Abstract
Kazal-type serine protease inhibitor is one of the most important and widely distributed protease inhibitor families. Pancreatic secretory trypsin inhibitor (PSTI), also known as serine protease inhibitor Kazal type I(SPINK1), binds rapidly to trypsin, inhibits its activity and is likely to protect the pancreas from prematurely activated trypsinogen. Therefore, it is an important factor in the onset of pancreatitis. Recent studies found that PSTI/SPINK1 is also involved in self-regulation of acinar cell phagocytosis, proliferation and growth of a variety of cell lines. In addition, it takes part in the response to inflammatory factor or injury and is highly related to adult type II citrullinemia.
Keywords: Pancreatic secretory trypsin inhibitor/serine protease inhibitor Kazal type I, Pancreatitis, Autophagy, Cell proliferation, Inflammatory factor, Adult-II citrullinemia.
INTRODUCTION
Human pancreatic secretory trypsin inhibitor (PSTI) is also known as serine protease inhibitor Kazal type I (SPINK1). It was originally isolated from the pancreas[1] and subsequently identified in mucus producing cells of the gastrointestinal tract and in a range of other tissues including lung, liver, kidney, ovary, breast and in the collecting tubules and transitional epithelium of the renal pelvis[2]. There are homologous PSTI/SPINK1 genes in mouse and rat. Serine protease inhibitor, Kazal type 3 (Spink3), the homologous gene in mouse, was recently discovered in the forebrain/midbrain junction region, mesonephric tubules and other tissues during mouse embryonic development[3]. In recent years, more and more attention has been devoted to PSTI/SPINK1 with identification of more unexpected functions for PSTI/SPINK1. In this review, we summarize the diverse roles of PSTI/SPINK1 in pancreatitis, embryonic development, cancer occurrence and development, acute phase response and adult-II citrullinemia.
STRUCTURE OF PSTI/SPINK1 AND ITS HOMOLOGS
Kazal-type serine protease inhibitor, usually with a signal peptide of about 20 amino acids at N-terminal, is normally composed of one or several Kazal domains which typically consist of 50-60 amino acid residues. Human PSTI gene, containing about 7.5 kb and four exons, is located on 5q32. Analysis of the PSTI gene reveals that a 40 bp DNA fragment located between kb -3.84 and -3.80 carries the element responsible for both transcriptional activity and IL-6-induced gene expression[4]. The protein of 79 amino acids encoded by human PSTI gene includes two parts. The first part of 56 amino acid residues contains three disulfide bonds and a trypsin-specific binding site formed by Lys-Ile; the second part is a 23 amino acid signal peptide. The mature PSTI is produced after being directed by signal peptide into the cavity of endoplasmic reticulum. In mouse, the homologous gene is designated Spink3. There are two types of cDNA, PSTI-I or SPINK3 and PSTI-II or SPINK1, which code for the two types of PSTIs in rat. The nucleotide sequences are 91% homologous between the two cDNAs, but 68% and 65% homologous respectively when compared with human PSTI cDNA. Both amino acid sequences consist of 79 amino acids with the secretion signal peptide consisting of 18 and 23 amino acids for PSTI-I and PSTI-II respectively. Therefore, mature PSTI-I consists of 61 (PSTI-61) and PSTI-II of 56 (PSTI-56)[5,6]. There may be no difference between two PSTIs in inhibitory properties. However, PSTI-I, also known as monitor peptide (MP), can sense the content of proteins in the intestine, promote peptide cholecystokinin (CCK) release and thereby contributes to pancreatic exocrine function, while PSTI-II has no effect on either CCK release or pancreatic secretion function[7-9].
