Abstract
Dysfunction of primary motor cortex (M1) is thought to contribute to the pathophysiology of parkinsonism. What specific aspects of M1 function are abnormal remains uncertain, however. Moreover, few models consider the possibility that distinct cortical neuron subtypes may be affected differently. Those questions were addressed by studying the resting activity of intratelencephalic-type corticostriatal neurons (CSNs) and distant-projecting lamina 5b pyramidal-tract type neurons (PTNs) in the macaque M1 before and after the induction of parkinsonism by administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Contrary to previous reports, the general population of M1 neurons (i.e., PTNs, CSNs, and unidentified neurons) showed reduced baseline firing rates following MPTP, attributable largely to a marked decrease in PTN firing rates. CSN firing rates were unmodified. Although burstiness and firing patterns remained constant in M1 neurons as a whole and CSNs in particular, PTNs became more bursty post-MPTP and less likely to fire in a regular-spiking pattern. Rhythmic spiking (found in PTNs predominantly) occurred at beta frequencies (14–32 Hz) more frequently following MPTP. These results indicate that MPTP intoxication induced distinct modifications in the activity of different M1 neuronal subtypes. The particular susceptibility of PTNs suggests that PTN dysfunction may be an important contributor to the pathophysiology of parkinsonian motor signs.
Keywords: basal ganglia, firing patterns, MPTP, Parkinson’s disease, primary motor cortex
Introduction
The primary motor cortex (M1) is a major destination for basal ganglia (BG) output by way of a disynaptic pallido-thalamo-cortical projection (Nambu et al. 1988; Hoover and Strick 1993). Thus, M1 may play an important role in transforming the abnormally patterned excessive neuronal activity, characteristic of output from the BG in Parkinson’s disease (PD), into motor signs (e.g., rigidity, bradykinesia, tremor) (Albin et al. 1989; DeLong 1990). A direct loss of the dopaminergic innervation of M1 may also contribute to parkinsonian motor dysfunctions (Gaspar et al. 1992; Jan et al. 2003). Despite its hypothesized importance, however, diverse views persist concerning what specific aspects of M1 function are abnormal in PD.
In idiopathic PD and in animal models of the disease, the resting activity of BG output neurons (i.e., during periods of attentive immobility) is marked by a variable combination of increased tonic firing rates, bursty firing, and low-frequency rhythmic modulations in firing rate that are synchronized across neurons (Miller and DeLong 1987; Filion and Tremblay 1991; Hutchison et al. 1994; Vila et al. 1997; Wichmann et al. 1999; Raz et al. 2000; Soares et al. 2004; Starr et al. 2008). Classical pathophysiologic models predict that the increased firing rate of these γ-aminobutyric acidergic (GABAergic) output neurons should inhibit activity in recipient thalamocortical circuits (Albin et al. 1989; DeLong 1990; Wichmann and DeLong 1996). While many subsequent studies have questioned the importance of altered firing rates in BG structures (Raz et al. 2000; Vitek and Giroux 2000; Montgomery 2007; Galvan and Wichmann 2008), the changes in firing rate at the cortical level and their clinical significance remain poorly understood. Relatively few studies have investigated single-unit activity in the M1 of parkinsonian animals (Doudet et al. 1990; Watts and Mandir 1992; Goldberg et al. 2002; Parr-Brownlie and Hyland 2005). Of those, only one, using a neuroleptic model of parkinsonism in rats, demonstrated a significant reduction in the baseline firing rate of M1 neurons (Parr-Brownlie and Hyland 2005). Similar uncertainty exists for the effects of parkinsonism on rest activity in the thalamic regions that link BG output to M1 (Schneider and Rothblat 1996; Molnar et al. 2005; Pessiglione et al. 2005; Rolland et al. 2007; Parr-Brownlie et al. 2009) and for the effects of parkinsonism on measures of resting metabolism in the vicinity of M1 (Crossman et al. 1985; Schwartzman and Alexander 1985; Schwartzman et al. 1988; Palombo et al. 1990; Eidelberg et al. 1994; Piert et al. 1996; Arahata et al. 1999; Hu et al. 2000; Berding et al. 2001; Brownell et al. 2003). The lack of consensus on how parkinsonism affects thalamocortical rest activity might be explained by: 1) differences in the animal model used; 2) changes in behavioral state of experimental subjects during data collection (Barraud et al. 2009); and 3) differences in data collection and sampling methods. The goal of the present study was to address these points by studying the resting firing rates of identified neuronal subtypes in the macaque M1 during well-defined periods of attentive immobility.
There is also little agreement on how the rhythmic bursty activity of the parkinsonian BG is propagated to motor cortex. Studies in patients and animal models of PD have documented a diffuse enhancement of low-frequency cortical potentials in theta and low-alpha (4–10 Hz) frequency bands (Tanaka et al. 2000; Bosboom et al. 2006; Sarnthein and Jeanmonod 2007; Stoffers et al. 2007; Moazami-Goudarzi et al. 2008) and in the beta-frequencies (14–32 Hz) (Brown 2003; Magill et al. 2004; Mallet et al. 2008). How such abnormalities in population-scale signals are reflected in the firing of single cortical neurons remains unclear. In their single-unit recording study, Goldberg et al. (2002) observed long-lasting bursts of M1 activity that were synchronized across multiple neurons, but the burst activity was aperiodic and had temporal dynamics that differed markedly from what is typical of the parkinsonian BG. Goldberg et al. (2002) also reported that the synchronized bursts of M1 activity occurred in the absence of overt movement or muscle activity, contrary to expectations for a brain area that innervates spinal motor nuclei directly. Other studies, however, found no abnormalities in firing pattern in the parkinsonian motor cortex (Doudet et al. 1990; Parr-Brownlie et al. 2007). A second goal of the present study was to determine if the resting activity of M1 neurons in the MPTP-treated macaque shows consistent abnormalities in firing pattern or rhythmicity.
M1 is composed of a complex collection of distinct cell types that differ with respect to intrinsic physiology (McCormick et al. 1985; Connors and Gutnick 1990; Stewart and Foehring 2000; Hattox and Nelson 2007) and afferent innervation (Swadlow 1994). These subtypes may be affected differently in the parkinsonian state. For example, intratelencephalic-projecting corticostriatal neurons (CSNs) and distant-projecting lamina 5b pyramidal tract-type neurons (PTNs) in the M1 are known to have markedly different intrinsic firing properties and task-related discharge in neurologically normal animals (Bauswein et al. 1989; Turner and DeLong 2000; Mallet et al. 2006; Ballion et al. 2008). PTNs are positioned to play a relatively direct role in the expression of parkinsonian motor signs due to their direct projections to spinal motor nuclei and collateral projections to the subthalamic nucleus (Magill et al. 2001; Orieux et al. 2002). CSNs, in contrast, provide an important glutamatergic input to motor regions of the striatum and, thus, are in a position to influence, for better or worse, the disordered physiology of the dopamine-depleted striatum (Mallet et al. 2006). Existing evidence is mixed on whether in parkinsonism the baseline activity of CSNs is reduced (Mallet et al. 2006; Ballion et al. 2008) or elevated (Barbeito et al. 1989; Lindefors and Ungerstedt 1990; Porter et al. 1994; Meshul et al. 1999).
Despite the frequent implication of M1 in the pathophysiology of parkinsonian signs, few studies have investigated single-unit activity in the M1 of MPTP-treated primates, and to our knowledge, no such study has examined identified CSN and PTN subpopulations. We hypothesized that the baseline activity of CSNs and PTNs is affected differently with the induction of parkinsonism. To address this issue, antidromically identified CSNs and PTNs were studied in the arm area of M1 in 2 rhesus monkeys. The animals performed a visuomotor step-tracking task, and recordings were obtained before and after induction of parkinsonism by unilateral intracarotid administration of MPTP. We tested the baseline activity of CSNs and PTNs for changes in: 1) discharge rate, 2) discharge pattern, and 3) rhythmic activity.
Materials and Methods
Animals, Apparatus, Tasks
Two female monkeys (Macaca mulatta) were used for these experiments (monkeys V and L). All aspects of animal care were in accord with the “Guide for the Care and Use of Laboratory Animals” (National Research Council, 1996), and all procedures were approved by the institutional animal care and use committee. Pre-MPTP data from one of the animals contributed to a previous report (Turner and DeLong 2000).
To achieve a controlled state of attentive immobility, the animals performed a visuomotor step-tracking task similar to one used in several previous studies of cortical and BG neuronal activity (Alexander 1987; Mitchell et al. 1987; Alexander and Crutcher 1990; Turner and DeLong 2000). The animal sat in a primate chair and faced a computer monitor. The animal’s right arm was placed in a 1D torquable manipulandum with the wrist (monkey L) or elbow (monkey V) joint aligned with the manipulandum’s axis of rotation. Flexion and extension movements rotated the manipulandum in the horizontal plane and thereby controlled the horizontal position of an onscreen cursor. On each behavioral trial, the animal was required to align the cursor with a series of targets displayed on the monitor. A trial began when a center target appeared and the monkey made the appropriate joint movement to align the cursor with the target. The monkey maintained this position for the duration of a start-position hold period (random duration, 2–5 s), during which the animal could not predict the location of the upcoming lateral target. The target then shifted to the left or right (chosen at random), and the animal moved the cursor to capture the lateral target. The animal received a drop of juice or food for successful completion of the task. Later aspects of the behavioral trial, which evaluated instruction-related neuronal activity, are irrelevant to the current study. The animals also performed trials in which torque perturbations were imposed, but those trials were not included in the current study.
Surgery
After training, each monkey was prepared surgically for recording using aseptic surgery under Isoflurane inhalation anesthesia. A cylindrical stainless steel chamber was implanted with stereotaxic guidance over a burr hole allowing access to the arm-related regions of the left M1 and the posterior putamen. The chamber was oriented parallel to the coronal plane at an angle of 35° so that electrode penetrations were orthogonal to the cortical surface. The chamber was fixed to the skull with bone screws and dental acrylic. Bolts were embedded in the acrylic to allow fixation of the head during recording sessions. Prophylactic antibiotics and analgesics were administered postsurgically.
Placement of Stimulating Electrodes
Several days after recovery from surgery, sites for implantation of stimulating electrodes were identified using standard electrophysiological mapping techniques (Turner and DeLong 2000). Arm-related areas of the putamen were identified by sensorimotor examination of striatal activity and microstimulation effects. The arm-related fiber tract in the prepontine cerebral peduncle (ventral to the substantia nigra) was located using similar techniques. (Defined strictly, pyramidal tract neurons are identified using stimulating electrodes implanted in the pyramidal tract below the pons. These neurons, however, belong to a relatively homogeneous class of large deep lamina 5 cells that send collaterals to both the spinal cord and a number of brainstem structures via the prepontine cerebral peduncle [Humphrey and Corrie 1978; Kuypers 1981; Groh et al. 2010]. Here, the term PTNs refers to that general class of neuron.)
Custom-built “floating” stimulating electrodes were implanted at identified arm-related sites in putamen and the peduncle (for details, see Turner and DeLong 2000). Each electrode consisted of 2 or 3 Teflon-coated PtIr microwires (each 50-μm dia, AM Systems) threaded through a short length of 30-ga stainless steel cannula. The cut ends of the microwires extended below the cannula tip by >1 mm at depths staggered by 0.5 mm. Electrodes were implanted transdurally at the desired sites in putamen and the peduncle through the chronic recording chamber using a guide-tube assembly mounted in the same microdrive as used for mapping. Electrodes were left in place with only the proximal ends of the insulated microwires exiting the dura. The wires were led through a port in the side of the recording chamber and soldered to a head-mounted connector. In both animals, 3 such electrodes were implanted in the posterior putamen between the planes of HC anterior 8 and 14, and one electrode was implanted in the arm-responsive portion of the prepontine peduncle. Histological reconstruction confirmed that the striatal and peduncle electrodes were at sites shown by anatomical studies to receive the bulk of M1 CS and PT projections, respectively (Brodal 1978; Flaherty and Graybiel 1991; Takada et al. 1998; Turner and DeLong 2000).
