesions, through which the cell attaches to the extracellular matrix (ECM)[47,48]. Intracellular signaling initiated by chemokines induces changes in the clustering and avidity of cell adhesion molecules called integrins. Integrins are heterodimeric transmembrane cell-surface receptors, comprised of α and β chains, which act as the principle mediators of the interaction between the ECM environment and the cell[49,50]. The α5 and β1 integrin subunits are selectively overexpressed on MSC and are thought likely to control MSC migration. This is supported by data indicating that MSC utilize β1 (also known as CD29) for migration to and engraftment into ischemic myocardium[51]. The α5β1 integrin complex is known to be a receptor for the ECM protein fibronectin, the major substrate of stromal cells connected with collagen fibers. Recently, we identified β1 integrin as an MSC surface receptor for the matricellular protein SPARC (secreted protein, acidic and rich in cysteine)[52], and demonstrated that SPARC induces de-adhesion and migration of MSC derived from white adipose tissue (WAT)[53]. Further studies will be needed to test whether MSC migration in vivo is triggered by SPARC/α5β1 signaling.
White adipose tissue as a source of mesenchymal stromal cells
Because the ability of the bone marrow progenitors to respond to mobilization stimuli appears to decline with age[54], there is likely to be a progressively decreased capacity of the bone marrow-derived cells to engage in injury repair. The role of other organs in supplying stromal progenitor cells for neovessels is also beginning to emerge[55]. For example, experiments in rodents have demonstrated that non-bone marrow-derived cells contribute to vascularization during wound repair[56]. As we have recently proposed, WAT represents a potential source of mobilizable progenitor cells[3]. Clinically, expansion of WAT leading to obesity results from adipocyte hypertrophy, as well as hyperplasia resulting from progenitor cell proliferation[30,57]. Multipotent progenitors of preadipocytes in WAT have been identified in the stromal/vascular fraction (SFV)[58]. Recently, elegant attempts to classify multipotent subpopulations within the SVF of WAT have been reported[30,59]. In addition to adipocytes, WAT contains vascular adipose endothelial cells (AEC) and perivascular adipose stromal cells (ASC) as well as infiltrating hematopoietic cells such as macrophages and lymphocytes[60].
We and others have shown that WAT mesenchymal progenitors correspond to CD34-positive CD45-negative CD31-negative (CD34+CD45-CD31-) ASC that comprise the majority of cells in the SVF and display multipotency and proliferation capacity comparable to those of bone marrow MSC, while also having clear unique features[31,59,61]. Consistent with depot-specific roles of WAT in pathology, the content and properties of cells composing WAT have been found to differ between visceral and subcutaneous (e.g. interscapular and inguinal) WAT depots[62]. Interestingly, visceral adipose progenitors appear to have comparatively high self-renewal capacity, consistent with abdominal WAT remaining in old age when subcutaneous depots are depleted[63].
Interestingly, accumulating evidence indicates that ASC, in addition to serving as stem cells, display characteristics of pericytes (mural cells) and cooperate with the endothelium during vascularization[31], which has been confirmed by others[30,59]. ASC promote endothelial cell proliferation and blood vessel formation at least in part via trophic effects of secreted vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and other angiogenic molecules[59,64]. It has been shown, however, that systemically administered ASC[64] and endogenous AEC[65] can physically localize to sites of injury and contribute to revascularization of injured mouse tissues. The clinical potential of WAT-derived MSC is demonstrated, for example, by studies showing that patient-derived ASC contribute to repair of ischemia-induced injury in vivo via secretion of growth factors supporting neovascularization[64]. Moreover, human ASC can induce angiogenesis and nerve sprouting following myocardial infarction, resulting in potent preservation of cardiac function[66]. This ASC function is mediated at least in part though their capacity to cooperate with the endothelium in forming vascular networks[67].
Mesenchymal stromal cells and cancer progression
Cancer progression, the multi-step process that involves tumor growth, invasion, and metastasis[68], relies on neovascularization[7-9]. The importance of cancer vasculogenesis, involving inflammation-associated infiltration of endothelial and hematopoietic cells, has been revealed recently[39,40,69]. While marked quantitative differences between human cancers and experimental mouse models with respect to the mechanisms and clinical relevance of vasculogenesis still remain to be reconciled[70], it has been shown that levels of circulating progenitor cells (CPC) and of mature EC are elevated in human cancer[17,18,71], and that circulating EC do engraft into the tumor vasculature[72], suggesting a possible role in disease progression. The role of various leukocyte populations migrating to tumors and promoting cancer progression is also well recognized[14,38,73-76].
