Abstract
Developing neocortical progenitors express transcription factors in gradients that induce programs of region-specific gene expression. Our previous work identified anteriorly upregulated expression gradients of a number of corticofugal neuron-associated gene probe sets along the anterior–posterior axis of the human neocortex (8-12 postconceptional weeks [PCW]). Here, we demonstrate by real-time polymerase chain reaction, in situ hybridization and immunohistochemistry that 3 such genes, ROBO1, SRGAP1, and CTIP2 are highly expressed anteriorly between 8-12 PCW, in comparison with other genes (FEZF2, SOX5) expressed by Layer V, VI, and subplate neurons. All 3 were prominently expressed by early postmitotic neurons in the subventricular zone, intermediate zone, and cortical plate (CP) from 8 to 10 PCW. Between 12 and 15 PCW expression patterns for ER81 and SATB2 (Layer V), TBR1 (Layer V/VI) and NURR1 (Layer VI) revealed Layer V forming. By 15 PCW, ROBO1 and SRGAP1 expression was confined to Layer V, whereas CTIP2 was expressed throughout the CP anteriorly. We observed ROBO1 and SRGAP1 immunoreactivity in medullary corticospinal axons from 11 PCW onward. Thus, we propose that the coexpression of these 3 markers in the anterior neocortex may mark the early location of the human motor cortex, including its corticospinal projection neurons, allowing further study of their early differentiation.
Keywords: cerebral cortex, corticospinal tract, regionalization
Introduction
The adult human neocortex is a multilayered structure along its radial dimension, while across the tangential axis, it is subdivided into functionally distinct areas with unique morphological, physiological, and neurochemical properties. During corticogenesis, progenitor cells express regulatory genes in graded or restricted patterns that drive the early phases of regionalization prior to innervation by thalamocortical afferent projections (O'Leary et al. 2007; Rakic et al. 2009) as they are migrating toward their final position within the cortical plate (CP) (Guillemot et al. 2006; Hevner et al. 2006). These regulatory genes control the expression of areal makers, such as cell adhesion molecules and axon guidance receptors, which eventually leads to further differentiation of primary areas by guiding input from the thalamic nuclei during later phases of regionalization (Bishop et al. 2002; Jones et al. 2002; Armentano et al. 2007; Sahara et al. 2007). In addition, a number of transcription factors have been identified that play a role in specifying the laminar distribution (Guillemot et al. 2006; Leone et al. 2008) and phenotype (Schuurmans and Guillemot 2002; Molnár and Cheung 2006; Shoemaker and Arlotta 2010) of cortical neurons.
Approximately 60% of fibers in the mature macaque corticospinal tract (CST) arise from the primary, premotor, and supplementary motor cortex in the frontal lobe with a further 15% arising from the prefrontal, cingulate, and insular cortex and 25% from the parietal cortex (Galea and Darian-Smith 1994). In human, the proportion arising from the frontal lobe may be even higher due to the increased importance of direct corticomotoneuronal connections from the primary motor cortex (Lemon 2008). In addition, the motor cortex is an important site of origin for cortical projections to cranial motor nuclei and also for corticopontine fibers (Glickstein et al. 1985). Both CST and motor cortex are common sites of developmental brain damage leading to cerebral palsy (Eyre 2007). A recent study suggests that lesions at early stages of human development may lead to subsequent substantial reorganization of the origins of the corticospinal output (Basu et al. 2010). The present study set out to explore how the cortical map is established, particularly the processes preceding motor cortex differentiation, in order to understand the mechanisms of reorganization as a basis for improving outcome after such lesions. In addition, understanding the genetic and epigenetic factors that determine acquisition of a corticospinal phenotype will guide efforts to produce corticospinal motor neurons from stem cells for brain repair.