Data Acquisition
Areas of M1 related to the primary joint used in the task were identified using microstimulation and sensorimotor mapping. Transdural extracellular recording was performed using single glass-coated PtIr microelectrodes mounted in a hydraulic microdrive (MO-95, Narishige Intl.). A cortical region was targeted for data collection if neurons responded to active and/or passive movement of the arm and microstimulation at low currents evoked contraction of forelimb muscles (<40 μA, 10 biphasic pulses at 300 Hz, Fig. 1A). Microelectrode penetrations were performed throughout the targeted cortical area while we searched for neurons activated antidromically from the putamen or peduncle stimulating electrodes. As an electrode was advanced, stimuli were delivered sequentially to each putamen and peduncle-stimulating site (biphasic current pulses of 700 μA, 0.2-ms duration separated by 0.1 ms, >1.5 s between successive biphasic shocks). Neurons were selected for data collection if they were activated antidromically or if they were located in close proximity to an antidromically activated neuron.
Figure 1.
(A) Surface map of electrode penetrations in M1. Separate maps are shown for each of the 3 cell types (CSN; PTNs; NA, not activated cells) in the 2 monkeys. The diameters of circles and crosses indicate the numbers of cells of each type sampled at each location in the normal state and after MPTP treatment, respectively. The maps were derived from photographs of the cortical surface taken after perfusion, histological sections, and the chamber locations for recording tracks. (B) Antidromic activation of M1 neurons from stimulating electrodes in the striatum (CSN) and peduncle (PTN). Antidromically elicited action potentials (*) occurred at a constant latency after stimulation (∇). Antidromic spikes collided (↓) with spontaneous spikes when stimulation was delivered after a spontaneous spike at any delay shorter that the cell’s antidromic latency plus the refractory period.
Standard tests for antidromic identification were used (see Fig. 1B): constant antidromic latency (<0.2-ms jitter), reliable following of a high-frequency train of stimuli (2 or 4 shocks at 200 Hz), and collision of antidromic spikes with spontaneously occurring spikes (Fuller and Schlag 1976). Antidromic latency was measured as the time from stimulation onset to the first inflection in the waveform of the antidromic spike. For most antidromically activated cells, the threshold current for activation was determined (∼50% probability of evoking a spike), and tests for antidromic identification and latency were typically performed at 2 times threshold or 700 μA, whichever was smaller.
Neuronal activity was collected while the animal performed the step-tracking task. The microelectrode signal was amplified × 104 and band-pass filtered (0.3–10 KHz, DAM-80, WPI Inc.). The action potentials of single neurons (sampled at 60 kHz) were discriminated online using template-based spike sorting (MultiSpike Detector, Alpha Omega Engineering). The timing of detected spikes and of relevant task events was sampled digitally at 1 kHz and saved to disk for offline analysis. For neurons that generated few spontaneous spikes, the isolation of action potentials was monitored during intertrial intervals using the spikes evoked by antidromic activation. Analog data from the manipulandum (i.e., arm position) were digitized at either 200 Hz (monkey L) or 500 Hz (monkey V).
Administration of MPTP
After completion of observations in the normal state, a hemiparkinsonian syndrome was induced by injection of MPTP into the left internal carotid artery (0.5 mg/kg, [Bankiewicz et al. 1986; Wu et al. 2007]). This model of parkinsonism was chosen to ensure that animals could maintain themselves and remain healthy for the months-long period of postintoxication recording. Use of this model also increased the likelihood that animals would continue performing the operant task following intoxication (Bankiewicz et al. 2001). The MPTP administration procedure was performed under general anesthesia (1–3% Isoflurane), and prophylactic antibiotics and analgesics were administered postsurgically. Both animals developed signs of parkinsonism contralateral to the infusion (i.e., on the right side of the body). Post-MPTP recording sessions started >30 days after MPTP administration.
Histology
After the last recording session, each monkey was given a lethal dose of sodium pentobarbital and was perfused transcardially with saline followed by 10% formalin in phosphate buffer and then sucrose. The brains were blocked in place in the coronal plane, removed, cryoprotected with sucrose, and cut into 50-μm sections. Sections at 0.5-mm intervals were stained with cresyl violet for localization of microelectrode tracks. Selected sections were processed for immunohistochemistry to visualize tyrosine hydroxylase (TH) for documentation of the loss of dopaminergic cells in the substantia nigra pars compacta (SNc).
Stereology
Cell counting was performed using Stereo Investigator (MBF Bioscience). One slice was used to estimate the number of TH neurons in the entire SNc of both hemispheres (MPTP vs. control). Cells that were clearly stained for TH with a visible nucleus were counted. The counting frame was 40 × 40 × 13 μm (height × width × dissector height), and the sampling grid was 125 × 125 μm. The final number of markers was divided by the estimated volume to obtain the density of TH-positive cells in both hemispheres.
Analysis of Behavioral Data
Digitized signals reflecting manipulandum position were filtered and differentiated (low-pass 25 Hz [Hamming 1983]). Movement initiation, peak velocity, and movement termination were detected automatically using position, velocity, and duration criteria. Reaction time (RT), peak velocity (Velmax), and movement amplitude (Ampl) were computed. Periods of immobility, “start-position stationary periods” (SPSPs), were detected as intervals during the start-position hold period that lasted at least 2048 ms and were separated in time from detectable movements of the manipulandum by at least 200 ms. A velocity threshold of 5 cm/s was used to detect movement for this purpose. (Thresholds lower than 5 cm/s gave unreliable results due to inherent noise in the systems used to sense and record manipulandum angular position). A neuron’s activity during SPSPs was defined as the neuron’s baseline activity.
To document an animal’s parkinsonian status, means were obtained for RT, Velmax, and Ampl across all trials performed during each data collection session before and following MPTP administration. The session-by-session means were entered into two-way analyses of variance (ANOVAs) to test for effects of MPTP administration and movement direction.
Analysis of Neuronal Data
Neuronal recordings were accepted for analysis based on the location, quality, and duration of the recording. Acceptable recording locations were restricted to regions of M1 within 3 mm of the anterior bank of the central sulcus from which movements of the arm could be evoked by microstimulation (Fig. 1). The quality of recording (i.e., single-unit isolation) was controlled by adjusting electrode position and spike sorting during data collection. Adequate unit isolation was verified offline by testing whether a neuron’s interspike intervals (ISIs) obeyed a refractory period (>2 ms). Neuronal data from periods of immobility (SPSPs) were concatenated across trials, and a minimum total record length of 20 s was required. Most records exceeded 50 s. The mean spontaneous firing rate of a neuron was calculated as the total number of spikes across all valid SPSPs divided by the summed duration of those SPSPs.
Discharge Pattern
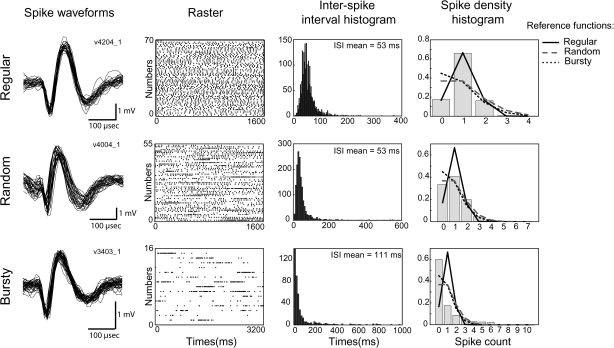
Neuronal data were submitted to 3 separate analyses to classify neuronal firing patterns and to measure the prevalence of burst discharges. The first analysis classified discharge patterns into 1 of 3 basic categories (regular, random, and bursty firing) according to how well a neuron’s spike density histogram (SDH) fit 3 corresponding reference functions (Kaneoke and Vitek 1996; Labarre et al. 2008). SDHs were constructed by breaking a neuronal spike train into n successive intervals of length t where t equals the neuron’s mean ISI. The fraction of n intervals that contained different numbers of spikes (spike count = 0, 1, 2, 3, etc.) was summarized in histogram form (Fig. 2). The SDH was then classified according to the reference function that fit the histogram best (according to least squared error). Regular tonic discharge is characterized by a symmetric distribution of ISIs around the mean ISI. To model this symmetry, the reference function for regular firing followed a Gaussian distribution (mean = 1, variance = 0.5). Random discharge patterns are characterized by a relatively broad distribution of ISIs around mean ISI. The random reference function modeled this feature using a Poisson distribution (mean = 1). Bursty discharge is characterized by the intermittent occurrence of clusters of action potentials at short ISIs separated by long intervals of lower discharge rate. The bursty reference function modeled the high incidence of low discharge rates using a Poisson function with mean = 0.8.
Figure 2.
Spike waveforms, raster diagrams, ISI histograms, and SDHs for 3 example M1 cells corresponding to a regular tonic discharge pattern (“top”), a random discharge pattern (“middle”), and a bursty discharge pattern (“bottom”). “Right column”: A cell’s discharge pattern was classified according to which of 3 reference functions fit the SDH best: a Gaussian distribution (“solid black”) for regular tonic; a Poisson distribution (mean = 1; “dashed gray”) for random; and a more skewed Poisson distribution (mean = 0.8; “dotted black”) for bursty.
Neuronal burst firing was quantified further using the Legendy surprise method (Legendy and Salcman 1985; Wichmann and Soares 2006). Bursts were defined as groups of 4 or more spikes whose ISIs were unusually short compared with other ISIs of a spike train. We used a surprise threshold of 5, which equates with alpha < 0.001 that the candidate burst would occur as a part of a Poisson-distributed sequence of spikes. To avoid possible biases introduced by our intermittent trial-by-trial sampling of spontaneous activity, burst-like events were counted as true bursts only if they did not coincide with a boundary, where 2 SPSPs were concatenated. Note that this correction may lead to a slight underestimation of a cell’s burstiness. The prevalence of bursts in a spike train was measured as: 1) the fraction of the total duration of recording that a spike train spent in bursts and 2) the fraction of the total number of spikes in a spike train contained within bursts. A possible influence of MPTP treatment on the time course and magnitude of bursts were investigated by constructing burst-triggered averages of a neuron’s instantaneous frequency of firing (i.e., ISI−1). The mean preburst firing rate (between −300 and −100 ms before burst onset) was subtracted from each burst-triggered average, and population means were constructed for different neuronal populations. Only neurons with >10 bursts were included in this analysis.
A similar periburst analysis was used to determine if bursts in M1 neuronal activity were associated with movements of the manipulandum. A low level of background noise was present in the records of manipulandum position and velocity. To test for the presence in that noise of small burst-related movement transients, records of rectified manipulandum velocity (|velocity|) were averaged around the onset times of bursts (as defined in the paragraph above). A periburst period (−100 ms before to 100 ms after burst onset) was tested for significant increases in |velocity| relative to the mean and standard deviation (SD) of |velocity| during a preburst control period (500–100 ms before burst onset). A significant periburst movement was defined as a >3 × SD above the control period mean that lasted at least 20 ms. The actual threshold used provided an omnibus sensitivity of P < 0.001 by correcting for multiple comparisons (actual P = 0.001/10 comparisons), where the number of independent comparisons was estimated from the duration of the analysis period divided by the minimum required response duration (i.e., 200 ms/20 ms). Relatively stringent threshold criteria were used in this analysis to reduce the likelihood of Type-1 (“false positive”) errors in light of a previous report that bursts of neuronal activity in the parkinsonian M1 were not associated with movement (Goldberg et al. 2002). Only neurons with >10 bursts were included in this analysis.
Finally, the general variability of a neuron’s firing rate was computed as the coefficient of variation of the spike train’s ISIs (i.e., SD ISI/mean ISI).