Because tumors secrete hypoxia and inflammation factors, it has been hypothesized that MSC may become recruited by tumors in cancer. Supporting this possibility, elevated circulation of CFU-F in cancer patients has been reported[77]. Recruitment of experimentally administered MSC by tumors has been demonstrated in animal models[78,79]. It has been proposed that MSC may be the progenitors of tumor stromal cells, which are also termed tumor-associated (or cancer-associated) fibroblasts (CAF)[78,80-83]. Upon tumor infiltration, MSC may re-establish the trophic microenvironment they normally maintain in the bone marrow[84,85]. Activation, and proliferation of stromal cells in tumors leads to their conversion into myofibroblasts/reactive stroma[83,86,87]. The resulting remodeling of the ECM, which the mesenchymal tumor cells execute, is an integral component of cancer progression[83,88,89]. There is evidence for epigenetically altered MSC cells driving both cancer initiation and progression[80,90,91]. The secretion of angiogenic and anti-apoptotic factors by MSC, as well as their ability to suppress T cell-mediated immune response has been proposed to account for the tumor-supporting role of MSC[37,42,79]. Consistent with the proposed importance of mesenchymal cells for cancer progression, experiments in animal models indicate that perivascular cells in tumors, supported by endogenously recruited mesenchymal progenitors[92] present a target complimentary to endothelial cells[93]. There is still no agreement in the field on the details of MSC localization upon homing. There are reports on both MSC incorporation into the endothelial cell layer and MSC perivascular engraftment[23,94,95]. It has been proposed that MSC incorporated into perivascular stroma might promote tumor growth via secretion of angiogenic factors, as previously demonstrated by tumor-associated fibroblasts[82,96]. Indeed, the ability of bone marrow MSC to favor tumor cell growth in rodent models has been demonstrated[97].
OBESITY AND CANCER: A PROGENITOR CELL LINK?
Identification of factors that can affect the clinical course of cancer is critical for strategizing the approaches to disease management. One such factor is obesity which is a progressively wide-spreading medical and social problem that has been reported to complicate a number of life-threatening pathologies[98]. Epidemiological studies have demonstrated that obesity, as measured by BMI (BMI= weight/height2) and waist-to-hip ratios, is associated with an increased risk of developing certain types of cancer[6,7]. Moreover, obesity has been shown to promote the progression of a number of cancers[2-5]. Increased physical activity and diet are the most logical measures against obesity although, unfortunately they have become increasingly insufficient given the lifestyle accepted in the modern world[99,100]. Thus, the prevalence of overweight and obese individuals which is increasing dramatically in most parts of the world, is likely to account for continuous changes in cancer epidemiology.
Colorectal cancer stands out as disease for which both risk and progression are positively associated with obesity. Anthropometric measures of adipose distribution have indicated an association between obesity and colon tumors and a similar association has also been noted for large or advanced adenomas, the proximate precursor to most colon cancers[101,102]. This risk of colon cancer in obese men may be as much as 2-fold that of leaner men. Interestingly, while body weight, and physical inactivity have been found to be positively related to risk of colon cancer in men, weaker or no associations have been reported for women. The lack of association between obesity and colorectal cancer in women[101] may be due to hormonal factors, since estrogens and hormone replacement therapy have been demonstrated to protect against colon cancer in both epidemiological and interventional studies. However, an alternative and unrecognized explanation is the difference of body fat distribution in men and women. Obesity in men is featured by comparatively high degree of visceral fat expansion, as compared to women typically showing outgrowth of subcutaneous hip fat, which suggest that the proximity of fat tissue to tumor site may be important. Interestingly, although a striking association between increased body mass index and colon cancer development has been found in numerous epidemiological studies, no consistent association has been found for rectal cancer[102].
The pathophysiology underlying the association between obesity and cancer is unclear. The regulatory mechanisms associated with both obesity and cancer are influenced by lifestyle (including diet and physical activity) and genetic factors (Figure 1). The metabolic syndrome[103], featured by insulin resistance and subsequent hyperinsulinemia, may be a critical consequence of obesity. This possibility is supported by studies showing that subjects with type-2 diabetes are at increased risk of colon cancer. Hyperinsulinemia has also been proposed as an underlying biological mechanism for the observed associations between sedentary behaviour, obesity, and colorectal cancer. Consistent evidence indicates that metabolic aspects of central and abdominal adiposity contribute to the influence of obesity on cancer. The possible association between diabetes mellitus, diets high in sugar, refined grains, high glycemic load, and colorectal cancer supports these observations. Conversely, energy restriction and its consequent insulin lowering effects seem to inhibit carcinogenesis in animal models, although the corresponding studies in patient populations have produced controversial results. Besides having its own growth-promoting effects, hyperinsulinemia enhances the growth hormone–stimulated expression of insulin-like growth factors (IGFs) and increases their bioavailability via decreased synthesis of IGF-binding proteins IGFBP-1, IGFBP-2, and IGFBP-3. In addition to their important role in normal growth and development, IGF-1 and IGF-2, through autocrine, paracrine, and endocrine actions, can promote proliferation of tumor cells. The IGF-1 receptor mediates the proliferative activity of the IGFs. Both epidemiologic and in vitro studies have shown that endogenous and exogenous estrogens reduce serum IGF-1. Although higher concentrations of insulin are directly mitogenic for certain tumor cells in vitro, the clinical relevance of the metabolic syndrome in cancer etiology requires further study.