PSTI/SPINK1 IN THE ONSET OF PANCREATITIS
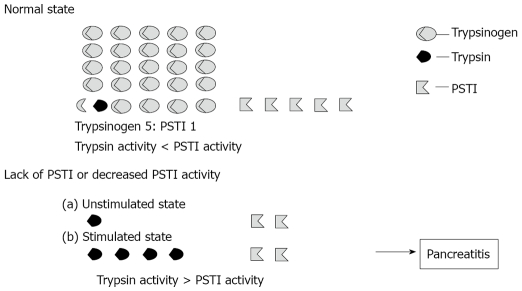
PSTI/SPINK1 is synthesized in acinar cells of the pancreas, packaged with digestive enzymes into the secretory granule and binds covalently with erroneously activated trypsin to form an inactive, stable complex[10]. Therefore, PSTI/SPINK1’s likely major role is to prevent premature activation of trypsinogen thereby ensuring the integrity of acinar cells to prevent autodigestion of the pancreas[11]. Because the SPINK1 and trypsinogen ratio is 1:5, SPINK1 can only inhibit up to 20% of the trypsin activity in the pancreas. Thus a lack of SPINK1 or decreased activity of SPINK1 may result in the premature conversion of trypsinogen into active trypsin leading to activation of various proteases that damage acinar cells and then ultimately the development of pancreatitis[12-14] (Figure 1).
Figure 1.
The importance of PSTI/SPINK1 in onset of pancreatitis (cited from Hirota et al[14] and revised).
There have been many reports of mutations of PSTI/SPINK1 gene in patients with pancreatitis and several hypothesized roles of these mutant proteins in pancreatitis. Pfutzer et al[12] compared the SPINK1 gene sequences in 112 pancreatitis patients with family history and 95 control persons and found SPINK1 mutations were very common (2%) in the population and closely related to pancreatitis. Drenth et al[15] showed SPINK1 gene mutations occurred in 12.2% of adult patients with alcohol-induced chronic pancreatitis and idiopathic chronic pancreatitis, indicating SPINK1 was a susceptible gene for chronic pancreatitis. In South India, SPINK1 gene mutations and environmental factors were found to be possible reasons that lead to tropical pancreatitis[16]. Furthermore, Witt et al[17] identified N34S missense mutation and four other kinds of mutations in SPINK1 gene in 18 of 96 (23%) patients with chronic pancreatitis. Bernardino pointed out the -253C allele for SPINK1 gene might represent a risk factor for the occurrence of pancreatitis in Brazilian population[18].
Interestingly, many previous studies have confirmed that the N34S and other SPINK1 mutations are more common in the general population. However, the incidence of pancreatitis is not very high. Lee et al revealed SPINK1 gene mutations only slightly increased the risk of alcohol-induced chronic pancreatitis (ACP) while the new genetic, environmental and triggering factors must be worth exploring the relationship with ACPs[19-21]. Therefore, the pathogenesis of pancreatitis may be more complex[12] and it is hypothesized that only the mutations that affect PSTI/SPINK1 binding to trypsin will contribute to the onset of pancreatitis. Recent research suggested that trypsinogen activation and trypsin activity were regulated by calcium and the combination mutations of calcium-sensing receptor (CASR) and SPINK1 gene may further increase the risk of pancreatitis[22]. In summary, SPINK1 gene mutations may be a key factor to determine the occurrence of pancreatitis while more attention should be paid to other genetic and external environmental factors.
To better understand the physiological roles of PSTI/SPINK1 in pancreatitis, Ohmuraya et al[23] carried out a study by means of Spink3 knockout mice and found that they died after birth due to excessive autophagy and impaired regeneration in pancreatic acinar cells, suggesting that Spink3 is involved in the regulation of autophagy and is essential for maintaining exocrine integrity of the pancreas. Subsequently, Ohmuraya et al[24] further demonstrated that autophagy is specific to pancreatic acinar cells and involved in trypsinogen activation in experimentally induced pancreatitis. These results suggest that Spink3 has protective roles in pancreatitis by dual mechanisms, one as a trypsin inhibitor and a second as a suppressor of autophagy.