Our previous work using Affymetrix gene chips to probe mRNA expression in human embryonic and fetal neocortical tissues between 8 and 12.5 postconceptional weeks (PCW) identified gene probe sets related to motor cortex and corticofugal axon development that were highly upregulated at the anterior pole of the neocortex compared with the posterior pole (see Table 1, also Ip et al. 2010). These included ROBO1, SRGAP1, and CTIP2; genes whose expression is not exclusive to corticospinal neurons but crucial to their development. In rodents, the transcription factor Ctip2 is expressed by subcerebrally projecting neurons in Layer V and is important for postmitotic differentiation of corticospinal motor neurons, including fasciculation, outgrowth, and pathfinding of their axons but not early specification since it is not expressed in the proliferative zones (Arlotta et al. 2005). Robo protein expression has been demonstrated throughout the developing corticofugal pathways including the CST (Sundaresan et al. 2004) and has been shown to be involved in human CST development (Jen et al. 2004). E18.5 Robo1−/−Robo2−/− double mutants exhibited severe misprojection and crossing over of corticospinal axons from the internal capsule toward the midline at the telencephalic level and a complete absence of cerebral peduncles in the mesencephalon (López-Bendito et al. 2007). Along with their downstream signaling molecule srGAP1, they play a role in axonal growth guidance by regulating actin polymerization (Wong et al. 2001). Other important functions of srGAPs have been proposed, such as regulating transcription factor activity or protein transcription as highly regulated shuttling of srGAPs between the nucleus, and the cytoplasm has been observed throughout neurogenesis (Yao et al. 2008).
Table 1.
Regionalized expression of genes in the human neocortex 8–12.5 PCW from Affymetrix gene chip study
| Gene | Folda | Function |
| Protocadherin 17 | 2.84 | Motor cortex/areal (Kim et al. 2007; Abrahams et al. 2008) |
| S100A10 | 2.48 | Layer V/subcerebral (Arlotta et al. 2005) |
| ER81 | 2.28 | Layer V (Hevner et al. 2003) |
| SRGAP1 | 2.15 | Axon guidance/ROBO1-related (Wong et al. 2001) |
| ROBO1 | 2.05 | Axon guidance/Layer V/subcerebral (Sundaresan et al. 2004; López-Bendito et al. 2007; Zembrzycki et al. 2007) |
| CNTNAP2 | 1.97 | Frontal (Abrahams et al. 2008) |
| CTIP2 | 1.94 | Layer V/subcerebral (Arlotta et al. 2005) |
Fold increase in expression anterior compared with posterior neocortex. A subset of the CST/frontal lobe-related probe sets are shown here. In full data set, they expressed >1.75-fold anterior compared with posterior neocortex. Adapted from Ip et al. (2010).
Another corticofugal-related transcription factor Fezf2 is expressed by progenitors and postmitotic Layer V subcerebral projecting neurons in rodents but is more important in earlier specification and differentiation of corticofugal neurons as it acts upstream of Ctip2 during development (Chen, Rašin, et al. 2005; Chen, Schaevitz, and McConnell 2005; Molyneaux et al. 2005; Chen et al. 2008). Sox5, a transcription factor acting even further upstream of Fezf2 and Ctip2, is expressed by postmitotic corticothalamic projecting neurons in Layer VI and subplate (SP) in rodents and is proposed to be responsible for determining the generation sequence and the molecular/laminar identity of corticofugal projection neurons (Kwan et al. 2008; Lai et al. 2008).
In order to test the hypothesis that the anterior pole of the neocortex may be an early site of origin of corticospinal motor neurons and other corticofugal motor projections, the present study characterized the high anterior to low posterior expression gradients of ROBO1, SRGAP1 and CTIP2 by real-time PCR, in situ hybridization (ISH) and immunohistochemistry (IHC), in comparison with FEZF2 and SOX5, utilizing human cortical tissue from 8 to 15 PCW, a period of time corresponding to when the CP first starts to form (7.5 PCW; Meyer et al. 2000) when the thalamocortical innervation of the SP occurs (13 PCW; Kostović and Rakic 1990) and to the appearance of distinct layers in the CP by 16 PCW (Bayatti, Moss, et al. 2008). Analysis of the laminar and cellular mRNA/protein expression patterns of these genes, in comparison with other layer-specific markers, that is, SATB2 (Layer V at early stages; Alcamo et al. 2008), ER81 (layer V; Yoneshima et al. 2006), NURR1 (Layer VI, SP; Hoerder-Suabedissen et al. 2009; Wang et al. 2010), TBR1 (Layer VI, SP; Hevner et al. 2003; Bayatti, Moss, et al. 2008), synaptophysin, and GAP43 (marginal zone [MZ], SP; Bayatti, Moss, et al. 2008) localized ROBO1, SRGAP1, and CTIP2 to the emerging Layer V. The expression of ROBO1 and SRGAP1 by human CST axons was also investigated.