Figure 1.

How does obesity promote cancer? In addition to obesity-associated lifestyle and diet which could influence cancer course, white adipose tissue (WAT) overgrowth may directly contribute to the pathogenesis of obesity and cancer progression. Adipokines (soluble molecules secreted from WAT) have been proposed to play a role, but other mechanisms may also be important.
The fact that obesity is the consequence of WAT expansion provides clues to the mechanistic links between obesity and cancer progression. It has been hypothesized that WAT itself, in addition to the metabolic syndrome and a state of chronic low-grade inflammation associated with obesity, contributes to cancer progression[104]. WAT has been revealed as a potent endocrine organ, which secrets into circulation various growth factors (IGF-1, IGFBPs, and TGF-β), cytokines (such as TNF-α and interleukin-6), and hormones (such as leptin)[60,105]. Expression of these adipokines increases in obesity and affects lipid metabolism, insulin sensitivity, the alternative complement system, vascular haemostasis, angiogenesis and regulation of energy balance[106-108]. Some of these adipokines have been shown to promote cancer in various animal and in vivo models, and could potentially be implicated in the effect of WAT on colon cancer progression in patients[109,110]. For example, interleukin-6 is strongly linked to inflammation-associated colorectal cancers, such as those associated with inflammatory bowel disease (IBD). Therefore, the endocrine/paracrine signaling by WAT could provide a mechanism by which obesity-related metabolic disorders drive cancer. However, the identity of the key cancer-promoting systemically circulating WAT-derived factors has remained controversial, and there is still no conclusive clinical data reported to prove that specific WAT-derived adipokines promote cancer by enhancing proliferation of tumor cells in vivo[111]. Therefore, the possibility of paracrine effects of WAT-derived cells in the vicinity of the tumors should also be considered.
A role of adipose mesenchymal stromal cells in cancer?
Despite the striking biological potency of WAT-resident progenitors[61], the possibility that MSC from WAT may undergo spontaneous mobilization and affect the progression of human disease has not so far been acknowledged. It is possible that adipose MSC can be mobilized in response to pathological signals and become recruited into the sites of disease, for instance serving as an extra CAF reserve in obese cancer patients (Figure 2). We have proposed that MSC from WAT (ASC) may become mobilized in obese patients in response to tumor signals and become recruited for tumor vasculogenesis[3]. Because the abundance of WAT cells is dramatically elevated in obese individuals, we hypothesize that high numbers of WAT-derived cells possibly mobilized and recruited by tumors in obese cancer patients may account for the epidemiological association between obesity and cancer progression.
Figure 2.
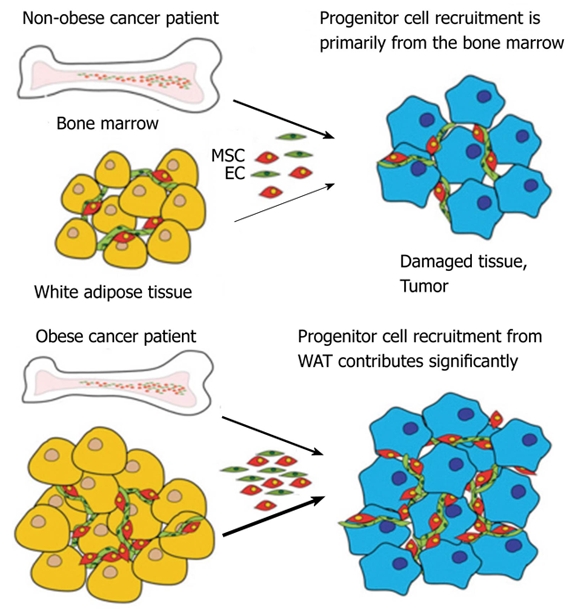
A model for the role of mesenchymal stromal cells of obese patients in cancer progression. Arrow thickness depicts relative contribution of progenitor cells from bone marrow and white adipose tissue. WAT: White adipose tissue; MSC: Mesenchymal stromal cells; EC: Endothelial cells.