Rhythmic Activity
ISI data sets were submitted to 2 complementary analyses to detect: 1) rhythmic modulations in firing rate (e.g., oscillatory bursting) and 2) the rhythmic occurrence of single spikes (otherwise known as “regular spiking” [Connors and Gutnick 1990; Wetmore and Baker 2004]). First, a “shuffled normalization” method detected rhythmic modulations in firing rate. The shuffled normalization algorithm has been used extensively to study oscillatory neuronal firing in the parkinsonian BG (Rivlin-Etzion et al. 2006; Starr et al. 2008; McCairn and Turner 2009). The discrete Fourier transform (FFT) was applied to nonoverlapping 2048-ms long segments of a spike train’s delta function smoothed with a Hanning window of the same length. (These spike train segments were extracted separately from each SPSP to avoid possible anomalies due to data discontinuities at SPSP concatenation boundaries.) The resulting “primary” spectral density estimate (0–500 Hz, 0.5-Hz resolution) was normalized by dividing it by a “control” spectrum. The control spectrum was the mean of 100 spectra computed after 100 shufflings of the same neuron’s ISIs. This normalization compensated for distortions in spectral estimates attributable to a neuron’s refractory period and thereby improved the detection of low-frequency oscillations (Rivlin-Etzion et al. 2006). The shuffle-normalization procedure yielded spectra that varied around a normalized value of 1. Because both primary and control spectra were computed from the same set of ISIs, the normalization procedure also removed spectral peaks attributable to rhythmic occurrences of single-action potentials, a firing pattern often found in regular-spiking cell types such as PTNs (Connors and Gutnick 1990; Wetmore and Baker 2004). To test for rhythmic occurrences of single spikes, “nonshuffle-normalized” spectra were also computed by omitting the shuffled normalization step used in the first analysis. (These will be referred to hereafter as “standard” spectra.) Spectral peaks in the standard spectra could arise from either the rhythmic occurrences of individual action potentials or rhythmic bursting. The standard spectra were normalized so that their mean spectral power 0–500 Hz equaled a value of 1. Other aspects of the analysis of standard spectra were identical to those used in the shuffle-normalized spectral analysis.
For both analyses, peaks in a spectrum between 3 and 60 Hz were tested for significance relative to the SD of the spectrum in the 300- to 500-Hz control range. The omnibus threshold for significance (α = 0.01) included a Bonferroni correction for multiple comparisons (0.01/114 spectral points tested between 3 and 60 Hz). If the spectrum exceeded the threshold at more than 1 frequency, the central rhythmic frequency was defined as the spectral peak with the highest power. To qualify for rhythmicity analysis, a spike train was required to contain at least 500 action potentials. The propensity of M1 cells to discharge rhythmically in the beta frequency band was quantified using the following ratio: mean power in the beta frequency range (14–32 Hz)/mean power in the gamma frequency range (33–60 Hz).
Neuronal Activity–to–Behavior Correlations
Two separate analyses were performed to determine if MPTP-induced alterations in neuronal activity correlated with the behavioral effects of MPTP. The first analysis tested for relationships across recording sessions. Mean measures of neuronal activity (firing rate, burst rate or beta rhythmicity) for a whole recording session were correlated with mean measures of task performance for those sessions (RT, Velmax, or Ampl). Spearman’s rank correlations were performed, and the threshold for significance was adjusted to account for multiple comparisons (P < 0.05/[12 comparisons] = 0.004).
The second analysis tested for relationships within individual recording sessions. A single neuron’s trial-to-trial firing rate and burst rate were correlated with an animal’s trial-to-trial performance of the behavioral task (RT, Velmax, or Ampl). Spearman’s rank correlations were performed for individual cells, and results were compared between populations recorded pre- and post-MPTP.
Results
Database
Electrode penetrations were made in the arm territory of the left M1 of 2 monkeys (Fig. 1A). For more anterior electrode penetrations, neurons were included only if they were at, or posterior to, sites where microstimulation-evoked arm movements at low threshold (i.e., <40 μA, 10 pulses at 300 Hz), thereby ensuring that neurons from the caudal premotor cortex were excluded (Weinrich and Wise 1982). Neurons were included if they were encountered in arm-related M1 and were either responsive to antidromic stimulation or were encountered within 0.5 mm of an antidromically activated neuron.
Of the 358 neurons studied in the normal state, 85 were activated antidromically from the putamen (CSNs; 51 in monkey V and 34 in monkey L), and 98 were activated from peduncle stimulation (PTNs; 76 in monkey V and 22 in monkey L). Among 245 neurons collected during the post-MPTP period, 60 responded to putamen stimulation (49 in monkey V and 11 in monkey L), and 68 were activated from peduncle stimulation (56 in monkey V and 12 in monkey L). Although neurons were often activated from several stimulating electrodes within the putamen, only 3 cells were activated from both the putamen and peduncle (0.5% of neurons studied). In the analyses below, these 3 cells were included in the “M1” category (which included all cells sampled, including PTNs, CSNs, and nonactivated cells) but were excluded from “CSN” and “PTN” categories. Figure 1B shows examples of the tests performed to determine antidromicity. These included observation of a highly consistent latency for stimulation-evoked action potentials and collision of the evoked action potentials when stimulation was preceded at short latency by a spontaneous spike. The action potentials of CSNs were typically of small amplitude and discriminable over a small range of electrode depths (<100 μm). Due to their small amplitude spikes and low discharge rates, CSNs would have composed a small fraction of the neuronal database if sampling had not been guided by antidromic activation. The remainder of the neurons (169 pre-MPTP and 109 post-MPTP) were not activated antidromically (NA) but were recorded either at the same time as CS or PT recordings or were encountered within 0.5 mm above or below an antidromically activated neuron along the same track (Fig.1A bottom).
Two Distinct Populations of M1 Cells
In addition to the observation that only 0.5% of neurons were activated antidromically from both the putamen and the peduncle, other observations reinforced the view that CSNs and PTNs belonged to distinct populations (Jones et al. 1977; Turner and DeLong 2000; Parent M and Parent A 2006; Ballion et al. 2008). The axonal conduction velocities of CSNs were slower than those of PTNs (Mann–Whitney U-test, P < 0.001). The antidromic spikes of CSNs had remarkably long latencies (range: 2.6–14.4 ms) compared with those of PTNs (range: 0.75–4.2 ms). Taking into account the estimated distances from stimulating sites in putamen and peduncle (20–25 mm and 31 mm, respectively), there was virtually no overlap between the conduction velocity distributions for CSNs and PTNs. Consistent with previous reports (Bauswein et al. 1989; Turner and DeLong 2000), spontaneous firing rates prior to MPTP administration were markedly lower for CSNs than for PTNs (Mann–Whitney U-test, P < 0.001). CSNs seldom fired >5 spikes/s (mean rate: 3.6 spikes/s, range: 0–22), whereas most PTNs exceeded 10 spikes/s (mean rate: 16.3 spikes/s, range: 0.2–45; Table 1). The spontaneous firing rates of these 2 neuronal populations did not differ between the 2 animals (Friedman test; χ2 < 2.4, P > 0.1).
Table 1.
Effects of MPTP on 2 distinct subpopulations of M1 cells
| M1 | CSN | PTN | ||
| Number of cells | Pre-MPTP | 358 | 85 | 98 |
| Post-MPTP | 245 | 60 | 68 | |
| Firing rate (sp/s) | Pre-MPTP | 11.6 ± 9.9 | 3.4 ± 3.6 | 18.9 ± 8.6 |
| Post-MPTP | 9.6 ± 9.9* | 3.8 ± 4.1 | 13.8 ± 9.5** | |
| % Time in bursts | Pre-MPTP | 4.1 ± 3.2 | 5.1 ± 3.1 | 2.9 ± 3.3 |
| Post-MPTP | 4.4 ± 3.9 | 4.7 ± 4 | 4.8 ± 4.9* | |
| % Spikes in bursts | Pre-MPTP | 21.7 ± 17.8 | 30.3 ± 16.3 | 9.9 ± 5.1 |
| Post-MPTP | 22.9 ± 19 | 26.5 ± 18 | 18.4 ± 20.9* | |
| Coefficient of variation | Pre-MPTP | 1.22 ± 0.44 | 1.35 ± 0.28 | 0.95 ± 0.4 |
| Post-MPTP | 1.25 ± 0.46 | 1.40 ± 0.49 | 1.05 ± 0.4* | |
| Rhythmic activity | Pre-MPTP | 134/358 (37%) | 2/85 (2%) | 74/98 (76%) |
| Post-MPTP | 77/254 (30%) | 4/60 (7%) | 40/68 (59%)*** |
Note: Mean values ± SD before and after MPTP treatment are calculated for all M1 cells (left), for CSNs (middle), and for PTNs (right).
*P < 0.05, **P < 0.001 (Mann–Whitney U-test) and ***P < 0.05 (χ2 test).
Task Performance
Intracarotid administration of MPTP rendered the animals moderately hemiparkinsonian as evidenced by the presence of bradykinesia, rigidity, and abnormal posture in the limbs contralateral to the infusion. Clinical examinations at regular intervals throughout the post-MPTP data collection period provided no evidence of tremor in either animal, consistent with previous observations that MPTP-treated macaques seldom exhibit parkinsonian tremor (Bankiewicz et al. 2001).
Performance of the step-tracking task was impaired following MPTP administration (Fig. 3). RTs were lengthened in both animals (two-way ANOVA; F > 221, P < 0.001). Consistent with previous reports of contralateral hemineglect following unilateral MPTP administration (Bankiewicz et al. 1986), the effect on RT was greatest for extension movements (i.e., for movements to the target contralateral to the lesioned hemisphere; ANOVA direction × treatment interaction; F > 14, P < 0.001). Movement velocities and amplitudes were also altered post-MPTP (F > 15 and P < 0.001), but the specific effect depended on the postural bias observed in the animal. In monkey V, flexion movements were particularly slow and hypometric, consistent with the extensor bias induced in that animal (Fig. 3, left column). In monkey L, extension movements were slow and hypometric, consistent with the flexor bias in that animal (Fig. 3, right column). MPTP-induced behavioral impairments persisted for the duration of the post-MPTP recording period (Supplementary Fig. 1), although a subset of performance measures (which differed between animals) showed evidence of recovery over time. Stereological analysis demonstrated a 67% loss of TH-positive neurons in the SNc of the MPTP-treated hemisphere (illustrated for monkey L in Fig. 4).
Figure 3.
Task performance was impaired following MPTP administration. Kinematic measures (cross-session means ± standard error of the mean) from pre-MPTP (“black”) and post-MPTP (“gray”) periods for flexion and extension movements in the visuomotor step-tracking task. RTs, peak velocities (Velmax), and movement amplitudes (Ampl) were compared between states (two-way ANOVA, MPTP × direction). ***Main effect of MPTP at P < 0.001. ###MPTP × direction interaction at P < 0.001.
Figure 4.
(A) Photomicrograph of a histologic section through the brainstem immunoreacted for TH (from monkey L). Compared with the control hemisphere (right), the studied hemisphere (left) displayed a severe loss of TH-positive neurons in the SNc. Subpanels on the right provide magnified views centered on the left and right SNc (top and bottom, respectively). The shaded line through the section marked by an asterisk reflects an artifact from histologic processing. (B) The density of TH-positive neurons in SNc of both hemispheres (control vs. MPTP) was estimated using unbiased stereological analysis.
Long periods of immobility (SPSPs) were identified during the start position hold period of the behavioral task. The total duration of SPSPs differed for recordings obtained pre- and post-MPTP (mean ± standard error of the mean: 96.3 ± 3.6 vs. 69.8 ± 2.8 s; F = 33.2, P < 0.001), primarily due to the fact that animals tended to perform fewer behavioral trials post-MPTP. None of the results reported below depended on this difference, however. Specifically, similar results were found when analyses for each cell were restricted to shorter time periods (≤50 s) that did not differ significantly in length between pre- and post-MPTP recordings.
Firing Rates
To facilitate comparison of our data with previous publications, we first examined the resting firing rates of all M1 cells combined (i.e., CSNs, PTNs, and NA cells). The mean firing rates of the general population of M1 cells decreased by 17% after MPTP treatment (Mann–Whitney U-test, P < 0.05). The distributions of neuronal firing rates for pre- and post-MPTP states are illustrated in Figure 5 (top), and mean values are summarized in Table 1. MPTP had markedly different effects on the 2 identified neuronal populations. The resting firing rates of CSNs remained unchanged following MPTP treatment (Fig. 5 middle, Table 1; Mann–Whitney U-test, P > 0.5). The resting rate of PTNs, however, was reduced by 27% following MPTP treatment (Mann–Whitney U-test, P < 0.001; Fig. 5 bottom, Table 1). The highly significant reduction in PTN firing rate was a major contributor to the significant reduction observed in the general M1 population. The general population of M1 cells excluding PTNs (i.e., CSNs and NAs) showed no evidence of a change in firing rate following MPTP (Mann–Whitney U-test, P > 0.5).