Materials and Methods
Human Embryonic and Fetal Brains
Brains were obtained from human embryos and fetuses ranging in age from 8 to 17 PCW for tissue ISH and IHC (8 PCW, n = 4; 9 PCW, n = 4; 10 PCW, n = 4; 12 PCW, n = 2; 14 PCW, n = 2; 15 PCW, n = 3; 17 PCW, n = 2) and quantitative rtPCR (8 PCW, n = 3; 10–10.5 PCW, n = 4; 12 PCW, n = 4). Age was estimated from measurements of foot length and heel to knee length compared with a standard growth chart (Hern 1984). Brains were dissected from terminations of pregnancy obtained from the MRC-Wellcome Trust Human Developmental Biology Resource at Newcastle University (http://www.hdbr.org), with appropriate maternal written consent and approval from the Newcastle and North Tyneside NHS Health Authority Joint Ethics Committee.
Prior to sectioning, brains were fixed for at least 24 h at 4 °C in 0.1 M phosphate-buffered saline (PBS) containing 4% paraformaldehyde (PFA; Sigma-Aldrich). Whole or half brains (divided sagittally) were transferred to 70% ethanol for storage at 4 °C prior to paraffin embedding. Eight-micrometer thick sections were cut sagittally or coronally, mounted on slides, and used for tissue ISH and IHC. Brainstems were cryoprotected in 30% sucrose/PBS after fixation in 4% PFA/PBS for at least 24 h. Seventy-micrometer thick sections were cut on a freezing microtome and collected in PBS for IHC.
RNA Isolation and Reverse Transcription
Right-sided cortices were dissected, subcortical structures, temporal lobes and meninges removed and 5-mm slices cut along the anterior–posterior axis. Total RNA was isolated from the anterior-most and posterior-most slices using the PeqGOLD RNAPure reagent (Peqlab) according to the manufacturer’s instructions. The cDNA templates were synthesized by reverse transcription first-strand synthesis reaction from the extracted RNA using random hexadeoxynucleotides primers (Promega) and SuperscriptTM III Reverse Transcriptase Kit (Invitrogen) following the manufacturer’s instructions for 2 μg of total RNA in a final volume of 50 μL. The transcribed cDNA template was further diluted 2-fold prior to the application of rtPCR.
Quantitative Real-time PCR
The expression levels of CTIP2, FEZF2, ROBO1, SOX5, and SRGAP1 in the anterior-most and posterior-most slices was determined by rtPCR. The sequence-specific primer sets (Eurofins MWG Operon) designed using Primer3 program (http://frodo.wi.mit.edu/primer3/), and amplicon sizes for all target genes are listed in Table 2. The identities of amplified PCR products were confirmed using restriction enzyme digestion and direct sequencing (Eurofin MWG Operon). Three housekeeping genes, β–ACTIN, GAPDH, and SDHA were used as internal references to normalize the cDNA template between different samples. A negative control was incorporated by replacing the cDNA template with Molecular Biology grade water (VWR International). The SYBR Green-based rtPCR assay was performed in 7900 HT Fast Real-Time PCR system (Applied Biosystems). A total volume of 10-μL qPCR reaction was set up in triplicates, containing 5 μL of 2 × SYBR Green qPCR Master Mix (Invitrogen), 1 μL of the diluted cDNA template, 0.5 μL of each primer (10 ρmol/μL), and 3 μL of Molecular Biology grade water. The thermal cycle protocol was 95 °C for 15 min followed by 40 cycles of 95 °C for 15 s, 60 °C for 30 s, 72 °C for 30 s, and 74 °C for 10 s. Amplification of a single PCR product was confirmed by dissociation curve analysis of PCR products (68 °C for 10 s followed by 99 °C for 10 s) after completion of each rtPCR reaction. Baseline was set automatically by the machine, and threshold was set manually above all the background signals. The data obtained were analyzed with the qBase software (Hellemans et al. 2007). Average normalized relative quantity (NRQ) of mRNA expression in the anterior and posterior region of the neocortex (±standard error of the mean) and average fold changes were calculated by qBase. A paired Student t-test was performed to determine the statistical significance of differences between the anterior and posterior region of the neocortex in the average NRQs.
Table 2.