Animal studies demonstrating the role of white adipose tissue-derived cells in cancer progression
The possibility that WAT cells may be mobilized in cancer and promote its progression has been tested for the first time in our recent work[112,113]. First, we tested whether mouse WAT cells can home to tumors. To analyze the short-term homing and engraftment capability of adipose cells, WAT SVF from mice expressing the green or red fluorescent proteins[114] (GFP or RFP) were iv-injected into mice bearing several types of model tumors[112]. Mice were sacrificed at 1 d and 3 d post-injection, and tissue sections were evaluated for the presence of GFP+ cells by immunofluorescence. At each time point, up to 50 GFP+ cells per cm2 were detected in tumor sections. Lungs, an established site of first-passage non-specific trapping of iv-injected cells, also contained occasional (1-5 per lung section) GFP+ cells, whereas no GFP+ cells were detected in control organs, including spleen, liver, and pancreas, as well as WAT. To assess recruitment of adipose cells by human tumors, we established xenografts of several human tumor cell lines in immunodeficient mice. Homing of adipose cells to tumors in these models similarly occurred within 1 d post-injection. Analysis of tissues with anti-Ki67 antibodies, demonstrated only rare proliferation of GFP+ cells.
To explore long-term engraftment of WAT cells, we injected 106 adipose SVF cells into mice bearing tumor grafts and analyzed tissues over 2 wk[112]. Over 80% of the GFP+ cells in tumors localized within the stroma, whereas the rest of the GFP+ cells appeared associated with blood vessels. At day 7, approximately 25% of tumor microvessels were found to contain threads of GFP+ cells, which at day 14 acquired morphology undistinguishable from the rest of the tumor blood microvessels. Confocal microscopy analysis showed that 5 to 10 % of the vessel-associated GFP+ expressed CD31, suggesting their endothelial identity. In many cases, endogenous GFP-/αSMA+ pericytes were observed as perivascular cells wrapped around GFP+ cells in capillaries, consistent with GFP+ cells being integrated into the lumen. Only about 1% of vessel-associated tumor-localized GFP+ cells were αSMA-positive, indicating that perivascular localization of donor cells is generally not related to αSMA expression. These data indicate that WAT-derived cells infiltrate different tumor compartments and suggest that both endothelial and stromal lineages are recruited by tumors.
Effect of adipose stem cells on tumor growth
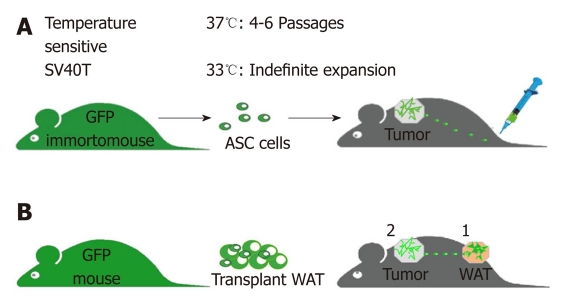
To generate a continuous ASC supply for metronomic cell administration in a long-term cancer progression study, we have developed a mouse model that has allowed us to establish a consistent source of readily available WAT-derived cells (Figure 3A). This new mouse strain termed ‘Immorto/GFP’, was created by crossing GFP mice[114] with ‘Immortomice’ that ubiquitously express a temperature-sensitive SV-40 T-antigen[115]. Cells from Immorto/GFP mice can be indefinitely cultured at permissive temperature (33°C), while at 37°C temperature the SV-40 T is degraded, thus rendering GFP+ cells physiologically normal in vivo. We have isolated ASC along with control stromal cells from lungs and bone marrow, immortalized and expanded them[112]. We reasoned that metronomically-repeated long-term treatment of experimental animals with low-doses of WAT cells would simulate the hypothetical chronic pathological recruitment of adipose cells by tumors[112]. To avoid invasive repetitive iv injections, we established that the sc administration route is compatible with migration of WAT cells to tumors. Analysis of peripheral blood mononuclear cells (PBMC) demonstrated that sc-injected WAT SVF cells were detectable in circulation as early as 15 min post-injection and began clearing the circulation after 6 h. Immunofluorescence analysis of tumors revealed comparable numbers of GFP+ cells in the stromal and perivascular compartments in sc-injected and iv-injected mice, indicating that sc-administered adipose cells efficiently home to tumors.
Figure 3.