Figure 5.
MPTP reduced baseline firing rates preferentially in PTNs. Frequency distributions and mean ± standard error of the mean comparisons of the spontaneous firing rates of M1 neurons in pre-MPTP (black) and post-MPTP (gray) states. Separate panels show results for all M1 cells (top), CSN (middle), and PTN (bottom). Horizontal box plots show the middle 2 quartiles of the firing rate distributions. The central white line within each box corresponds to the median value and horizontal “whiskers” outside each box show the extent of the overall distribution (excluding outliers). In the post-MPTP state, there was a significant decrease of the mean spontaneous firing rate for the general population of M1 neurons (*Mann–Whitney U-test, P < 0.05) and a much larger reduction for PTNs (***Mann–Whitney U-test, P < 0.001).
Firing Patterns
To test for MPTP-induced effects on neuronal firing patterns, we first classified neuronal firing patterns into 3 general categories (regular, random, and bursty firing; Fig. 2). Nineteen cells were excluded from this analysis because extremely low firing rates prevented reliable fitting to an SDH. For the general population of M1 cells, the proportion of neurons in different firing pattern categories did not change following MPTP (χ2 = 4.5, P = 0.11; Fig. 6). In both pre- and post-MPTP periods, approximately 20% of M1 cells discharged in a regular tonic pattern, 32% discharged in a random pattern, and 48% discharged in a bursty pattern. Similarly, MPTP did not alter the distribution of firing patterns of CSNs (χ2 = 2.9, P = 0.23). Most CSNs (∼70%) had a bursty pattern of discharge both before and following MPTP. Note that very few CSN had a regular tonic discharge pattern. For PTNs, however, the proportion of neurons with a regular discharge pattern decreased significantly following MPTP administration (−15%; χ2 = 5.9, P = 0.05) accompanied by reciprocal increases in random and bursty discharge patterns (+7% and +8%, respectively). A selective effect of MPTP on the firing patterns of PTNs was corroborated by a significant increase in the mean ISI coefficient of variation for PTNs post-MPTP (Mann–Whitney U-test, P < 0.05; Table 1).
Figure 6.
MPTP altered the firing patterns of PTNs. The fraction of neurons that discharged with regular (white), random (gray), and burst (black) patterns did not change follow MPTP administration for the general population of M1 cells (top) or for CSNs (middle). For PTNs (bottom), the fraction of neurons firing in a regular pattern decreased significantly and cells firing in random or bursty patterns increased proportionately (*; χ2 test, P < 0.05).
A more focused analysis of burst firing using the Legendy surprise method revealed a clear MPTP-induced increase in the mean fraction of time that PTNs spent in bursts. The general population of M1 cells spent similar fractions of time in bursts pre- and post-MPTP (Fig. 7 top). CSNs also showed similar fractions of time in bursts pre- and post-MPTP (Fig. 7 middle). Parallel analyses of the fraction of spikes in bursts yielded very similar results for general population and CSN categories (Table 1). For PTNs, however, the mean fraction of time in bursts increased 1.7-fold following MPTP (Mann–Whitney U-test, P < 0.05; Fig. 7 bottom) and the fraction of spikes in bursts increased 1.9-fold (Mann–Whitney U test, P < 0.05). These measures of PTN burstiness were inversely correlated with the mean firing rates (Spearman rho = −0.5, P < 0.001), suggesting that the MPTP-induced reductions in firing rate and increases in burst prevalence shared a common substrate (Supplementary Fig. 2A). For CSNs, in contrast, higher baseline firing rates were associated with greater burstiness (rho = 0.3, P < 0.001; data not shown). Results from the categorical classification of discharge pattern and the Poisson surprise analysis were complimentary. The fraction of time in bursts differed significantly between the 3 discharge pattern categories (Kruskal–Wallis test, P < 0.001; Supplementary Fig. 2B). As expected, cells categorized as “bursty” spent much more time in bursts than “regular” cells. “Random” cells spent an intermediate fraction of time in bursts.
Figure 7.
PTNs fired action potentials in bursts more frequently following MPTP. Distributions and mean ± standard error of the mean comparisons of the percent of time in bursts of M1 neurons in the normal (black) and MPTP (gray) states. PTNs showed a significant increase in the percent of time in bursts following MPTP administration (*Mann–Whitney U-test, P < 0.05). The figure follows the conventions outlined for Figure 5.
MPTP also altered the time-magnitude structure of bursts in M1 activity. Figure 8 shows the periburst firing rates for CSN and PTN populations and Table 2 summarizes the individual burst parameters for those 2 populations. For CSNs, the magnitude and duration of bursts did not differ substantially between pre- and post-MPTP periods (Fig. 8B left). For PTNs, however, intraburst firing rate was depressed post-MPTP (Mann–Whitney U-test, P < 0.05; Fig. 8B right) and burst duration increased (Mann–Whitney U-test, P < 0.05). Measures of a PTN’s burst morphology correlated closely with its mean firing rate in the post-MPTP data (burst duration vs. mean rate, Spearman |rho| = 0.7 and P < 0.001; intraburst firing rate vs. mean rate, rho = 0.8 and P < 0.001) suggesting that alterations in burst morphology were likely related to the MPTP-induced reduction in PTN mean firing rates.
Figure 8.
MPTP altered the magnitude and time course of burst discharges in PTNs. (A) Raster representation of typical M1 activity with bursts. (Example is from a PTN post-MPTP.) Black vertical ticks indicate times of individual action potentials. Horizontal red bars show times of bursts as determined by the Legendy surprise method. (B) Population averages of the burst-triggered mean frequency of firing (±95% confidence intervals) for CSNs and PTNs in normal (“black trace”) and MPTP (“red trace”) states. The mean instantaneous frequency of firing functions were aligned on burst onset times for all bursts detected by the Legendy surprise method. Mean preburst firing rates (between −300 and −100 ms) were subtracted from individual cell averages to aid comparison of the burst characteristics. Inset: P values from t-tests comparing pre- and post-MPTP periburst population averages bin-by-bin (1 ms). Green horizontal line: P = 0.05.
Table 2.
Effects of MPTP on burst parameters
| M1 | CSN | PTN | ||
| Mean spikes per burst | Pre-MPTP | 8.4 ± 4 | 6.9 ± 3.3 | 9.9 ± 5.1 |
| Post-MPTP | 7.7 ± 3.6* | 6.7 ± 3.2 | 9.5 ± 4 | |
| Intraburst firing rate (sp/s) | Pre-MPTP | 39.5 ± 31.2 | 17.4 ± 19.3 | 48.2 ± 27.2 |
| Post-MPTP | 43.7 ± 68.2 | 30.9 ± 55.7 | 41.8 ± 31.8* | |
| Burst duration (ms) | Pre-MPTP | 383 ± 709 | 756 ± 1297 | 218 ± 139 |
| Post-MPTP | 364 ± 369 | 540 ± 515 | 360 ± 330* | |
| Intraburst coefficient of variation | Pre-MPTP | 1.12 ± 0.59 | 1.32 ± 0.39 | 0.77 ± 0.49 |
| Post-MPTP | 1.1 ± 0.56 | 1.19 ± 0.59 | 0.74 ± 0.4 | |
| Neurons with burst-associated movements | Pre-MPTP | 52/297 (17%) | 4/40 (10%) | 11/54 (20%) |
| Post-MPTP | 20/141 (14%) | 2/16 (12%) | 5/30 (16%) |
Note: Mean values ± SD before and after MPTP treatment are calculated for all M1 cells (left), for CSNs (middle), and for PTNs (right).
*P < 0.05 (Mann–Whitney U-test).
Next, we tested whether bursts in M1 activity were associated with small movements of the manipulandum, consistent with traditional views of the role of M1 in motor function, and if that relationship broke down in parkinsonian animals as reported previously (Goldberg et al. 2002). Averages of |velocity| were constructed around the onset times of bursts for all cells that emitted >10 bursts. For a minority of M1 neurons (17%, 50 of the 298 cells), bursts were associated with small but significant transients in |velocity| (Table 2, Supplementary Fig. 3). Velocity transients associated with individual burst occurrences were indistinguishable from the background noise of the recording system, but when |velocity| was averaged across all burst occurrences the transients combined to reveal a significant peak in the periburst average. The proportion of cells with burst-associated |velocity| transients did not differ significantly between pre- and post-MPTP recording periods (18% and 14%, respectively; χ2 = 2.2, P > 0.3) or between the 3 cell populations (general M1, CSN, and PTN; χ2 = 1.9, P > 0.3). In particular, similar fractions of PTNs from pre- and post-MPTP periods showed significant periburst |velocity| peaks (20% and 17%, respectively; χ2 = 0.77, P > 0.6). Periburst movements began at a range of latencies relative to the time of burst onset (range: −96 to 82 ms; mean = −23 ms). The distribution of latencies did not differ between pre- and post-MPTP periods or between cell populations (Mann–Whitney U-test, P > 0.1). No obvious relationship was found between the MPTP-induced increase in PTN burstiness (reported above) and the association of bursts with velocity transients.
To summarize, MPTP increased the prevalence of irregular firing and bursts in PTNs and altered burst structure, but the firing patterns of CSNs remained unaffected. MPTP had no apparent effect on the relationship between bursts in M1 activity and the occurrence of small movements of the arm.
Firing Rhythmicity
The presence of rhythmic spiking in M1 neurons was revealed as periodic peaks and valleys in a spike train’s autocorrelation function and as a significant peak in the standard spectral density function (Fig. 9). It is important to note that spectra produced using the shuffled normalization method yielded no significant spectral peaks for any cortical neuron studied pre- or post-MPTP (i.e., 0% of 603 neurons). Given that the shuffled normalization analysis specifically detected rhythmic modulations in firing rate (e.g., oscillatory bursting) (Rivlin-Etzion et al. 2006; McCairn and Turner 2009), the negative result indicated: 1) that rhythmic modulations in firing rate or bursting were not present in M1 neurons pre- or post-MPTP and 2) that any significant periodicity detected in the standard spectra arose from the rhythmic generation of individual action potentials (i.e., the presence of highly regular ISIs).
Figure 9.
Examples of rhythmic firing in 2 PTNs. Autocorrelation functions of the spike trains and power spectra illustrate the tendency of many PTNs to fire individual action potentials at regular ISIs, leading to significant peaks in the standard spectra (right, see Materials and Methods). Horizontal dashed line: threshold for statistical significance relative to the mean ± SD between 300 and 500 Hz.
Rhythmic firing was far more common in PTNs than in CSNs (69% vs. 4% of neurons, respectively, combining pre- and post-MPTP populations; χ2 = 9.5, P < 0.001; Table 1). As expected, rhythmic firing was found most often in spike trains classified as having a regular discharge pattern (77% of M1 cells; Supplementary Fig. 4), but spike trains categorized as random and bursty could also contain significant rhythmicity (48% and 10% of M1 cells, respectively; Supplementary Fig. 4). Consistent with the previously described increase in irregular and bursty firing in PTNs post-MPTP (Figs 6–7 and Table 1), the fraction of PTNs expressing rhythmic spiking decreased by 17% following MPTP administration (χ2 = 7.5, P < 0.05; Table 1). In contrast, the fraction of rhythmic-firing CSNs was not affected post-MPTP (χ2 = 3.2, P = 0.2; Table 1).
Although fewer PTNs fired rhythmically post-MPTP, those that did were more likely to fire rhythmically in the beta frequency range (14–32 Hz). Prior to MPTP administration, spectral peaks were distributed evenly from 15 to 58 Hz for both the general population of M1 neurons and for PTNs in particular (Fig. 10). For both categories, a substantial fraction of the rhythmic activity occurred in the low gamma frequencies (33–60 Hz). Following MPTP, spectral peaks became more concentrated in the beta frequency range for both M1 neurons in general (Kolmogorov–Smirnov test; D = 2.1, P < 0.05) and for PTNs in particular (D = 7.5, P < 0.05). (The small number of rhythmically firing CSNs [n = 2 in pre- and n = 4 in post-MPTP] prevented a similar analysis for those neurons.)
Figure 10.