PSTI/SPINK1 IN CELL PROLIFERATION AND GROWTH
PSTI/SPINK1 and embryonic development
In 1986, Fukayama et al[25] first detected SPINK1 in developing buds of the pancreas during the 8th gestational week and found that pancreatic proteinases appeared in acinar cells during the 14th wk of gestation before trypsinogen started to be produced. Thus, the development of the pancreas may be related to the earlier appearance of SPINK1 which perhaps works as a growth factor. Wang et al[3] detected the expression of Spink3 during development of fetal mouse and observed Spink3 in the foregut, midgut, hindgut and the forebrain/midbrain junction region at 9.5 d post coitus (dpc), in the pancreas, large intestine, mesonephric tubules and urogenital ridge at 11.5 dpc, in the acinar cell, small intestine and genital swelling at 13.5 dpc, in the the ductus epididymis at 17.5 dpc and in the seminal vesicle at 8 wk. These data suggest that PSTI/SPINK1 may play important roles in proliferation and/or differentiation of various cell types during development.
PSTI/SPINK1 and cancers
Many previous studies have shown PSTI/SPINK1 were highly expressed in the liver and serum of hepatocellular carcinoma (HCC) patients. Ohmachi et al[26] early discovered the blood level of PSTI in 27 patients with HCC was significantly increased and positively correlated with tumor size, suggesting that elevated blood level of PSTI without inflammation indicates the presence of HCC. Human PSTI is also known as tumor-associated trypsin inhibitor (TATI). The results of Lee et al[27] concluded that TATI overexpression contributed to cell growth advantage and enhanced the metastatic potential of tumors and suggested using TATI, AFP and osteopontin as combined markers for molecular staging, the detection of HCC and for the prediction of early tumor recurrence. PSTI is highly expressed not only in HCC but also in many other tumors[28]. By means of DNA microarray analysis, quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry, PSTI was identified in the cytoplasm of intrahepatic cholangiocarcinoma (ICC) cells and had been suggested as a potential marker for identifying ICC patients with an increased risk of early recurrence after surgical resection[29]. Moreover, high TATI expression was associated with liver metastasis and was an independent predictor of poor prognosis in cancer of the colon and the breast[30,31]. Paju et al[32] demonstrated for the first time that TATI was expressed in the benign and malignant prostate and androgens can regulate TATI protein expression in high-grade tumors.
High expression of PSTI/SPINK1 in cancers may be highly related to its roles in cell proliferation and migration[33]. In addition, there are structural similarities between SPINK1 and epidermal growth factor (EGF) in terms of the number of amino acid residues and the presence of 3 intrachain disulfide bridges. Hence, SPINK1 may bind to EGF receptor (EGFR) to activate its downstream signaling. Recently, Ozaki first showed that SPINK1 induced proliferation and growth of NIH 3T3 cells and pancreatic cancer cell lines and this effect of EGF or SPINK1 can be completely inhibited by EGFR and MAPK/ERKK inhibitor. Taken together, these results suggest SPINK1 stimulates the proliferation of pancreatic cancer cells through the EGFR/MAPK cascade[34]. Rat PSTI-61 in vitro induced the growth and proliferation of rat intestinal epithelial cells IEC-6 in a dose-dependent manner and can effectively enhance the activity of ornithine decarboxylase, indicating polyamine metabolism may be involved in the mechanism of growth induction[35].