List of primers for real-time PCR
| Primer set | 5' to 3' Sequence |
Amplicon size (bp) | |
| Forward | Reverse | ||
| β-ACTINa | CTACAATGAGCTGCGTGTGGC | CAGGTCCAGACGCAGGATGGC | 271 |
| CTIP2 (also known as BCL11B, RIT1) | CAGAGCAGCAAGCTCACG | GGTGCTGTAGACGCTGAA GG | 102 |
| FEZF2 (FEZL, ZNF312, ZFP312, TOF, FKSG36, FLJ10142) | ACACGCATATCCGCATCC | AGGCCTTGTTGCAGATGG | 147 |
| GAPDHa | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG | 86 |
| ROBO1 (DUTT1, SAX3 FLJ21882, MGC131599, MGC133277) | TTGTGAGGGCAGCTAATGC | TCTCTGGACCTGCTTGTGG | 116 |
| SDHAa | TGGGAACAAGAGGGCATCTG | CCACCACTGCATCAAATTCATG | 84 |
| SOX5 (MGC35153) | ATGACCATGATGCTGTCACC | TTCACAACAGCCACCTTCC | 103 |
| SRGAP1 (KIAA1304, ARHGAP13, FLJ22166) | CCAACATTGATGCCTGTCC | TCTCATAAACAGGGCCATCC | 139 |
Housekeeping genes.
Manufacturing of Probes
Total RNAs from human fetal whole brain or neocortex aged between 8 and 12 PCW were isolated with Trizol (Invitrogen) according to the manufacturer’s instructions and reverse transcribed into cDNA templates. Gene-specific primer sets (Eurofins MWG Operon) were designed using Primer 3 program (http://frodo.wi.mit.edu/primer3/) and were incorporated with either SP6 (forward) or T7 (reverse) consensus sequences. The primer sequences, amplicon size, and the manufactured probes target locations are listed in Table 3. Procedures and conditions for PCR and subsequent gel extraction, in vitro transcription Digoxigenin (DIG)-labeling reaction, labeled probes purification, and quality controls were carried out as previously described (Bayatti, Sarma, et al. 2008). SRGAP1 probes were for this study but did not give satisfactory results and are not reported on.
Table 3.
Primer sequences for ISH probes
| Genes | GenBank accession numbers | Probe sequences | Primer sequencesa | Product size (bp) |
| CTIP2 | NM_138576.2 | 1892–2351 | SP6 (FP) 5′-AAT ACG ATT TAG GTG ACA CTA TAG AAT ACA GGA GCT GCT ACT GGA GAA CGA GA-3′ | 517 |
| NM_022898.1 | 1679–2138 | T7 (RP) 5′-TAA GTT AAT ACG ACT CAC TAT AGG GCG ATC CAG GTC CTT CTC CAC CTT GAT-3′ | ||
| ER81 | NM_004956.4 | 1609–2173 | SP6 (FP) 5′-AAT ACG ATT TAG GTG ACA CTA TAG AAT ACT GAC ACC TGT GTT GTC CCA GAA A-3′ | 464 |
| NM_001163147.1 | 1343–1907 | |||
| NM_001163148.1 | 1151–1715 | T7 (RP) 5′-TAA GTT AAT ACG ACT CAC TAT AGG GCG ACC ATG TCT GTC TTC AGC AGT GGA-3′ | ||
| NM_001163149.1 | 1022–1586 | |||
| NM_001163150.1 | 1088–1652 | |||
| NM_001163151.1 | 1034–1598 | |||
| NM_001163152.1 | 899–1463 | |||
| FEZF2 | NM_018008.3 | 1120–1531 | SP6 (FP) 5′-AAT ACG ATT TAG GTG ACA CTA TAG AAT ACA AAC TTC ACC TGC GAG GTG TGC-3′ | 469 |
| T7 (RP) 5′-TAA GTT AAT ACG ACT CAC TAT AGG GCG AGT TGT GGG TGT GCA TAT GGA AGG-3′ | ||||
| ROBO1 | NM_002941.2 | 4013–4499 | SP6 (FP) 5′-AAT ACG ATT TAG GTG ACA CTA TAG AAT ACT AGC CAA GAT GCA AAC CAG AAG G-3′ | 544 |
| T7 (RP) 5′-TAA GTT AAT ACG ACT CAC TAT AGG GCG ATG TGT CTT GGA TTG GGC AGT AGG-3′ | ||||
| NM_133631.1 | 4859–5345 | |||
| SATB2 | NM_015265.2 | 711–1262 | SP6 (FP) 5′-AAT ACG ATT TAG GTG ACA CTA TAG AAT ACT TAG CCA AAG AAT GCC CTC TCT CC-3′ | 552 |
| T7 (FP) 5′-TAA GTT AAT ACG ACT CAC TAT AGG GCG ACC TCT TCA GCT CAT CTC TGA CTT GC-3′ | ||||
| SOX5 | NM_006940.4 | 1832–2257 | SP6 (FP) 5′-AAT ACG ATT TAG GTG ACA CTA TAG AAT ACC CTT TCC TGA CAT GCA CAA CTC C-3′ | 483 |
| NM_152989.2 | 2062–2487 | |||
| T7 (RP) 5′-TAA GTT AAT ACG ACT CAC TAT AGG GCG ACC TCT CCT TTC ACA CCG TAA GTG C-3′ | ||||
| NM_178010.1 | 594–1019 |
Gene-specific sequence underlined. FP, forward primer; RP, reverse primer.