Rhythmically spiking cortical neurons were more likely to fire at beta frequencies following MPTP. Distributions of the preferred frequency of rhythmic firing in the general population of M1 neurons and in PTNs pre-MPTP (black) and post-MPTP (gray). Cumulative frequency distributions (bottom) illustrate the increased concentration of rhythmic frequencies in the beta frequency band (light-gray rectangle) following MPTP administration (*P < 0.05; Kolmogorov–Smirnov 2-sample test). Although this plot also appeared to show a post-MPTP increase in rhythmic firing at frequencies <13 Hz, the small number of cells (7 cells total) prevented reliable statistical tests of this possibility.
Abnormalities in Neuronal Activity Seldom Correlated with the Severity of Parkinsonism
Although the resting activity of PTNs and task performance were both affected by MPTP administration, the modifications in resting M1 activity were not correlated with variations in motor impairment (Supplementary Fig. 5). That question was addressed first by testing for correlations between session-by-session measures of mean PTN activity and mean measures of task performance during those sessions (RT, Velmax, and Ampl). The analysis was restricted to PTNs so that the tests were performed on a relatively homogenous population of neurons whose firing was known to be affected by MPTP administration. The mean resting firing rates of PTNs did not correlate with Velmax (Spearman |rho| < 0.2, P > 0.4; Supplementary Fig. 5A) or Ampl (Spearman |rho| < 0.3, P > 0.2; data not shown) either before or after MPTP administration. PTN firing rates were also uncorrelated with RTs during the pre-MPTP period (rho = −0.1, P > 0.4) but showed a modest positive correlation with RTs post-MPTP (rho = 0.4, P < 0.003; i.e., RTs were lengthened when PTN rates were higher). Measures of burstiness (fraction of time in bursts and fraction of spikes in bursts) and beta rhythmicity did not correlate with any of the measures of task performance (all Spearman |rho| < 0.2; P > 0.05; results for %-time-in-bursts and beta rhythmicity vs. Velmax are shown in Supplementary Fig. 5B–C).
A second approach searched for correlations between the trial-to-trial baseline firing rate of a neuron and single-trial task performance (RT, Velmax, and Ampl). Resting rates correlated with one or another measure of task performance in a small number of PTNs (<9% of the cells studied pre- or post-MPTP) and the fraction of cells with significant correlations did not change pre- vs. post-MPTP (χ2 < 8.0, P > 0.1). Moreover, the mean correlation coefficient (Spearman rho averaged across cells) did not differ significantly between PTNs recorded pre- and post-MPTP (T-statistic < 1.0, P > 0.1). Similar results were obtained in correlation analyses of the general population of M1 cells and of CSNs (results not shown).
Discussion
We studied the effects of MPTP-induced parkinsonism on the resting activity of antidromically identified neurons in the primate M1. Following MPTP administration, PTNs as a population showed reduced resting firing rates, an increased tendency to fire action potentials in irregular patterns and bursts, and an increased propensity for rhythmic spiking in the beta frequency range. The spontaneous activity of CSNs and other unidentified neurons in M1, in contrast, was unaffected by MPTP administration. Given that the primary efferent pathway that conveys motor commands from M1 to the spinal cord originates in PTNs, the observed abnormalities in PTN activity may contribute to parkinsonian motor signs. The observation of PTN-selective effects of MPTP intoxication may explain at least some of the discrepancies between previous studies of motor cortical function in parkinsonism.
Spontaneous Firing Rates
Our results are in partial agreement with the classical Albin/DeLong model in which parkinsonian pathophysiology is associated with reduced excitatory drive to the motor cortices and insufficient activation of the corticospinal tract (Albin et al. 1989; DeLong 1990). Although numerous studies of idiopathic PD and experimental parkinsonism have reported increased spontaneous firing rates in BG output neurons (Miller and DeLong 1987; Filion and Tremblay 1991; Bergman et al. 1994; Vila et al. 1997; Boraud et al. 1998; Wichmann et al. 1999; Soares et al. 2004; Starr et al. 2008), not all studies have observed such changes, and current opinion holds that altered firing rates in BG output neurons cannot, by themselves, account for parkinsonian pathophysiology (Raz et al. 2000; Vitek and Giroux 2000; Montgomery 2007; Galvan and Wichmann 2008). Changes in firing rate at the thalamic and cortical level have been studied far less often, however, and their relations to parkinsonian signs are less well understood. Several studies have reported a reduction in spontaneous activity in the BG-recipient thalamus in animal models of parkinsonism (Voloshin et al. 1994; Schneider and Rothblat 1996; Elder and Vitek 2001; Rolland et al. 2007) and in intraoperative recording studies in humans (Molnar et al. 2005; Miao et al. 2009). A recent single-unit recording study in the MPTP-treated primate, however, reported no significant modification of resting firing rates in the BG-recipient thalamus (Pessiglione et al. 2005). Similarly, several studies failed to find alterations in firing rate in a general sample of M1 neurons recorded before and after MPTP (Doudet et al. 1990; Watts and Mandir 1992; Goldberg et al. 2002).
Few models of the pathophysiology of PD consider the fact that cortex contains diverse neuronal cell types that differ with respect to morphology, laminar distribution, afferent inputs, and efferent projections (Creutzfeldt 1995). These divergent characteristics suggest that different cortical cell types perform distinct functions. Indeed, abundant evidence indicates that 2 distinct cell types in M1, intratelencephalic-projecting CSNs and distant-projecting PTNs, transmit markedly different types of task information to different targets (Bauswein et al. 1989; Swadlow 1994; Turner and DeLong 2000; Beloozerova et al. 2003). Correspondingly, not all neuronal subtypes of a functionally diverse cortex may be affected in the same way by the induction of parkinsonism. The present study supports that conjecture by showing that the induction of parkinsonism led to a large (27%), but selective, decrease in the spontaneous firing rate of PTNs (Fig. 5 and Table 1). The mean discharge rate of CSNs, in contrast, remained unchanged. The general population of M1 neurons showed a 17% reduction in firing rate, but that change was attributable in large part to the PTN component of the population.
How do we reconcile the present results with multiple previous reports (references above) that mean firing rates in M1 were not changed in the parkinsonian state? Several significant differences in experimental approach are worth considering. First, all earlier studies reported on general samples of cortical neurons with no attempt to distinguish neuronal subtypes. Antidromic identification helped us avoid sampling biases (Towe and Harding 1970) including an undersampling of low-firing rate PTNs (a category that grew markedly following MPTP). Second, although the effects of behavioral state on resting firing rates is well recognized for cortex, thalamus, and the BG (Evarts 1963; DeLong 1969; Detari et al. 1987; Bezdudnaya et al. 2006; Stefani et al. 2006), the severity of parkinsonian symptoms prevented some previous studies from using a task to control behavioral state (Goldberg et al. 2002; but see Doudet et al. 1990). Finally, there were substantial differences between studies in the mode of toxin administration (systemic in Goldberg et al. [2002] vs. unilateral intracarotid here) and the severity and duration of dopamine depletion (<60 days following MPTP in Goldberg et al. [2002] vs. 36–117 days post-MPTP here). Little is known about how these differences might affect the model of parkinsonism produced and the attendant pathophysiology.
One particular discrepancy worth highlighting is the relatively high firing rates found for PTNs here (>10 sp/s) compared with the low firing rates reported previously (∼5 sp/s in Goldberg et al. [2002]; Rivlin-Etzion et al. [2008]) for both pre- and post-MPTP periods. One possible explanation for this disparity is that some aspect of the behavioral task used in our study, or the mere fact that the animals were engaged in a task, increased baseline firing rates significantly. Other studies have also reported high rates for a general sample of M1 neurons recorded during task performance (∼10 sp/s, Doudet et al. 1990) and low rates for lamina 5 corticofugal neurons in animals at rest (∼5 sp/s, Swadlow 1994; Beloozerova et al. 2003). Based on these observations, one might question whether firing rate during immobile periods of a behavioral task reflects a neuron’s true “rest state activity.” Despite this limitation, however, engagement in a well-defined behavioral task remains the best-established method for achieving relative control over a subject’s behavioral state (Bergman et al. 1994).
The observation that MPTP depressed resting activity in PTNs selectively may explain the diverse results from studies of cortical metabolism in PD patients and animal models of the disease (Crossman et al. 1985; Schwartzman and Alexander 1985; Schwartzman et al. 1988; Palombo et al. 1990; Eidelberg et al. 1994; Piert et al. 1996; Arahata et al. 1999; Hu et al. 2000; Berding et al. 2001; Brownell et al. 2003). Low resolution imaging approaches such as positron emission tomography and postmortem 2-deoxyglucose cannot resolve cell-type specific changes in activity. Studies using methods that have cell-level resolution have provided clear evidence that metabolic markers are significantly depressed in PTNs following dopamine depletion (Steiner and Kitai 2001; Orieux et al. 2002). The absence of a significant change in CSN firing rates observed here disagrees with previous reports that CSN activity was selectively reduced in an anesthetized rat model of PD (Mallet et al. 2006; Ballion et al. 2008). Others have argued, however, that the baseline activity of CSNs is actually elevated with the induction of parkinsonism, based on observed increases in striatal glutamatergic tone (Barbeito et al. 1989; Lindefors and Ungerstedt 1990; Porter et al. 1994; Meshul et al. 1999) and alterations in the morphology of CSN synapses (Meshul et al. 1999; Muriel et al. 2001; Villalba and Smith 2009).
Firing Pattern
Another neurophysiologic hallmark of parkinsonism is an increased propensity for neurons in BG nuclei to fire action potentials in bursts (Miller and DeLong 1987; Filion and Tremblay 1991; Bergman et al. 1994; Boraud et al. 1998; Wichmann et al. 1999; Wichmann and Soares 2006). Several influential models have proposed that spread of bursty firing patterns from the BG to thalamocortical circuits contributes to the genesis of parkinsonian signs (Vitek 2002; Rubin and Terman 2004; Wichmann and Soares 2006; Guo et al. 2008). The existing literature, however, contains reports of normal neuronal discharge patterns in BG-recipient thalamus (Pessiglione et al. 2005) and M1 (Doudet et al. 1990) following the induction of parkinsonism, in addition to reports that bursting discharge is increased in those areas (Elder and Vitek 2001; Goldberg et al. 2002; Postupna and Anderson 2007). The second major finding of the current study is that MPTP-induced parkinsonism was associated with a marked increase in burst-like discharges in PTNs (Fig. 7 and Table 1). Here again, the induction of parkinsonism had different effects on different cortical neuron subtypes. The incidence of bursts remained unchanged in the general population of M1 neurons and in CSNs. As discussed above for firing rates, the discordance between our results and previous studies may be attributed to differences in neuronal sampling, the animal model used, and possible changes in the behavioral state of the animal subjects.
We also found that the time–magnitude structure of PTN bursts was changed following MPTP administration (Fig. 8 and Table 2). The fact that alterations in burst occurrence and burst morphology correlated well with PTN firing rate (Supplementary Fig. 2A) suggests that a common mechanism mediated the effects of MPTP on firing rates and bursts.
It is unlikely, however, that the post-MPTP increase in PTN burstiness arose from direct propagation of burst discharges from the parkinsonian BG via thalamus to cortex. First, the simple fact that BG efferents are GABAergic (Uno and Yoshida 1975; Penney and Young 1981) means that synchronized bursts in BG output neurons should cause synchronized waves of “inhibition” in BG-recipient thalamocortical circuits, followed, perhaps, by disinhibition-related rebound bursting (Person and Perkel 2005; Kojima and Doupe 2009). In addition, the characteristics of bursts in M1 activity (arhythmic and long duration, see also Goldberg et al. 2002) differed substantially from the often rhythmic short-duration bursts described for the parkinsonian BG (Bergman et al. 1994; Wichmann and Soares 2006). A variety of indirect mechanisms may contribute to the increase in PTN burstiness post-MPTP, ranging from postinhibitory rebound bursting in BG-recipient thalamocortical neurons (Jeanmonod et al. 1996) to circuit-level interactions (DeLong and Wichmann 2007). Loss of dopamine locally within cortex (Gaspar et al. 1992; Jan et al. 2003) and abnormal suppression of intracortical inhibition (Ridding et al. 1995; Chu et al. 2009) may also contribute to the observed abnormalities in PTN firing pattern.