PSTI/SPINK1 IN RESPONSE TO INFLAMMATORY FACTOR OR INJURY
The early stages of the host response to infection, inflammation, tissue damage and other stressors include a number of physiologic changes collectively known as the acute phase response. The acute phase response is comprised of many acute-phase reactants which rapidly increase in the serum. PSTI has been suggested to be an acute-phase reactant in humans in previous studies. Yasuda reported LPS-stimulated macrophage conditioned medium and IL-6 markedly stimulated the secretion of PSTI by cultured hepatoblastoma cells and suggested IL-6 induced PSTI secretion is mediated by cAMP dependent protein kinase A[36]. Jönsson also found the median plasma level of PSTI increased significantly at the fourth day following subtotal pancreatoduodenectomy with no increase in pancreatic juice or trypsinogen levels in pancreatic juice and plasma. Furthermore, he detected the levels of PSTI in culture medium from endotoxin-stimulated hepatocellular carcinoma cells and pancreatic cancer cells and demonstrated that the liver is the probable source of extrapancreatic origin of plasma PSTI during the acute-phase reaction[37]. There are two PSTIs (PSTI-61 and -56) purified from rat pancreatic juice. However, only the serum PSTI-61 had increased approximately 17-fold over the initial level at 48 h after the injection in the turpentine-induced acute inflammation model and this immunoreactive PSTI-61 was detected in the liver after induction of inflammation[38]. Thus, PSTI is highly expressed mainly in the liver after stimulation by inflammatory cytokines or other stressors and thereby leads to elevated levels of plasma PSTI.
In addition, SPINK3 was found highly expressed in the pancreas and induced after pancreatic injury in mouse. Because SPINK3 may be an important serine protease inhibitor, its up-regulation may reflect an important endogenous cytoprotective mechanism in preventing further injury[39]. Marchbank et al[40] demonstrated that PSTI can reduce the release of cytokine in lipopolysaccharide-stimulated dendritic cells and therefore transgenic mice overexpressing human PSTI within the jejunum reduced indomethacin-induced injury by 42% and systemic recombinant hPSTI truncated injurious effects in rat models of dextran-sodium-sulfate-induced colitis. Human milk containing high concentration of PSTI stimulated migration and proliferation of intestinal HT29 cells about three fold, reduced indomethacin-induced apoptosis by about 70%-80% and gastric damage in rats by about 75%[41]. Thus, we conclude that PSTI may be involved in stabilizing intestinal mucosa against noxious agents and stimulating repair after injury. We used Rat Genome 230 2.0 array to detect the expression of rat PSTI-61 mRNA in isolated hepatocytes, stellate cells, dendritic cells, sinusoidal endothelial cells, Kupffer cells and pit cells from regenerating livers and found its expressions were significantly up-regulated in some kinds of hepatic cells, with the highest 449-fold over the control at 2 h after partial hepatectomy (PH) in isolated stellate cells and 132-fold at 2 h after PH in hepatocytes, which implies PSTI-61 may be highly related to inflammation or injury during liver regeneration.
PSTI/SPINK1 IN ADULT-II CITRULLINEMIA
Citrin deficiency caused by SLC25A13 gene mutations usually develops into adult-onset type II citrullinemia (CTLN2). Previous studies have shown that the expression of hPSTI mRNA increased significantly and the concentration of hPSTI protein was higher in the liver of type II patients than controls. Furthermore, a significant increase in serum hPSTI level with no change in the other serum markers was found, suggesting serum hPSTI is useful as a diagnostic marker for adult-onset type II citrullinemia[42,43]. Further study indicated that CTLN2 patients may also have weight loss, hepatic steatosis, steatohepatitis and a history of pancreatitis and serum concentration of PSTI was higher when compared with non-alcoholic fatty liver disease (NAFLD) (non-SLC25A13 gene mutations). Therefore, serum PSTI may be a useful indicator for distinguishing CTLN2 patients from conventional NAFLD[44]. However, the regulatory mechanism and physiological role of high expression of PSTI in the liver remain to be elucidated.
CONCLUSION
The roles of PSTI/SPINK1, especially its relationship to the onset of pancreatitis, have been particularly described and we have shown that activation of trypsinogen and PSTI/SPINK1 regulates the onset of pancreatitis while other genetic and external environmental factors should be considered more in future. We need to further explore possible signal pathways for PSTI/SPINK1 involved in cell proliferation, growth and migration which may contribute to a better understanding of its roles in embryonic development or types of cancers. Furthermore, we do not clearly know the mechanism involved in high expression of PSTI/SPINK1 in livers when stimulated by inflammatory factors or partial hepatectomy. In addition, the relationship between PSTI/SPINK1 and adult type II citrullinemia remains unclear. In short, much further work is required in order to comprehensively understand the functions of PSTI/SPINK1.