Design of experiments testing tumor homing of white adipose tissue cells and their effect on tumor growth. A: metronomic green fluorescent proteins (GFP) + white adipose tissue (WAT) administration: cultured cells are sc-injected (lower back) using metronomic cell injection regimen (104 cells/d) into mice xenografted with human tumors (upper back). B: WAT transplants: GFP+ WAT is implanted (1) into lean mice prior to tumor grafting (2), which allows us to track recruitment of implant-derived cells by tumor and to measure the resulting effect on cancer progression. Tumor growth is compared to that in control mice.
To test the effect of ASC on tumor growth, we used immunodeficient Foxn1nu/nu mice, which have only residual endogenous WAT, as a model. Daily injections of 104 immortalized ASC or control cells were performed sc (distantly to tumor) for 6 wk starting 1 d after tumor grafting. After 2 wk of treatment, tumor growth rate accelerated in ASC-injected mice, but not in mice injected with control stromal cells. Analysis of tumors recovered from representative mice at week 6 revealed ASC accumulation in tumors, but not in control tissues.
Finally, we tested whether recruitment of endogenous WAT cells by tumors is associated with accelerated tumor growth. To determine whether migration of adipose cells to tumors could take place, we have developed an experimental model based on transplantation of traceable WAT cells into Foxn1nu/nu mice (Figure 3B). As a source of WAT for transplantation we used mice that ubiquitously express GFP.” To test whether WAT cells can be endogenously attracted by tumors, once GFP-labeled WAT implants had assimilated in the host 10 d post-implantation, we sc-grafted mouse tumor cells 2 cm away from the implant. For each model, tumors grew significantly faster in mice carrying WAT implants. Consistent with adipose cell mobilization into circulation, GFP+ cells were detected in blood by flow cytometry. Immunofluorescence analysis of tissues from WAT-grafted animals with anti-GFP and anti-CD31 antibodies demonstrated the presence of vascular and perivascular GFP+ cells within the tumors.
CONCLUSION
The studies outlined above suggest that advanced cancer progression is associated the recruitment of WAT-derived cells to tumors. These results are consistent with other reports indicating positive effect of ASC on tumor growth[116]. Collectively, data from these studies suggest that the recruitment of WAT-derived cells by tumors may at least partially account for advanced cancer progression in obese individuals (Figure 4). How exactly MSC (including ASC) are recruited to the diseased site is currently unclear. In theory, they could locally migrate to the sites of inflammation from the surrounding solid tissues (such as WAT), arrive through the systemic circulation, or both[42]. MSC are virtually absent in the peripheral circulation of healthy individuals although hypoxia and inflammation signals are believed to result in MSC mobilization and migration from their niche[94,117,118]. The possibility of spontaneous MSC mobilization in disease has been widely speculated upon, and could underlie their recruitment by tumors although this remains to be tested. Historically, quantification of tissue-resident progenitors has been performed based on the colony forming unit (CFU) assay based on the established ability of bone marrow-derived progenitors to attach to plastic[26,117]. Several groups have reported elevated CFU-F circulation in pathological conditions including cancer[77,119,120]. However, no careful immunophenotyping of freshly isolated cells was performed in some of these studies. It appears that in many cases hematopoietic cells, capable of EC and MSC mimicry in culture[121], may have been mistaken for MSC possibly accounting for conflicting published results. For example, based on CFU-F enumeration in blood, G-CSF treatment had been reported to mobilize MSC in humans[122], while in mice growth factors are incapable of mobilizing MSC without a perturbation in chemokine gradients[45]. Our unpublished results confirm that the abundance of attachable monocytes in blood is incompatible with the CFU assays for enumeration of MSC in circulation.
Figure 4.
Increased circulation of white adipose tissue-derived cells in obese individuals? A hypothetical model predicts mobilization of adipose EC and MSC from residual adipose tissue into the peripheral blood, which may also occur in non-obese patients with cancer and is predicted to be the highest in obese cancer patients. EC: Endothelial cells; MSC: Mesenchymal stromal cells.
It should be noted that plastic attachment is unlikely to be optimal for quantifying circulating stromal cells. This is because the CFU-F assay is designed to detect attachment of cells isolated from solid tissues, in which stromal cells maintain the state of firm adhesion. In contrast, cells undergoing mobilization are known to have key adhesion molecules, including β1[123], modulated to overcome matrix adherence and this is likely to reduce the ability of stromal cells to attach. More importantly, the dominating hematopoietic cells that attach to plastic upon PBMC plating for the CFU-F assay have been observed to inhibit MSC adherence and proliferation[124]. Therefore, capacity of MSC to undergo mobilization is still unclear and new methods are needed to quantify this phenomenon. Future studies will need to establish the identity of cell populations mobilized in obese cancer patients, compare the relative contribution of adipose and bone marrow-derived cells to tumors and investigate the mechanisms through which adipose cells promote cancer progression. This will allow us to determine whether cancer induces mobilization of cells from adipose tissue in obesity, to identify the populations of adipose cells that are recruited by tumors, and to determine functional contribution of ASC to tumor growth.