In a minority of M1 neurons (∼17% of cells with bursts), bursts were associated consistently with slight shifts in joint position (Supplementary Fig. 3). Such burst-related velocity transients appeared in similar fractions of cells studied before and after MPTP administration and in roughly equal proportions of all neuronal subtypes (Table 2). Burst-associated movements began at a range of latencies, some preceding and others following burst onset, suggesting that bursts could be both a product of somatosensory input to cortex and a driver of motor output. Although these results are consistent with established roles of M1 in sensorimotor function (Evarts 1966; Lemon and Porter 1976; Cheney and Fetz 1984), they differ from Goldberg et al.’s (2002) report that, in parkinsonian animals, bursts in M1 activity were not associated with movement. In light of that report, we used stringent statistical criteria to reduce the likelihood of false positives. In addition, we sampled only one of the many dimensions of forelimb movement that might be associated with bursts of neuronal activity in arm-related M1. Despite those restrictions, burst-related movements were found in small but significant fractions of the M1 neurons studied pre- and post-MPTP. These results are not consistent with the idea that M1 activity and movement are somehow decoupled in parkinsonian animals (Goldberg et al. 2002).
Rhythmic Firing
The parkinsonian state has been associated with increased low-frequency oscillatory activity studied at the macroscale (local field potentials, electroencephalography, and magnetoencephalography) (i.e., in the alpha-, theta- [Tanaka et al. 2000; Bosboom et al. 2006; Sarnthein and Jeanmonod 2007; Stoffers et al. 2007; Moazami-Goudarzi et al. 2008], and beta-frequency [Brown 2003] bands). Those observations have prompted proposals that increased entrainment of cortical activity to low frequency oscillations, accompanied perhaps by deficient rhythmicity in the gamma frequency band, plays an important role in the genesis of parkinsonian signs (Gatev et al. 2006; Brown 2007; Hammond et al. 2007). The relationship between abnormalities in population-scale potentials and changes in the spike trains of single cortical neurons remains unclear, however. Indeed, Goldberg et al. (2002) found no increase in rhythmic bursting in the M1 of parkinsonian animals. The present study corroborated that observation and also identified an increase in ISI variability (Fig. 6 and Table 1). In addition, our spectral analysis found that fewer PTNs generated rhythmic spiking following the administration of MPTP (Table 1). Together, these observations appear to conflict with the idea that M1 spike activity becomes more organized and entrained to low-frequency rhythms in the parkinsonian state.
A possible explanation for how activity in M1 may be both less organized and more rhythmic in parkinsonism comes from our observation that the extant rhythmic firing became more concentrated in the beta-frequencies following MPTP administration (Fig. 10). A propensity for M1 neurons to fire rhythmically is well recognized (Schwindt and Crill 1999; Wetmore and Baker 2004; Chen and Fetz 2005), although that rhythmicity was not examined in previous studies in parkinsonian animals. An increased concentration of rhythmic firing in a narrow band of beta frequencies is likely to be associated with greater millisecond-scale synchronization of spiking across neurons (Baker et al. 2001), both of which would increase beta power in population-scale cortical potentials. Emerging evidence suggests that beta synchronization in M1 promotes postural stabilization (Baker et al. 2001; Gilbertson et al. 2005; Kristeva et al. 2007). Exaggerated beta synchronization may promote rigidity and impede efficient mobilization of the limb. Beta rhythmicity may also block synchronization at higher (gamma) frequencies, thereby interfering with the efficient recruitment of task-specific neuronal ensembles (Brown and Marsden 1998; Omlor et al. 2007).
Pathophysiology and Functional Considerations
In summary, we found that PTNs were particularly sensitive to MPTP treatment in that their spontaneous discharge rates were reduced, and their propensity to fire action potentials irregularly and in bursts was increased. M1 PTNs are the source of the strongest and most direct motor pathway from cortex to spinal motor nuclei (He et al. 1993; Porter and Lemon 1993). Because of this, the particular sensitivity of M1 PTNs to the loss of dopamine may be an important contributor to the motor impairments of PD. Considered separately, some motor signs suggest insufficient motor activity (akinesia and bradykinesia), while others suggest excessive activity (rigidity and tremor). Accordingly, one might speculate that the decreased baseline firing rate of PTNs after MPTP treatment contributes to akinesia and bradykinesia, while the increased incidence of burst firing contributes to muscle rigidity. This account is conjectural, however. We found little evidence that variations in the severity of parkinsonian signs (across recording sessions or across individual behavioral trials) could be explained by variations in M1 rest activity (Supplementary Fig. 5). It is quite possible that abnormalities in M1 activity not explored here (e.g., responsiveness to active movement [Parr-Brownlie and Hyland 2005]) also contribute to parkinsonian signs. Our results are unlikely to be relevant to the pathophysiology of tremor for 2 reasons: 1) MPTP injections did not induce tremor in our animals and 2) no significant tremor-frequency periodicity was observed in M1 activity.
What physiologic mechanisms can account for the different effects of dopamine depletion on PTNs and CSNs? PTNs are known to receive direct synaptic input from motor regions of the thalamus (Strick and Sterling 1974), including direct inputs from BG-recipient thalamic regions (Rathelot and Strick 2009). Several studies indicate that CSNs, in contrast, receive few thalamocortical synapses (Kitai et al. 1976; Jinnai and Matsuda 1979; Hersch and White 1982). PTNs and CSNs also differ with respect to laminar distribution, morphology, intrinsic membrane properties, and firing patterns (Stewart and Foehring 2000; Morishima and Kawaguchi 2006; Hattox and Nelson 2007; Groh et al. 2010). Some combination of the differences listed here may allow abnormal inhibitory outflow from the parkinsonian BG to affect PTN activity strongly while leaving the activity of CSN relatively unchanged.
It is important to recognize that the nonlinear characteristics of inhibitory pallidothalamocortical synaptic transmission (Person and Perkel 2005; Kojima and Doupe 2009) make it possible that one abnormality in pallidal firing (e.g., synchronized bursts) may be translated into other forms of firing abnormality at the cortical level (e.g., altered firing rates). The physiology of pallidothalamocortical transmission must be studied in more detail to understand the relationship between specific PD-related changes in pallidal activity and their attendant thalamic and cortical effects. It is also important to note that local pathologic changes may contribute to the abnormalities in cortical function observed in parkinsonism (Jan et al. 2003; Moore et al. 2008). Future studies are required to determine the importance of these local changes, which include reduced dopaminergic innervation of M1 (Pifl et al. 1990; Jan et al. 2003; Hosp et al. 2009) and intrinsic neurochemical dysfunctions (Ferrer 2009).
In summary, our results are in partial agreement with the classical Albin/DeLong model of parkinsonism in that the resting firing rates of PTNs was reduced following MPTP administration. Our results are also consistent with the common current view that altered “patterns” of neuronal activity play important roles in PD pathophysiology (DeLong and Wichmann 2007; Israel and Bergman 2008). Following MPTP, PTNs were less likely to fire in a regular-spiking pattern, and in agreement with previous results (Goldberg et al. 2002), burst firing became more frequent. A novel finding was that cells that did fire rhythmically following MPTP had an increased propensity to fire in the beta frequency range, consistent with the idea that beta-frequency oscillations, synchronized across the BG and motor cortex, play an important role in the genesis of parkinsonian signs (Gatev et al. 2006; Brown 2007; Hammond et al. 2007). In contrast to the effects on PTN activity, the activity of CSNs was unaffected by MPTP, likely due to the unique connectivity and intrinsic properties of those neurons.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institute of Neurological Disorders and Stroke at the National Institutes of Health (grant numbers NS044551 and NS055197 to R.S.T.).
Supplementary Material
Acknowledgments
We thank Dr J. Timothy Greenamyre and Dr Victor Tapias for their assistance with the stereological analysis. Conflict of Interest: None declared.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Alexander GE. Selective neuronal discharge in monkey putamen reflects intended direction of planned limb movements. Exp Brain Res. 1987;67:623–634. doi: 10.1007/BF00247293. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol. 1990;64:133–150. doi: 10.1152/jn.1990.64.1.133. [DOI] [PubMed] [Google Scholar]
- Arahata Y, Hirayama M, Ieda T, Koike Y, Kato T, Tadokoro M, Ikeda M, Ito K, Sobue G. Parieto-occipital glucose hypometabolism in Parkinson's disease with autonomic failure. J Neurol Sci. 1999;163:119–126. doi: 10.1016/s0022-510x(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Baker SN, Spinks R, Jackson A, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. i. Task-dependent modulation in single-unit synchrony. J Neurophysiol. 2001;85:869–885. doi: 10.1152/jn.2001.85.2.869. [DOI] [PubMed] [Google Scholar]
- Ballion B, Mallet N, Bezard E, Lanciego JL, Gonon F. Intratelencephalic corticostriatal neurons equally excite striatonigral and striatopallidal neurons and their discharge activity is selectively reduced in experimental parkinsonism. Eur J Neurosci. 2008;27:2313–2321. doi: 10.1111/j.1460-9568.2008.06192.x. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Oldfield EH, Chiueh CC, Doppman JL, Jacobowitz DM, Kopin IJ. Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Life Sci. 1986;39:7–16. doi: 10.1016/0024-3205(86)90431-5. [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Sanchez-Pernaute R, Oiwa Y, Kohutnicka M, Cummins A, Eberling J. Preclinical models of Parkinson's disease. Curr Protoc Neurosci. 2001;9:9.4.1–9.4.32. doi: 10.1002/0471142301.ns0904s09. [DOI] [PubMed] [Google Scholar]
- Barbeito L, Girault JA, Godeheu G, Pittaluga A, Glowinski J, Cheramy A. Activation of the bilateral corticostriatal glutamatergic projection by infusion of GABA into thalamic motor nuclei in the cat: an in vivo release study. Neuroscience. 1989;28:365–374. doi: 10.1016/0306-4522(89)90183-8. [DOI] [PubMed] [Google Scholar]
- Barraud Q, Lambrecq V, Forni C, McGuire S, Hill M, Bioulac B, Balzamo E, Bezard E, Tison F, Ghorayeb I. Sleep disorders in Parkinson's disease: the contribution of the MPTP non-human primate. model. Exp Neurol. 2009;219:574–582. doi: 10.1016/j.expneurol.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Bauswein E, Fromm C, Preuss A. Corticostriatal cells in comparison with pyramidal tract neurons: contrasting properties in the behaving monkey. Brain Res. 1989;493:198–203. doi: 10.1016/0006-8993(89)91018-4. [DOI] [PubMed] [Google Scholar]
- Beloozerova IN, Sirota MG, Swadlow HA. Activity of different classes of neurons of the motor cortex during locomotion. J Neurosci. 2003;23:1087–1097. doi: 10.1523/JNEUROSCI.23-03-01087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berding G, Odin P, Brooks DJ, Nikkhah G, Matthies C, Peschel T, Shing M, Kolbe H, van Den Hoff J, Fricke H, et al. Resting regional cerebral glucose metabolism in advanced Parkinson's disease studied in the off and on conditions with [(18)F]FDG-PET. Mov Disord. 2001;16:1014–1022. doi: 10.1002/mds.1212. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Bezdudnaya T, Cano M, Bereshpolova Y, Stoelzel CR, Alonso JM, Swadlow HA. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Boraud T, Bezard E, Guehl D, Bioulac B, Gross C. Effects of L-DOPA on neuronal activity of the globus pallidus externalis (GPe) and globus pallidus internalis (GPi) in the MPTP-treated monkey. Brain Res. 1998;787:157–160. doi: 10.1016/s0006-8993(97)01563-1. [DOI] [PubMed] [Google Scholar]