Footnotes
Supported by the National Basic Research 973 Pre-research Program of China, No. 2006CB708506
Peer reviewers: Christoph Michalski, MD, Chirurgische Klinik und Poliklinik, TU München, Ismaningerstrasse 22, Munich 81675, Germany; Brian KP Goh, MBBS, MMed, MSc, FRCSEd, Department of Surgery, Singapore General Hospital, Outram Road, Singapore 169608, Singapore
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Kazal La, Spicer Ds, Brahinsky Ra. Isolation of a crystalline trypsin inhibitor-anticoagulant protein from pancreas. J Am Chem Soc. 1948;70:3034–3040. doi: 10.1021/ja01189a060. [DOI] [PubMed] [Google Scholar]
- 2.Shibata T, Ogawa M, Takata N, Matsuda K, Niinobu T, Uda K, Wakasugi C, Mori T. Distribution of pancreatic secretory trypsin inhibitor in various human tissues and its inactivation in the gastric mucosa. Res Commun Chem Pathol Pharmacol. 1987;55:243–248. [PubMed] [Google Scholar]
- 3.Wang J, Ohmuraya M, Hirota M, Baba H, Zhao G, Takeya M, Araki K, Yamamura K. Expression pattern of serine protease inhibitor kazal type 3 (Spink3) during mouse embryonic development. Histochem Cell Biol. 2008;130:387–397. doi: 10.1007/s00418-008-0425-8. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda T, Ogawa M, Murata A, Ohmachi Y, Yasuda T, Mori T, Matsubara K. Identification of the IL-6-responsive element in an acute-phase-responsive human pancreatic secretory trypsin inhibitor-encoding gene. Gene. 1993;131:275–280. doi: 10.1016/0378-1119(93)90306-n. [DOI] [PubMed] [Google Scholar]
- 5.Uda K, Ogawa M, Shibata T, Murata A, Mori T, Kikuchi N, Yoshida N, Tsunasawa S, Sakiyama F. Purification, characterization and amino-acid sequencing of two pancreatic secretory trypsin inhibitors in rat pancreatic juice. Biol Chem Hoppe Seyler. 1988;369 Suppl:55–61. [PubMed] [Google Scholar]
- 6.Horii A, Tomita N, Yokouchi H, Doi S, Uda K, Ogawa M, Mori T, Matsubara K. On the cDNA’s for two types of rat pancreatic secretory trypsin inhibitor. Biochem Biophys Res Commun. 1989;162:151–159. doi: 10.1016/0006-291x(89)91975-x. [DOI] [PubMed] [Google Scholar]
- 7.Miyasaka K, Funakoshi A, Nakamura R, Kitani K, Uda K, Murata A, Ogawa M. Differences in stimulatory effects between rat pancreatic secretory trypsin inhibitor-61 and -56 on rat pancreas. Jpn J Physiol. 1989;39:891–899. doi: 10.2170/jjphysiol.39.891. [DOI] [PubMed] [Google Scholar]
- 8.Fukuoka S, Scheele GA. Rapid and selective cloning of monitor peptide, a novel cholecystokinin-releasing peptide, using minimal amino acid sequence and the polymerase chain reaction. Pancreas. 1990;5:1–7. doi: 10.1097/00006676-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Tsuzuki S, Fushiki T, Kondo A, Murayama H, Sugimoto E. Effect of a high-protein diet on the gene expression of a trypsin-sensitive, cholecystokinin-releasing peptide (monitor peptide) in the pancreas. Eur J Biochem. 1991;199:245–252. doi: 10.1111/j.1432-1033.1991.tb16116.x. [DOI] [PubMed] [Google Scholar]
- 10.Marchbank T, Freeman TC, Playford RJ. Human pancreatic secretory trypsin inhibitor. Distribution, actions and possible role in mucosal integrity and repair. Digestion. 1998;59:167–174. doi: 10.1159/000007485. [DOI] [PubMed] [Google Scholar]