Tissue ISH
ISH was performed as previously described (Bayatti, Sarma, et al. 2008) with some modifications. Briefly, paraffin sections were dewaxed in xylene and rehydrated gradually in decreasing concentrations of ethanol before incubated with proteinase K (20 μg/mL; Sigma-Aldrich) for 8 min at room temperature. Sections were fixed in 4% PFA/PBS for 20 min, washed in PBS, and treated with 0.1 M triethanolamine (Sigma-Aldrich, pH 8.0)/0.25% acetic anhydride (Sigma-Aldrich)/0.2% HCl for 10 min, dehydrated in increasing concentrations of ethanol and air-dried by filtered air stream. DIG-labeled probes (300 ng) were used per 100 μL of DIG Easy Hyb mixture (Roche). Probe/Hyb mix (200 μL) was used per slide, covered with glass coverslips. Slides were incubated in a hybridization chamber overnight at 68 °C, rinsed in 5× standard sodium citrate (SSC, pH 7.2) at 65 °C to remove coverslips, followed by 3 washes at 50 °C (2× SSC twice and 0.2× SSC once), followed by one wash with 0.2× SSC once at room temperature. After briefly rinsing in 0.1 M Tris (pH 7.6)/0.15 M NaCl (Buffer 1) and blocking with 10% fetal calf serum (FCS; Invitrogen)/Buffer 1 for 1 h at room temperature, sections were incubated with anti-DIG antibody (Roche; diluted 1: 1000 in 2% FCS/Buffer 1) overnight at 4 °C. Sections were washed in Buffer 1 for 6 × 30 min. Detection of probes/anti-DIG antibody was achieved by addition of NBT/BCIP solution (Roche; 20 μL/mL) in 0.1 M Tris (pH 9.5)/0.1 M NaCl (Buffer 2). The color reaction was developed in the dark for several hours to overnight and terminated by rinsing slides in Buffer 2 and then distilled water. Sections were mounted in Aquamount. Comparison of staining between sense and antisense probes was carried out to ensure specificity (Supplementary Fig. 1).
IHC
Immunoperoxidase histochemistry was carried out according to standard protocols as previously described (Bayatti, Moss, et al. 2008). Paraffin sections of forebrain were immunostained on slides, frozen sections of medulla were immunostained while free-floating. Primary antibodies used on paraffin and frozen (Fr) sections were diluted in 0.1% Triton-X-100/PBS (PBS-T) with 3% of the appropriate serum (Vector Laboratories) as follows: CTIP2 1/1000, Abcam ab18465; GAP43 1/1000, 1/5000 (Fr), Sigma-Aldrich G9264; NURR1 1/200, R & D Systems AF2156; ROBO1 1/4000, 1/10000 (Fr), Abcam ab7279; SATB2 1/400, Abcam ab51502; SOX5 1/200, Sigma-Aldrich AV33323; SRGAP1 1/900, 1/1000 (Fr), Abcam ab57504; Synaptophysin 1/1000, Sigma-Aldrich S5768, TBR1 1/500, Abcam ab31940. The appropriate biotinylated-secondary antibodies (Vector Labs) were used at 1/200 dilution in 0.1% PBS-T. For immunofluorescence histochemistry, paraffin sections were dewaxed and rehydrated then boiled in 10 mM citrate buffer and blocked in 10% appropriate serum (Vector Labs)/0.1% PBS-T for 1 h at room temperature before incubation with primary antibodies (CTIP2 diluted 1/500; ROBO1 diluted 1/100; SATB2 diluted 1/100; SRGAP1 diluted 1/100; TBR2 diluted 1/200, Abcam ab23345; in 0.1% PBS-T with 3% serum). Sections were incubated in a moist chamber at 4 °C overnight. Sections were washed and incubated with Cy3-horse anti-mouse IgG (Sigma-Aldrich), Cy3-sheep anti-rabbit IgG (Sigma-Aldrich), Alexa Fluor 488-goat anti-rat IgG (Invitrogen), and Alexa Fluor 488-goat anti-rabbit IgG (Invitrogen) secondary antibodies (1/200 in 0.1% PBS-T) at room temperature for half an hour in the dark. Sections were then incubated with DAPI nucleic acid stain (Invitrogen, 1/10000 in PBS-T) for 5 min at room temperature in the dark, washed with PBS-T, and rinsed once in distilled water. Sections were mounted in Vectashield mounting medium (Vector Labs) and kept in the dark at 4 °C until examination.