Footnotes
Peer reviewers: Umberto Galderisi, PhD, Associate Professor, Department of Experimental Medicine, Second University of Naples, Via L. De Crecchio 7, 80138 Napoli, Italy; M Quamrul Islam, PhD, CellAmp Laboratory, Fanjunkaregatan 86, S-582 16 Linkoping, Sweden
Supported by Komen for the Cure Award KG080782 and the American Heart Association Grant 0835434N to Kolonin MG
S- Editor Wang JL L- Editor Hughes D E- Editor Yang C
References
- 1.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 2.Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolonin MG, Simmons PJ. Combinatorial stem cell mobilization. Nat Biotechnol. 2009;27:252–253. doi: 10.1038/nbt0309-252. [DOI] [PubMed] [Google Scholar]
- 4.Pelus LM. Peripheral blood stem cell mobilization: new regimens, new cells, where do we stand. Curr Opin Hematol. 2008;15:285–292. doi: 10.1097/MOH.0b013e328302f43a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn G, Stuhlmüller B, Hain N, Kalden JR, Pfizenmaier K, Burmester GR. Modulation of monocyte activation in patients with rheumatoid arthritis by leukapheresis therapy. J Clin Invest. 1993;91:862–870. doi: 10.1172/JCI116307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 8.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 10.Jin SW, Patterson C. The opening act: vasculogenesis and the origins of circulation. Arterioscler Thromb Vasc Biol. 2009;29:623–629. doi: 10.1161/ATVBAHA.107.161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, Hicklin DJ, Chaplin D, Foster FS, Benezra R, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–1787. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 12.Kopp HG, Ramos CA, Rafii S. Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Opin Hematol. 2006;13:175–181. doi: 10.1097/01.moh.0000219664.26528.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- 14.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzone M, Dettori D, Leite de Oliveira R, Loges S, Schmidt T, Jonckx B, Tian YM, Lanahan AA, Pollard P, Ruiz de Almodovar C, et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell. 2009;136:839–851. doi: 10.1016/j.cell.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Böhm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 17.Goon PK, Lip GY, Boos CJ, Stonelake PS, Blann AD. Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia. 2006;8:79–88. doi: 10.1593/neo.05592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835–845. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 19.McDonald DM, Foss AJ. Endothelial cells of tumor vessels: abnormal but not absent. Cancer Metastasis Rev. 2000;19:109–120. doi: 10.1023/a:1026529222845. [DOI] [PubMed] [Google Scholar]
- 20.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, Holtz DO, Jenkins A, Na H, Zhang L, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;10:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 21.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 22.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- 24.Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029–2031. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- 25.Cottler-Fox MH, Lapidot T, Petit I, Kollet O, DiPersio JF, Link D, Devine S. Stem cell mobilization. Hematology Am Soc Hematol Educ Program. 2003:419–437. doi: 10.1182/asheducation-2003.1.419. [DOI] [PubMed] [Google Scholar]
- 26.Friedenstein AJ. Stromal mechanisms of bone marrow: cloning in vitro and retransplantation in vivo. Haematol Blood Transfus. 1980;25:19–29. doi: 10.1007/978-3-642-67319-1_3. [DOI] [PubMed] [Google Scholar]
- 27.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 29.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 30.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 32.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, Scheding S, Bengzon J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17:183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 35.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 36.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 37.Jones BJ, McTaggart SJ. Immunosuppression by mesenchymal stromal cells: from culture to clinic. Exp Hematol. 2008;36:733–741. doi: 10.1016/j.exphem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 40.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 41.Pelus LM, Fukuda S. Chemokine-mobilized adult stem cells; defining a better hematopoietic graft. Leukemia. 2008;22:466–473. doi: 10.1038/sj.leu.2405021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox JM, Chamberlain G, Ashton BA, Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491–502. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 43.Chamberlain G, Wright K, Rot A, Ashton B, Middleton J. Murine mesenchymal stem cells exhibit a restricted repertoire of functional chemokine receptors: comparison with human. PLoS One. 2008;3:e2934. doi: 10.1371/journal.pone.0002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuevas BD, Abell AN, Johnson GL. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26:3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 45.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 47.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz MA, Horwitz AR. Integrating adhesion, protrusion, and contraction during cell migration. Cell. 2006;125:1223–1225. doi: 10.1016/j.cell.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 50.Moissoglu K, Schwartz MA. Integrin signalling in directed cell migration. Biol Cell. 2006;98:547–555. doi: 10.1042/BC20060025. [DOI] [PubMed] [Google Scholar]