- 11.Graf R, Klauser S, Fukuoka SI, Schiesser M, Bimmler D. The bifunctional rat pancreatic secretory trypsin inhibitor/monitor peptide provides protection against premature activation of pancreatic juice. Pancreatology. 2003;3:195–206. doi: 10.1159/000070729. [DOI] [PubMed] [Google Scholar]
- 12.Pfützer RH, Barmada MM, Brunskill AP, Finch R, Hart PS, Neoptolemos J, Furey WF, Whitcomb DC. SPINK1/PSTI polymorphisms act as disease modifiers in familial and idiopathic chronic pancreatitis. Gastroenterology. 2000;119:615–623. doi: 10.1053/gast.2000.18017. [DOI] [PubMed] [Google Scholar]
- 13.Hirota M, Ohmuraya M, Baba H. Genetic background of pancreatitis. Postgrad Med J. 2006;82:775–778. doi: 10.1136/pgmj.2006.050591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota M, Ohmuraya M, Baba H. The role of trypsin, trypsin inhibitor, and trypsin receptor in the onset and aggravation of pancreatitis. J Gastroenterol. 2006;41:832–836. doi: 10.1007/s00535-006-1874-2. [DOI] [PubMed] [Google Scholar]
- 15.Drenth JP, te Morsche R, Jansen JB. Mutations in serine protease inhibitor Kazal type 1 are strongly associated with chronic pancreatitis. Gut. 2002;50:687–692. doi: 10.1136/gut.50.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tandon RK, Garg PK. Tropical pancreatitis. Dig Dis. 2004;22:258–266. doi: 10.1159/000082797. [DOI] [PubMed] [Google Scholar]
- 17.Witt H, Luck W, Hennies HC, Classen M, Kage A, Lass U, Landt O, Becker M. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 18.Bernardino AL, Guarita DR, Mott CB, Pedroso MR, Machado MC, Laudanna AA, Tani CM, Almeida FL, Zatz M. CFTR, PRSS1 and SPINK1 mutations in the development of pancreatitis in Brazilian patients. JOP. 2003;4:169–177. [PubMed] [Google Scholar]
- 19.Lee KH, Ryu JK, Yoon WJ, Lee JK, Kim YT, Yoon YB. Mutation analysis of SPINK1 and CFTR gene in Korean patients with alcoholic chronic pancreatitis. Dig Dis Sci. 2005;50:1852–1856. doi: 10.1007/s10620-005-2950-9. [DOI] [PubMed] [Google Scholar]
- 20.Perri F, Piepoli A, Stanziale P, Merla A, Zelante L, Andriulli A. Mutation analysis of the cystic fibrosis transmembrane conductance regulator (CFTR) gene, the cationic trypsinogen (PRSS1) gene, and the serine protease inhibitor, Kazal type 1 (SPINK1) gene in patients with alcoholic chronic pancreatitis. Eur J Hum Genet. 2003;11:687–692. doi: 10.1038/sj.ejhg.5201035. [DOI] [PubMed] [Google Scholar]
- 21.Whitcomb DC. Genetic predisposition to alcoholic chronic pancreatitis. Pancreas. 2003;27:321–326. doi: 10.1097/00006676-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Mahurkar S, Reddy DN, Rao GV, Chandak GR. Genetic mechanisms underlying the pathogenesis of tropical calcific pancreatitis. World J Gastroenterol. 2009;15:264–269. doi: 10.3748/wjg.15.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohmuraya M, Hirota M, Araki M, Mizushima N, Matsui M, Mizumoto T, Haruna K, Kume S, Takeya M, Ogawa M, et al. Autophagic cell death of pancreatic acinar cells in serine protease inhibitor Kazal type 3-deficient mice. Gastroenterology. 2005;129:696–705. doi: 10.1016/j.gastro.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 24.Ohmuraya M, Hirota M, Araki K, Baba H, Yamamura K. Enhanced trypsin activity in pancreatic acinar cells deficient for serine protease inhibitor kazal type 3. Pancreas. 2006;33:104–106. doi: 10.1097/01.mpa.0000226889.86322.9b. [DOI] [PubMed] [Google Scholar]