Densitometry
Quantification of expression levels of ROBO1, SRGAP1, CTIP2, FEZF2, and SOX5 was performed to confirm their tangential expression gradients, using the image processing program ImageJ (NIH; http://rsbweb.nih.gov/ij/). Photographs were taken from the anterior- and posterior-most extents of sagittal sections at 8, 9, and 10 PCW for comparison (Embryo number n = 2 for each stage and at least 3 sections for each embryo were used for statistical analysis). All photographs were taken with the same exposure time and cropped to similar widths. The average optical density of histological staining in the CP (expression detected in this layer throughout all investigated stages) and ventricular zone (VZ) (considered background staining throughout all investigated stages) was measured in rectangular boxes of equal widths, which spanned the thickness of the CP or the VZ, and were placed adjacently. To take into account of background staining, the ratio of mean gray values CP/VZ was calculated. A Student’s paired T-test was performed to determine the significance of expression level differences between the anterior and posterior regions.
Results
rtPCR and ISH/IHC Confirmation of ROBO1, SRGAP1/SRGAP1, and CTIP2 Tangential Gradients
After normalization to 3 housekeeping genes (β-ACTIN, GAPDH, and SDHA) and calculating an average over the developmental period of 8–12 PCW, rtPCR showed expression of ROBO1 and CTIP2 to be significantly higher in anteriorly- rather than posteriorly-dissected tissue (n = 11) with similar fold changes (2.14-fold and 2.00-fold, respectively, Fig. 1) to those found by our previous Affymetrix gene chip analysis (Table 1). SRGAP1 was expressed higher anteriorly but to a lesser degree (1.28-fold) nevertheless this was statistically significant (Fig. 1). rtPCR revealed expression of FEZF2 (1.30-fold) and SOX5 (1.25-fold) to be higher posteriorly; although these differences were small they were statistically significant (Fig. 1).
Figure 1.
rtPCR confirmation of gradients of ROBO1, SRGAP1, and CTIP2 expression during 8–12 PCW. Table showingfold changes (A) and a graphical representation (B) of relative expression (NRQ) of ROBO1, SRGAP1, CTIP2, FEZF2, and SOX5 determined by rtPCR from RNA extracted from anterior and posterior regions of developing human neocortex aged between 8 and 12 PCW (n = 11). ROBO1 and CTIP2 exhibited statistically significant, large fold changes, indicating a high anterior to low posterior gradient. SRGAP1 showed a small but significant fold change corresponding to a high anterior to low posterior gradient. FEZF2 and SOX5 exhibited high posterior, low anterior gradients of small but significant fold changes. *P < 0.05 (paired Student’s T-test); A, anterior; P, posterior.
ISH carried out for ROBO1 and CTIP2 and IHC for SRGAP1 at 8, 9, and 10 PCW was performed on sagittal sections. Expression of all 3 appeared consistently higher in anterior compared with posterior regions of the neocortex at all ages studied (Fig. 2A,B). The expression levels observed were quantified by measuring the relative optical densities of histological staining and confirmed significant differences between the anterior and posterior poles in expression levels for all 3 genes at all stages investigated (Fig. 2C). In addition, a statistically significant high posterior to low anterior gradient for FEZF2 was detected by ISH at 9 and 10 PCW but not 8 PCW, but SOX5 exhibited no statistical significant difference in intensity of expression either anteriorly or posteriorly at all investigated stages (Fig. 2C).
Figure 2.
ROBO1, SRGAP1, and CTIP2 gradients in sections from the developing human neocortex. ISH of sagittal sections for ROBO1, CTIP2, FEZF2 and SOX5 and IHC for SRGAP1 (A, B) revealed that ROBO1, SRGAP1, and CTIP2 were expressed at high levels anteriorly within the SVZ, IZ, and CP between 8–10 PCW. A high posterior, low anterior expression of FEZF2 was detected but at 9 and 10 PCW only. No gradient was detected for SOX5 at any stage investigated. Adjacent sections were selected for each gene/protein at each developmental stage. Higher magnification images, outlined with boxes, were taken at the anterior (At) and posterior regions (Pt) of the neocortex. (C) The optical density of histological staining for ROBO1, CTIP2, FEZF2, SOX5 mRNA and SRGAP1-immunoreactivity at the anterior-most neocortex (gray bars) was measured in the CP and expressed relative to background staining in the VZ (see Materials and Methods) and compared with the same ratios measured at the posterior pole (black bars). *P < 0.05 (paired Student’s T-test). Scale bars represent 200 μm.