- 51.Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukunaga-Kalabis M, Herlyn M. Unraveling mysteries of the multifunctional protein SPARC. J Invest Dermatol. 2007;127:2497–2498. doi: 10.1038/sj.jid.5701050. [DOI] [PubMed] [Google Scholar]
- 53.Nie J, Chang B, Traktuev DO, Sun J, March K, Chan L, Sage EH, Pasqualini R, Arap W, Kolonin MG. IFATS collection: Combinatorial peptides identify alpha5beta1 integrin as a receptor for the matricellular protein SPARC on adipose stromal cells. Stem Cells. 2008;26:2735–2745. doi: 10.1634/stemcells.2008-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thijssen DH, Vos JB, Verseyden C, van Zonneveld AJ, Smits P, Sweep FC, Hopman MT, de Boer HC. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell. 2006;5:495–503. doi: 10.1111/j.1474-9726.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 55.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 56.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fändrich F, Siebert R, Cooke JP, Dimmeler S, Heeschen C. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–589. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 57.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 58.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 59.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 60.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 61.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Harmelen V, Röhrig K, Hauner H. Comparison of proliferation and differentiation capacity of human adipocyte precursor cells from the omental and subcutaneous adipose tissue depot of obese subjects. Metabolism. 2004;53:632–637. doi: 10.1016/j.metabol.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 63.Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol. 2007;42:463–471. doi: 10.1016/j.exger.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 65.Grenier G, Scimè A, Le Grand F, Asakura A, Perez-Iratxeta C, Andrade-Navarro MA, Labosky PA, Rudnicki MA. Resident endothelial precursors in muscle, adipose, and dermis contribute to postnatal vasculogenesis. Stem Cells. 2007;25:3101–3110. doi: 10.1634/stemcells.2006-0795. [DOI] [PubMed] [Google Scholar]
- 66.Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, March KL. IFATS collection: Human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009;27:230–237. doi: 10.1634/stemcells.2008-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, Johnstone BH, Ingram DA, March KL. Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res. 2009;104:1410–1420. doi: 10.1161/CIRCRESAHA.108.190926. [DOI] [PubMed] [Google Scholar]
- 68.Weinberg RA. Mechanisms of malignant progression. Carcinogenesis. 2008;29:1092–1095. doi: 10.1093/carcin/bgn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Gerald WL, Benezra R. Utilization of bone marrow-derived endothelial cell precursors in spontaneous prostate tumors varies with tumor grade. Cancer Res. 2004;64:6137–6143. doi: 10.1158/0008-5472.CAN-04-1287. [DOI] [PubMed] [Google Scholar]
- 70.Purhonen S, Palm J, Rossi D, Kaskenpää N, Rajantie I, Ylä-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duda DG, Cohen KS, di Tomaso E, Au P, Klein RJ, Scadden DT, Willett CG, Jain RK. Differential CD146 expression on circulating versus tissue endothelial cells in rectal cancer patients: implications for circulating endothelial and progenitor cells as biomarkers for antiangiogenic therapy. J Clin Oncol. 2006;24:1449–1453. doi: 10.1200/JCO.2005.04.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, et al. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- 73.Kim SJ, Kim JS, Papadopoulos J, Wook Kim S, Maya M, Zhang F, He J, Fan D, Langley R, Fidler IJ. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am J Pathol. 2009;174:1972–1980. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;18:372–378. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barnett BG, Rüter J, Kryczek I, Brumlik MJ, Cheng PJ, Daniel BJ, Coukos G, Zou W, Curiel TJ. Regulatory T cells: a new frontier in cancer immunotherapy. Adv Exp Med Biol. 2008;622:255–260. doi: 10.1007/978-0-387-68969-2_20. [DOI] [PubMed] [Google Scholar]
- 77.Fernández M, Simon V, Herrera G, Cao C, Del Favero H, Minguell JJ. Detection of stromal cells in peripheral blood progenitor cell collections from breast cancer patients. Bone Marrow Transplant. 1997;20:265–271. doi: 10.1038/sj.bmt.1700890. [DOI] [PubMed] [Google Scholar]
- 78.Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 79.Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22:559–574. doi: 10.1101/gad.1636908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galiè M, Konstantinidou G, Peroni D, Scambi I, Marchini C, Lisi V, Krampera M, Magnani P, Merigo F, Montani M, et al. Mesenchymal stem cells share molecular signature with mesenchymal tumor cells and favor early tumor growth in syngeneic mice. Oncogene. 2008;27:2542–2551. doi: 10.1038/sj.onc.1210920. [DOI] [PubMed] [Google Scholar]