- Bosboom JL, Stoffers D, Stam CJ, van Dijk BW, Verbunt J, Berendse HW, Wolters E. Resting state oscillatory brain dynamics in Parkinson's disease: an MEG study. Clin Neurophysiol. 2006;117:2521–2531. doi: 10.1016/j.clinph.2006.06.720. [DOI] [PubMed] [Google Scholar]
- Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978;101(Pt 2):251–283. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- Brown P. Oscillatory nature of human basal ganglia activity: relationship to the pathophysiology of Parkinson's disease. Mov Disord. 2003;18:357–363. doi: 10.1002/mds.10358. [DOI] [PubMed] [Google Scholar]
- Brown P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr Opin Neurobiol. 2007;17:656–664. doi: 10.1016/j.conb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Brown P, Marsden CD. What do the basal ganglia do? Lancet. 1998;351:1801–1804. doi: 10.1016/s0140-6736(97)11225-9. [DOI] [PubMed] [Google Scholar]
- Brownell AL, Canales K, Chen YI, Jenkins BG, Owen C, Livni E, Yu M, Cicchetti F, Sanchez-Pernaute R, Isacson O. Mapping of brain function after MPTP-induced neurotoxicity in a primate Parkinson's disease model. Neuroimage. 2003;20:1064–1075. doi: 10.1016/S1053-8119(03)00348-3. [DOI] [PubMed] [Google Scholar]
- Chen D, Fetz EE. Characteristic membrane potential trajectories in primate sensorimotor cortex neurons recorded in vivo. J Neurophysiol. 2005;94:2713–2725. doi: 10.1152/jn.00024.2005. [DOI] [PubMed] [Google Scholar]
- Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol. 1984;349:249–272. doi: 10.1113/jphysiol.1984.sp015155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Wagle-Shukla A, Gunraj C, Lang AE, Chen R. Impaired presynaptic inhibition in the motor cortex in Parkinson disease. Neurology. 2009;72:842–849. doi: 10.1212/01.wnl.0000343881.27524.e8. [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990;13:99–103. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O. Cortex cerebri: performance, structural and functional organization of the cortex. New York: Oxford University Press; 1995. [Google Scholar]
- Crossman AR, Mitchell IJ, Sambrook MA. Regional brain uptake of 2-deoxyglucose in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the macaque monkey. Neuropharmacology. 1985;24:587–591. doi: 10.1016/0028-3908(85)90070-x. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons in the monkey during movement and sleep. Physiologist. 1969;12:207. [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Detari L, Juhasz G, Kukorelli T. Neuronal firing in the pallidal region: firing patterns during sleep-wakefulness cycle in cats. Electroencephalogr Clin Neurophysiol. 1987;67:159–166. doi: 10.1016/0013-4694(87)90039-3. [DOI] [PubMed] [Google Scholar]
- Doudet DJ, Gross C, Arluison M, Bioulac B. Modifications of precentral cortex discharge and EMG activity in monkeys with MPTP-induced lesions of DA nigral neurons. Exp Brain Res. 1990;80:177–188. doi: 10.1007/BF00228859. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- Elder CM, Vitek JL. The motor thalamus: alteration of neuronal activity in the parkinsonian state. In: Kultas-Ilinsky K, Ilinsky IA, editors. Basal ganglia and thalamus in health and movement disorders. New York: Kluwer Academic; 2001. pp. 257–265. [Google Scholar]
- Evarts E. Temporal patterns of discharge of pyramidal tract neurons during sleep and waking in the monkey. J Neurophysiol. 1963;27:152–171. doi: 10.1152/jn.1964.27.2.152. [DOI] [PubMed] [Google Scholar]
- Evarts E. Pyramidal tract activity associated with a conditioned hand movement in the monkey. J Neurophysiol. 1966;29:1011–1027. doi: 10.1152/jn.1966.29.6.1011. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Early involvement of the cerebral cortex in Parkinson's disease: convergence of multiple metabolic defects. Prog Neurobiol. 2009;88:89–103. doi: 10.1016/j.pneurobio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. J Neurophysiol. 1991;66:1249–1263. doi: 10.1152/jn.1991.66.4.1249. [DOI] [PubMed] [Google Scholar]
- Fuller JH, Schlag JD. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976;112:283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Stepniewska I, Kaas JH. Topography and collateralization of the dopaminergic projections to motor and lateral prefrontal cortex in owl monkeys. J Comp Neurol. 1992;325:1–21. doi: 10.1002/cne.903250102. [DOI] [PubMed] [Google Scholar]
- Gatev P, Darbin O, Wichmann T. Oscillations in the basal ganglia under normal conditions and in movement disorders. Mov Disord. 2006;21:1566–1577. doi: 10.1002/mds.21033. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 2005;25:7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Boraud T, Maraton S, Haber SN, Vaadia E, Bergman H. Enhanced synchrony among primary motor cortex neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine primate model of Parkinson's disease. J Neurosci. 2002;22:4639–4653. doi: 10.1523/JNEUROSCI.22-11-04639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh A, Meyer HS, Schmidt EF, Heintz N, Sakmann B, Krieger P. Cell-type specific properties of pyramidal neurons in neocortex underlying a layout that is modifiable depending on the cortical area. Cereb Cortex. 2010;20:826–836. doi: 10.1093/cercor/bhp152. [DOI] [PubMed] [Google Scholar]
- Guo Y, Rubin JE, McIntyre CC, Vitek JL, Terman D. Thalamocortical relay fidelity varies across subthalamic nucleus deep brain stimulation protocols in a data-driven computational model. J Neurophysiol. 2008;99:1477–1492. doi: 10.1152/jn.01080.2007. [DOI] [PubMed] [Google Scholar]
- Hamming RW. Digital filters. Englewood Cliffs (NJ): Prentice Hall; 1983. [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, White EL. A quantitative study of the thalamocortical and other synapses in layer IV of pyramidal cells projecting from mouse SmI cortex to the caudate–putamen nucleus. J Comp Neurol. 1982;211:217–225. doi: 10.1002/cne.902110302. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Molina-Luna K, Hertler B, Atiemo CO, Luft AR. Dopaminergic modulation of motor maps in rat motor cortex: an in vivo study. Neuroscience. 2009;159:692–700. doi: 10.1016/j.neuroscience.2008.12.056. [DOI] [PubMed] [Google Scholar]
- Hu MT, Taylor-Robinson SD, Chaudhuri KR, Bell JD, Labbe C, Cunningham VJ, Koepp MJ, Hammers A, Morris RG, Turjanski N, et al. Cortical dysfunction in non-demented Parkinson's disease patients: a combined (31)P-MRS and (18)FDG-PET study. Brain. 2000;123:340–352. doi: 10.1093/brain/123.2.340. [DOI] [PubMed] [Google Scholar]
- Humphrey DR, Corrie WS. Properties of pyramidal tract neuron system within a functionally defined subregion of primate motor cortex. J Neurophysiol. 1978;41:216–243. doi: 10.1152/jn.1978.41.1.216. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Lozano AM, Davis KD, Saint-Cyr JA, Lang AE, Dostrovsky JO. Differential neuronal activity in segments of globus pallidus in Parkinson's disease patients. Neuroreport. 1994;5:1533–1537. doi: 10.1097/00001756-199407000-00031. [DOI] [PubMed] [Google Scholar]
- Israel Z, Bergman H. Pathophysiology of the basal ganglia and movement disorders: from animal models to human clinical applications. Neurosci Biobehav Rev. 2008;32:367–377. doi: 10.1016/j.neubiorev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Jan C, Pessiglione M, Tremblay L, Tande D, Hirsch EC, Francois C. Quantitative analysis of dopaminergic loss in relation to functional territories in MPTP-treated monkeys. Eur J Neurosci. 2003;18:2082–2086. doi: 10.1046/j.1460-9568.2003.02946.x. [DOI] [PubMed] [Google Scholar]
- Jeanmonod D, Magnin M, Morel A. Low-threshold calcium spike bursts in the human thalamus. Common physiopathology for sensory, motor and limbic positive symptoms. Brain. 1996;119:363–375. doi: 10.1093/brain/119.2.363. [DOI] [PubMed] [Google Scholar]
- Jinnai K, Matsuda Y. Neurons of the motor cortex projecting commonly on the caudate nucleus and the lower brain stem in the cat. Neurosci Lett. 1979;13:121–126. doi: 10.1016/0304-3940(79)90028-4. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Burton H, Porter R. Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol. 1977;173:53–80. doi: 10.1002/cne.901730105. [DOI] [PubMed] [Google Scholar]
- Kaneoke Y, Vitek JL. Burst and oscillation as disparate neuronal properties. J Neurosci Methods. 1996;68:211–223. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Kocsis JD, Wood J. Origin and characteristics of the cortico-caudate afferents: an anatomical and electrophysiological study. Brain Res. 1976;118:137–141. doi: 10.1016/0006-8993(76)90848-9. [DOI] [PubMed] [Google Scholar]
- Kojima S, Doupe AJ. Activity propagation in an avian basal ganglia-thalamocortical circuit essential for vocal learning. J Neurosci. 2009;29:4782–4793. doi: 10.1523/JNEUROSCI.4903-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeva R, Patino L, Omlor W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage. 2007;36:785–792. doi: 10.1016/j.neuroimage.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Kuypers HG. Anatomy of the descending pathways. In: Brookhart JM, Mountcastle VB, Brooks VB, Geiger SR, editors. Handbook of physiology. The nervous system. Motor control. Sect. 1, Vol. II, Part 1. Bethesda (MD): American Physiological Society; 1981. pp. 597–666. [Google Scholar]
- Labarre D, Meissner W, Boraud T. Measure of the regularity of events in stochastic point processes: application to neuron activity analysis. Proc IEEE Int Conf Acoust Speech Signal Process. 2008;1:489–492. [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Porter R. Afferent input to movement-related precentral neurones in conscious monkeys. Proc R Soc Lond B Biol Sci. 1976;194:313–339. doi: 10.1098/rspb.1976.0082. [DOI] [PubMed] [Google Scholar]
- Lindefors N, Ungerstedt U. Bilateral regulation of glutamate tissue and extracellular levels in caudate-putamen by midbrain dopamine neurons. Neurosci Lett. 1990;115:248–252. doi: 10.1016/0304-3940(90)90463-j. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. 2001;106:313–330. doi: 10.1016/s0306-4522(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Sharott A, Bolam JP, Brown P. Brain state-dependency of coherent oscillatory activity in the cerebral cortex and basal ganglia of the rat. J Neurophysiol. 2004;92:2122–2136. doi: 10.1152/jn.00333.2004. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Pogosyan A, Sharott A, Csicsvari J, Bolam JP, Brown P, Magill PJ. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J Neurosci. 2008;28:4795–4806. doi: 10.1523/JNEUROSCI.0123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCairn KW, Turner RS. Deep brain stimulation of the globus pallidus internus in the parkinsonian primate: local entrainment and suppression of low-frequency oscillations. J Neurophysiol. 2009;101:1941–1960. doi: 10.1152/jn.91092.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Emre N, Nakamura CM, Allen C, Donohue MK, Buckman JF. Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience. 1999;88:1–16. doi: 10.1016/s0306-4522(98)00189-4. [DOI] [PubMed] [Google Scholar]
- Miao SH, Zhuang P, Li JY, Li YJ. Neuronal activity of ventrolateral thalamus in patients with essential tremor. Zhonghua Yi Xue Za Zhi. 2009;89:620–624. [PubMed] [Google Scholar]
- Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism. In: Carpenter MB, Jayaraman A, editors. The basal ganglia II. New York: Plenum Press; 1987. pp. 415–427. [Google Scholar]
- Mitchell SJ, Richardson RT, Baker FH, DeLong MR. The primate globus pallidus: neuronal activity related to direction of movement. Exp Brain Res. 1987;68:491–505. doi: 10.1007/BF00249793. [DOI] [PubMed] [Google Scholar]