In addition, we studied expression patterns of CTIP2, ROBO1, SRGAP1, FEZF2, and SOX5 in coronal sections at 8 and 12 PCW. Laminar staining patterns resembled those seen in sagittal sections, but no lateral to medial gradients of expression were observed (Supplementary Fig. 2).
Laminar and Cellular Expression of ROBO1/ROBO1, SRGAP1, and CTIP2/CTIP2
From 8 to 10 PCW, the majority of ROBO1 and CTIP2/CTIP2 expression was found in cell bodies in the CP and the subventricular zone (SVZ). To a lesser extent, they were also expressed in the migrating neurons passing through the intermediate zone (IZ) of the dorsolateral frontal cortex (Fig. 3A,B). The very low-density band of CTIP2-positive cells just beneath the CP that appeared at 10 PCW corresponds to the newly emerging SP. Although the IZ and SP contained few ROBO1-positive cell bodies, immunoreactivity for ROBO1 protein and SRGAP1 was high in these regions showing that receptor complexes containing these proteins were highly expressed on growing axons passing through these regions.
Figure 3.
Laminar localization of ROBO1, SRGAP1, and CTIP2 during early human neocortical development (8–10 PCW). ISH and IHC on paraffin sections revealed expression of ROBO1 mRNA and protein, SRGAP1 protein and CTIP2 mRNA and protein in the SVZ, IZ/SP, and CP between 8 and 10 PCW (A, B). All sections were taken from the dorsolateral region of the neocortex, and adjacent sections were selected for each gene/protein at each developmental stage. Note the higher expression of ROBO1 and SRGAP1 protein in the IZ and SP compared with ROBO1 mRNA, which was more highly expressed in the CP. This suggests a predominant localization of the proteins to receptor complexes in axons compared with the predominant localization of mRNA in the cell body. CTIP2, a transcription factor, was predominantly localized to the nucleus of positive cells. Scale bars represent 200 μm.
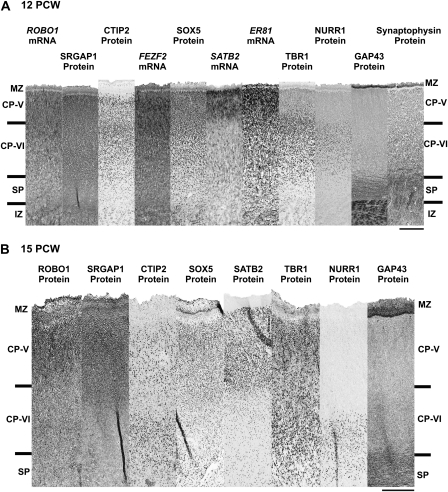
The expression patterns of ER81, SATB2, TBR1, NURR1, GAP43, and synaptophysin observed at 12 PCW lead us to propose that Layer V of the CP is formed at around this time point (Fig 4A). The CP was found to be delineated by the absence of GAP43 and synaptophysin expression, which were prominently detected in the SP, IZ, and MZ (Fig. 4A). The segregation of layers in the CP was revealed by strong ER81 and SATB2 expression in the upper CP (Fig. 4A), presumably Layer V, and strong TBR1 and NURR1 expression in the lower CP (Fig. 4A), presumably Layer VI. ROBO1, SRGAP1, and CTIP2 together with FEZF2 and SOX5 were expressed by cortical neurons in both layers of the CP (Fig. 4A). In the upper CP, discrete populations of CTIP2-positive and SATB2-positive cells were observed with only a small number of them coexpressing both markers (Fig. 5B). However, a larger proportion of cells in the upper CP coexpressed both ROBO1 and SATB2 (Fig. 5C).
Figure 4.