- 25.Fukayama M, Hayashi Y, Koike M, Ogawa M, Kosaki G. Immunohistochemical localization of pancreatic secretory trypsin inhibitor in fetal and adult pancreatic and extrapancreatic tissues. J Histochem Cytochem. 1986;34:227–235. doi: 10.1177/34.2.3511141. [DOI] [PubMed] [Google Scholar]
- 26.Ohmachi Y, Murata A, Matsuura N, Yasuda T, Yasuda T, Monden M, Mori T, Ogawa M, Matsubara K. Specific expression of the pancreatic-secretory-trypsin-inhibitor (PSTI) gene in hepatocellular carcinoma. Int J Cancer. 1993;55:728–734. doi: 10.1002/ijc.2910550505. [DOI] [PubMed] [Google Scholar]
- 27.Lee YC, Pan HW, Peng SY, Lai PL, Kuo WS, Ou YH, Hsu HC. Overexpression of tumour-associated trypsin inhibitor (TATI) enhances tumour growth and is associated with portal vein invasion, early recurrence and a stage-independent prognostic factor of hepatocellular carcinoma. Eur J Cancer. 2007;43:736–744. doi: 10.1016/j.ejca.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Paju A, Stenman UH. Biochemistry and clinical role of trypsinogens and pancreatic secretory trypsin inhibitor. Crit Rev Clin Lab Sci. 2006;43:103–142. doi: 10.1080/10408360500523852. [DOI] [PubMed] [Google Scholar]
- 29.Tonouchi A, Ohtsuka M, Ito H, Kimura F, Shimizu H, Kato M, Nimura Y, Iwase K, Hiwasa T, Seki N, et al. Relationship between pancreatic secretory trypsin inhibitor and early recurrence of intrahepatic cholangiocarcinoma following surgical resection. Am J Gastroenterol. 2006;101:1601–1610. doi: 10.1111/j.1572-0241.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 30.Taccone W, Mazzon W, Belli M. Evaluation of TATI and other markers in solid tumors. Scand J Clin Lab Invest Suppl. 1991;207:25–32. doi: 10.3109/00365519109104622. [DOI] [PubMed] [Google Scholar]
- 31.Gaber A, Johansson M, Stenman UH, Hotakainen K, Pontén F, Glimelius B, Bjartell A, Jirström K, Birgisson H. High expression of tumour-associated trypsin inhibitor correlates with liver metastasis and poor prognosis in colorectal cancer. Br J Cancer. 2009;100:1540–1548. doi: 10.1038/sj.bjc.6605047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paju A, Hotakainen K, Cao Y, Laurila T, Gadaleanu V, Hemminki A, Stenman UH, Bjartell A. Increased expression of tumor-associated trypsin inhibitor, TATI, in prostate cancer and in androgen-independent 22Rv1 cells. Eur Urol. 2007;52:1670–1679. doi: 10.1016/j.eururo.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 33.Gouyer V, Fontaine D, Dumont P, de Wever O, Fontayne-Devaud H, Leteurtre E, Truant S, Delacour D, Drobecq H, Kerckaert JP, et al. Autocrine induction of invasion and metastasis by tumor-associated trypsin inhibitor in human colon cancer cells. Oncogene. 2008;27:4024–4033. doi: 10.1038/onc.2008.42. [DOI] [PubMed] [Google Scholar]