- 81.Hu M, Yao J, Carroll DK, Weremowicz S, Chen H, Carrasco D, Richardson A, Violette S, Nikolskaya T, Nikolsky Y, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 83.Baglole CJ, Ray DM, Bernstein SH, Feldon SE, Smith TJ, Sime PJ, Phipps RP. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol Invest. 2006;35:297–325. doi: 10.1080/08820130600754960. [DOI] [PubMed] [Google Scholar]
- 84.Chantrain CF, Feron O, Marbaix E, DeClerck YA. Bone marrow microenvironment and tumor progression. Cancer Microenviron. 2008;1:23–35. doi: 10.1007/s12307-008-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 87.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 89.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 91.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–879. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585–597. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 95.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 96.Heil M, Ziegelhoeffer T, Mees B, Schaper W. A different outlook on the role of bone marrow stem cells in vascular growth: bone marrow delivers software not hardware. Circ Res. 2004;94:573–574. doi: 10.1161/01.RES.0000124603.46777.EB. [DOI] [PubMed] [Google Scholar]
- 97.Zhu W, Xu W, Jiang R, Qian H, Chen M, Hu J, Cao W, Han C, Chen Y. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Exp Mol Pathol. 2006;80:267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 98.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 99.Hofbauer KG, Nicholson JR, Boss O. The obesity epidemic: current and future pharmacological treatments. Annu Rev Pharmacol Toxicol. 2007;47:565–592. doi: 10.1146/annurev.pharmtox.47.120505.105256. [DOI] [PubMed] [Google Scholar]
- 100.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 101.Shike M. Body weight and colon cancer. Am J Clin Nutr. 1996;63:442S–444S. doi: 10.1093/ajcn/63.3.442. [DOI] [PubMed] [Google Scholar]
- 102.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132:2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 103.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev. 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 105.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 107.Onuma M, Bub JD, Rummel TL, Iwamoto Y. Prostate cancer cell-adipocyte interaction: leptin mediates androgen-independent prostate cancer cell proliferation through c-Jun NH2-terminal kinase. J Biol Chem. 2003;278:42660–42667. doi: 10.1074/jbc.M304984200. [DOI] [PubMed] [Google Scholar]
- 108.Dizdar O, Alyamaç E. Obesity: an endocrine tumor? Med Hypotheses. 2004;63:790–792. doi: 10.1016/j.mehy.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 109.McTiernan A. Obesity and cancer: the risks, science, and potential management strategies. Oncology (Williston Park) 2005;19:871–881; discussion 881-882, 885-886. [PubMed] [Google Scholar]
- 110.Baillargeon J, Rose DP. Obesity, adipokines, and prostate cancer (review) Int J Oncol. 2006;28:737–745. [PubMed] [Google Scholar]
- 111.Hursting SD, Nunez NP, Varticovski L, Vinson C. The obesity-cancer link: lessons learned from a fatless mouse. Cancer Res. 2007;67:2391–2393. doi: 10.1158/0008-5472.CAN-06-4237. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Y, Daquinag A, Traktuev DO, Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W, Kolonin MG. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kiberstis PA. Fat feeds tumors. Science. 2009;324:1621. [Google Scholar]
- 114.Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 115.Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, Devarajan E, et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30:589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 117.He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 2007;25:69–77. doi: 10.1634/stemcells.2006-0335. [DOI] [PubMed] [Google Scholar]
- 118.Rochefort GY, Delorme B, Lopez A, Hérault O, Bonnet P, Charbord P, Eder V, Domenech J. Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells. 2006;24:2202–2208. doi: 10.1634/stemcells.2006-0164. [DOI] [PubMed] [Google Scholar]
- 119.Wang Y, Johnsen HE, Mortensen S, Bindslev L, Ripa RS, Haack-Sørensen M, Jørgensen E, Fang W, Kastrup J. Changes in circulating mesenchymal stem cells, stem cell homing factor, and vascular growth factors in patients with acute ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2006;92:768–774. doi: 10.1136/hrt.2005.069799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mansilla E, Marín GH, Drago H, Sturla F, Salas E, Gardiner C, Bossi S, Lamonega R, Guzmán A, Nuñez A, et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: new evidence for their use in regenerative medicine. Transplant Proc. 2006;38:967–969. doi: 10.1016/j.transproceed.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 121.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967–976. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- 123.Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, Mortier C, Bron D, Lagneaux L. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]