- Moazami-Goudarzi M, Sarnthein J, Michels L, Moukhtieva R, Jeanmonod D. Enhanced frontal low and high frequency power and synchronization in the resting EEG of parkinsonian patients. Neuroimage. 2008;41:985–997. doi: 10.1016/j.neuroimage.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Molnar GF, Pilliar A, Lozano AM, Dostrovsky JO. Differences in neuronal firing rates in pallidal and cerebellar receiving areas of thalamus in patients with Parkinson's disease, essential tremor, and pain. J Neurophysiol. 2005;93:3094–3101. doi: 10.1152/jn.00881.2004. [DOI] [PubMed] [Google Scholar]
- Montgomery EB., Jr Basal ganglia physiology and pathophysiology: a reappraisal. Parkinsonism Relat Disord. 2007;13:455–465. doi: 10.1016/j.parkreldis.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Moore RY, Whone AL, Brooks DJ. Extrastriatal monoamine neuron function in Parkinson's disease: an 18F-dopa PET study. Neurobiol Dis. 2008;29:381–390. doi: 10.1016/j.nbd.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 2006;26:4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriel MP, Agid Y, Hirsch E. Plasticity of afferent fibers to striatal neurons bearing D1 dopamine receptors in Parkinson's disease. Mov Disord. 2001;16:435–441. doi: 10.1002/mds.1103. [DOI] [PubMed] [Google Scholar]
- Nambu A, Yoshida S, Jinnai K. Projection on the motor cortex of thalamic neurons with pallidal input in the monkey. Exp Brain Res. 1988;71:658–662. doi: 10.1007/BF00248759. [DOI] [PubMed] [Google Scholar]
- National Research Council. Washington (DC): National Academy Press; 1996. Guide for the care and use of laboratory animals. [Google Scholar]
- Omlor W, Patino L, Hepp-Reymond MC, Kristeva R. Gamma-range corticomuscular coherence during dynamic force output. Neuroimage. 2007;34:1191–1198. doi: 10.1016/j.neuroimage.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Orieux G, Francois C, Feger J, Hirsch EC. Consequences of dopaminergic denervation on the metabolic activity of the cortical neurons projecting to the subthalamic nucleus in the rat. J Neurosci. 2002;22:8762–8770. doi: 10.1523/JNEUROSCI.22-19-08762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo E, Porrino LJ, Bankiewica KS, Crane AM, Sokoloff L, Kopin IJ. Local cerebral glucose utilization in monkeys with hemiparkinsonism induced by intracarotid infusion of the neurotoxin MPTP. J Neurosci. 1990;10:860–869. doi: 10.1523/JNEUROSCI.10-03-00860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent M, Parent A. Single-axon tracing study of corticostriatal projections arising from primary motor cortex in primates. J Comp Neurol. 2006;496:202–213. doi: 10.1002/cne.20925. [DOI] [PubMed] [Google Scholar]
- Parr-Brownlie LC, Hyland BI. Bradykinesia induced by dopamine D2 receptor blockade is associated with reduced motor cortex activity in the rat. J Neurosci. 2005;25:5700–5709. doi: 10.1523/JNEUROSCI.0523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr-Brownlie LC, Poloskey SL, Bergstrom DA, Walters JR. Parafascicular thalamic nucleus activity in a rat model of Parkinson's disease. Exp Neurol. 2009;217:269–281. doi: 10.1016/j.expneurol.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr-Brownlie LC, Poloskey SL, Flanagan KK, Eisenhofer G, Bergstrom DA, Walters JR. Dopamine lesion-induced changes in subthalamic nucleus activity are not associated with alterations in firing rate or pattern in layer V neurons of the anterior cingulate cortex in anesthetized rats. Eur J Neurosci. 2007;26:1925–1939. doi: 10.1111/j.1460-9568.2007.05814.x. [DOI] [PubMed] [Google Scholar]
- Penney JB, Young AB. GABA as the pallidothalamic neurotransmitter: implications for basal ganglia function. Brain Res. 1981;207:195–199. doi: 10.1016/0006-8993(81)90693-4. [DOI] [PubMed] [Google Scholar]
- Person AL, Perkel DJ. Unitary IPSPs drive precise thalamic spiking in a circuit required for learning. Neuron. 2005;46:129–140. doi: 10.1016/j.neuron.2004.12.057. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci. 2005;25:1523–1531. doi: 10.1523/JNEUROSCI.4056-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piert M, Koeppe RA, Giordani B, Minoshima S, Kuhl DE. Determination of regional rate constants from dynamic FDG-PET studies in Parkinson's disease. J Nucl Med. 1996;37:1115–1122. [PubMed] [Google Scholar]
- Pifl C, Bertel O, Schingnitz G, Hornykiewicz O. Extrastriatal dopamine in symptomatic and asymptomatic rhesus monkeys treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Neurochem Int. 1990;17:263–270. doi: 10.1016/0197-0186(90)90148-m. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal function and voluntary movement. Oxford: Oxford University Press; 1993. [Google Scholar]
- Porter RH, Greene JG, Higgins DS, Jr, Greenamyre JT. Polysynaptic regulation of glutamate receptors and mitochondrial enzyme activities in the basal ganglia of rats with unilateral dopamine depletion. J Neurosci. 1994;14:7192–7199. doi: 10.1523/JNEUROSCI.14-11-07192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postupna N, Anderson ME. Thalamic bursting activity in the parkinsonian monkey during different behavioral states. Neuroscience meeting planner. San Diego (CA): Society for Neuroscience; 2007. [Google Scholar]
- Rathelot JA, Strick PL. Direct thalamic input to corticomotoneuronal cells. Chicago (IL): Society for Neuroscience; 2009. Soc Neurosci Abstr. Program No. 863.1. 2009 Neuroscience meeting planner. [Google Scholar]
- Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Ann Neurol. 1995;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Marmor O, Saban G, Rosin B, Haber SN, Vaadia E, Prut Y, Bergman H. Low-pass filter properties of basal ganglia cortical muscle loops in the normal and MPTP primate model of parkinsonism. J Neurosci. 2008;28:633–649. doi: 10.1523/JNEUROSCI.3388-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Ritov Y, Heimer G, Bergman H, Bar-Gad I. Local shuffling of spike trains boosts the accuracy of spike train spectral analysis. J Neurophysiol. 2006;95:3245–3256. doi: 10.1152/jn.00055.2005. [DOI] [PubMed] [Google Scholar]
- Rolland AS, Herrero MT, Garcia-Martinez V, Ruberg M, Hirsch EC, Francois C. Metabolic activity of cerebellar and basal ganglia-thalamic neurons is reduced in parkinsonism. Brain. 2007;130:265–275. doi: 10.1093/brain/awl337. [DOI] [PubMed] [Google Scholar]
- Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J Comput Neurosci. 2004;16:211–235. doi: 10.1023/B:JCNS.0000025686.47117.67. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Jeanmonod D. High thalamocortical theta coherence in patients with Parkinson's disease. J Neurosci. 2007;27:124–131. doi: 10.1523/JNEUROSCI.2411-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Rothblat DS. Alterations in intralaminar and motor thalamic physiology following nigrostriatal dopamine depletion. Brain Res. 1996;742:25–33. doi: 10.1016/s0006-8993(96)00988-2. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM. Changes in the local cerebral metabolic rate for glucose in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) primate model of Parkinson's disease. Brain Res. 1985;358:137–143. doi: 10.1016/0006-8993(85)90957-6. [DOI] [PubMed] [Google Scholar]
- Schwartzman RJ, Alexander GM, Ferraro TN, Grothusen JR, Stahl SM. Cerebral metabolism of parkinsonian primates 21 days after MPTP. Exp Neurol. 1988;102:307–313. doi: 10.1016/0014-4886(88)90224-5. [DOI] [PubMed] [Google Scholar]
- Schwindt P, Crill W. Mechanisms underlying burst and regular spiking evoked by dendritic depolarization in layer 5 cortical pyramidal neurons. J Neurophysiol. 1999;81:1341–1354. doi: 10.1152/jn.1999.81.3.1341. [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, Kang GA, Heath S, Shimamoto S, Turner RS. Pallidal neuronal discharge in Huntington's disease: support for selective loss of striatal cells originating the indirect pathway. Exp Neurol. 2008;211:227–233. doi: 10.1016/j.expneurol.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Galati S, Peppe A, Bassi A, Pierantozzi M, Hainsworth AH, Bernardi G, Orlacchio A, Stanzione P, Mazzone P. Spontaneous sleep modulates the firing pattern of parkinsonian subthalamic nucleus. Exp Brain Res. 2006;168:277–280. doi: 10.1007/s00221-005-0175-y. [DOI] [PubMed] [Google Scholar]
- Steiner H, Kitai ST. Unilateral striatal dopamine depletion: time-dependent effects on cortical function and behavioural correlates. Eur J Neurosci. 2001;14:1390–1404. doi: 10.1046/j.0953-816x.2001.01756.x. [DOI] [PubMed] [Google Scholar]
- Stewart A, Foehring RC. Calcium currents in retrogradely labeled pyramidal cells from rat sensorimotor cortex. J Neurophysiol. 2000;83:2349–2354. doi: 10.1152/jn.2000.83.4.2349. [DOI] [PubMed] [Google Scholar]
- Stoffers D, Bosboom JL, Deijen JB, Wolters EC, Berendse HW, Stam CJ. Slowing of oscillatory brain activity is a stable characteristic of Parkinson's disease without dementia. Brain. 2007;130:1847–1860. doi: 10.1093/brain/awm034. [DOI] [PubMed] [Google Scholar]
- Strick PL, Sterling P. Synaptic termination of afferents from the ventrolateral nucleus of the thalamus in the cat motor cortex. A light and electron microscopy study. J Comp Neurol. 1974;153:77–106. doi: 10.1002/cne.901530107. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Efferent neurons and suspected interneurons in motor cortex of the awake rabbit: axonal properties, sensory receptive fields, and subthreshold synaptic inputs. J Neurophysiol. 1994;71:437–453. doi: 10.1152/jn.1994.71.2.437. [DOI] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res. 1998;120:114–128. doi: 10.1007/s002210050384. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Koenig T, Pascual-Marqui RD, Hirata K, Kochi K, Lehmann D. Event-related potential and EEG measures in Parkinson's disease without and with dementia. Dement Geriatr Cogn Disord. 2000;11:39–45. doi: 10.1159/000017212. [DOI] [PubMed] [Google Scholar]
- Towe AL, Harding GW. Extracellular microelectrode sampling bias. Exp Neurol. 1970;29:366–381. doi: 10.1016/0014-4886(70)90065-8. [DOI] [PubMed] [Google Scholar]
- Turner R, DeLong M. Corticostriatal activity in primary motor cortex of the macaque. J Neurosci. 2000;20:7096–7108. doi: 10.1523/JNEUROSCI.20-18-07096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno M, Yoshida M. Monosynaptic inhibition of thalamic neurons produced by stimulation of the pallidal nucleus in cats. Brain Res. 1975;99:377–380. doi: 10.1016/0006-8993(75)90040-2. [DOI] [PubMed] [Google Scholar]
- Vila M, Levy R, Herrero MT, Ruberg M, Faucheux B, Obeso JA, Agid Y, Hirsch EC. Consequences of nigrostriatal denervation on the functioning of the basal ganglia in human and nonhuman primates: an in situ hybridization study of cytochrome oxidase subunit I mRNA. J Neurosci. 1997;17:765–773. doi: 10.1523/JNEUROSCI.17-02-00765.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba RM, Smith Y. A comparative 3D ultrastructural analysis of corticostriatal and thalamostriatal axo-spinous synapses in control and MPTP-treated monkeys. 2009 Neuroscience meeting planner. Chicago (IL): Society for Neuroscience; 2009. [Google Scholar]
- Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17(Suppl 3):S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann Neurol. 2000;47:S131–S140. [PubMed] [Google Scholar]
- Voloshin M, Lukhanina EP, Kolomietz BP, Prokopenko VF, Rodionov VA. Electrophysiological investigation of thalamic neuronal mechanisms of motor disorders in parkinsonism: an influence of D2ergic transmission blockade on excitation and inhibition of relay neurons in motor thalamic nuclei of cat. Neuroscience. 1994;62:771–781. doi: 10.1016/0306-4522(94)90475-8. [DOI] [PubMed] [Google Scholar]
- Watts RL, Mandir AS. The role of motor cortex in the pathophysiology of voluntary movement deficits associated with parkinsonism. Neurol Clin. 1992;10:451–469. [PubMed] [Google Scholar]
- Weinrich M, Wise SP. The premotor cortex of the monkey. J Neurosci. 1982;2:1329–1345. doi: 10.1523/JNEUROSCI.02-09-01329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore DZ, Baker SN. Post-spike distance-to-threshold trajectories of neurones in monkey motor cortex. J Physiol. 2004;555:831–850. doi: 10.1113/jphysiol.2003.048918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Wu AK, McCairn KW, Zada G, Wu T, Turner RS. Motor cortex stimulation: mild transient benefit in a primate model of Parkinson disease. J Neurosurg. 2007;106:695–700. doi: 10.3171/jns.2007.106.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.