- 34.Ozaki N, Ohmuraya M, Hirota M, Ida S, Wang J, Takamori H, Higashiyama S, Baba H, Yamamura K. Serine protease inhibitor Kazal type 1 promotes proliferation of pancreatic cancer cells through the epidermal growth factor receptor. Mol Cancer Res. 2009;7:1572–1581. doi: 10.1158/1541-7786.MCR-08-0567. [DOI] [PubMed] [Google Scholar]
- 35.Fukuda M, Fujiyama Y, Sasaki M, Andoh A, Bamba T, Fushiki T. Monitor peptide (rat pancreatic secretory trypsin inhibitor) directly stimulates the proliferation of the nontransformed intestinal epithelial cell line, IEC-6. Digestion. 1998;59:326–330. doi: 10.1159/000007510. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda T, Ogawa M, Murata A, Oka Y, Uda K, Mori T. Response to IL-6 stimulation of human hepatoblastoma cells: production of pancreatic secretory trypsin inhibitor. Biol Chem Hoppe Seyler. 1990;371 Suppl:95–100. [PubMed] [Google Scholar]
- 37.Jönsson P, Linder C, Genell S, Ohlsson K. Extrapancreatic origin of the pancreatic secretory trypsin inhibitor as an acute-phase reactant. Pancreas. 1996;12:303–307. doi: 10.1097/00006676-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 38.Uda K, Murata A, Nishijima J, Doi S, Tomita N, Ogawa M, Mori T. Elevation of circulating monitor peptide/pancreatic secretory trypsin inhibitor-I (PSTI-61) after turpentine-induced inflammation in rats: hepatocytes produce it as an acute phase reactant. J Surg Res. 1994;57:563–568. doi: 10.1006/jsre.1994.1183. [DOI] [PubMed] [Google Scholar]
- 39.Neuschwander-Tetri BA, Fimmel CJ, Kladney RD, Wells LD, Talkad V. Differential expression of the trypsin inhibitor SPINK3 mRNA and the mouse ortholog of secretory granule protein ZG-16p mRNA in the mouse pancreas after repetitive injury. Pancreas. 2004;28:e104–e111. doi: 10.1097/00006676-200405000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Marchbank T, Mahmood A, Fitzgerald AJ, Domin J, Butler M, Goodlad RA, Elia G, Cox HM, van Heel DA, Ghosh S, et al. Human pancreatic secretory trypsin inhibitor stabilizes intestinal mucosa against noxious agents. Am J Pathol. 2007;171:1462–1473. doi: 10.2353/ajpath.2007.070192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marchbank T, Weaver G, Nilsen-Hamilton M, Playford RJ. Pancreatic secretory trypsin inhibitor is a major motogenic and protective factor in human breast milk. Am J Physiol Gastrointest Liver Physiol. 2009;296:G697–G703. doi: 10.1152/ajpgi.90565.2008. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi K, Nakata M, Terazono H, Shinsato T, Saheki T. Pancreatic secretory trypsin inhibitor gene is highly expressed in the liver of adult-onset type II citrullinemia. FEBS Lett. 1995;372:69–73. doi: 10.1016/0014-5793(95)00948-9. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi K, Horiuchi M, Saheki T. Pancreatic secretory trypsin inhibitor as a diagnostic marker for adult-onset type II citrullinemia. Hepatology. 1997;25:1160–1165. doi: 10.1002/hep.510250519. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu M, Yazaki M, Tanaka N, Sano K, Hashimoto E, Takei Y, Song YZ, Tanaka E, Kiyosawa K, Saheki T, et al. Citrin deficiency as a cause of chronic liver disorder mimicking non-alcoholic fatty liver disease. J Hepatol. 2008;49:810–820. doi: 10.1016/j.jhep.2008.05.016. [DOI] [PubMed] [Google Scholar]