Laminar localization of ROBO1, SRGAP1, and CTIP2 at 12 and 15 PCW. (A) Comparison of expression of ROBO1, SRGAP1, and CTIP2 with various laminar-specific markers (SATB2, ER81, TBR1, NURR1 and GAP43 and Synaptophysin) revealed the emergence of Layer V by 12 PCW. The CP was identified by GAP43- and Synaptophysin-negative immunoreactivity, proximal to the MZ. Within the CP, strong SATB2 and ER81 expression was detected in the Layer V above the TBR1- and NURR1-expressing Layer VI. ROBO1, SRGAP1, and CTIP2 together with FEZF2 and SOX5 expression were observed throughout the CP (Layer V and VI). (B) At 15 PCW, NURR1-expressing cells were observed in Layer VI and also in the upper parts of the GAP43-positive SP. Expression of CTIP2 together with SOX5, SATB2, and TBR1 were detected in both Layer V and VI with different intensities, whereas intense immunostaining of ROBO1 and SRGAP1 were observed mostly in the Layer V. All sections were taken from the dorsolateral region of the neocortex, and adjacent sections were selected for each gene/protein at each foetal stage. Scale bars represent 200 μm.
Figure 5.
Cellular localization of ROBO1, SRGAP1, and CTIP2 from 10 to 12 PCW. (A) At 10 PCW, within the SVZ, most CTIP2-positive cells (green) did not express TBR2 (red) a marker for INPs, indicating that CTIP2 positive cells are predominantly postmitotic neurons. (B) At 12 PCW, within Layer V of the CP, postmitotic neurons predominantly expressed either CTIP2 (green) or SATB2 (red), and very few coexpressed both markers (arrow). However, there were many more ROBO1 and SATB2 coexpressing cells (arrow). (C–E) At 10 PCW, within the CP, a subset of postmitotic neurons coexpressed CTIP2, ROBO1, and SRGAP1 (arrows). Scale bars represent 100 μm.
At 15 PCW, a similar expression pattern was observed with some refinements (Fig. 4B). SATB2 remained strongly expressed by cortical neurons located in the upper CP (Layer V, Fig. 4B). Layer VI neurons continued to express TBR1 and NURR1, however, a subset of NURR1-expressing cells were observed in the GAP43-positive SP (Wang et al. 2010). CTIP2 and SOX5 continued to be expressed by cortical neurons in both Layer V and VI of the CP (Fig. 4B). ROBO1 and SRGAP1 were expressed strongly by cortical neurons in Layer V (Fig. 4B); however, ROBO1 and SRGAP1 expression in the SP was weaker between 12 and 15 PCW (Fig. 4) than at earlier stages. This suggests that axon growth cones expressing the ROBO1 receptor complex were not present in the SP by this stage.
The human SVZ, constituting a heterogeneous population of cells, including radial glia-like cells, intermediate neuronal precursors (INPs) and early postmitotic, immature neurons (Bayatti, Moss, et al. 2008; Hansen et al. 2010) is profoundly different from the thin rodent SVZ (Martinez-Cerdeno et al. 2006) at comparable developmental stages. At 10 PCW, double-labeling of CTIP2 with INPs marker, TBR2 (Hevner et al. 2006) showed that although CTIP2 expression was detected in the SVZ, it was not expressed by INPs (Fig. 5A). Within the CP, double-labeling of CTIP2/ROBO1, CTIP2/SRGAP1, and ROBO1/SRGAP1 showed that a substantial subpopulation of postmitotic neurons coexpressed CTIP2, ROBO1, and SRGAP1 (Fig. 5C–E). The intracellular location of CTIP2 was distinct from that of ROBO1 and SRGAP1; CTIP2 was predominantly expressed within the nuclei, whereas ROBO1 and SRGAP1 showed cytoplasmic localization (Fig. 5C–E).
ROBO1 and SRGAP1 Expression in Corticospinal Axons
At 11 PCW, GAP43 immunoreactivity revealed growing axons in multiple pathways through the medulla at the level of olives (Fig. 6A), whereas both ROBO1 and SRGAP1 immunoreactivity were restricted to the 2 small regions at the ventral surface of the medulla (Fig. 6B,C). When examining sections at the level of decussation of corticospinal axons, small numbers of fibers immunoreactive for GAP43 were found extending from the ventral surface of the medulla crossing over the decussation (Fig. 6D). However, expression of ROBO1 and SRGAP1 was not detected in fibers crossing over the decussation but was present in fibers on the ventral surface at this level (arrows, Fig. 6E,F).
Figure 6.
ROBO1 and SRGAP1 are CST markers. (A-C) Show caudal medulla sections at the level of olives at 11 PCW. GAP43 expression was detected in all growing axons of different pathways revealed in this section (A). However, ROBO1- (B) and SRGAP1- (C) immunoreactive fibers were observed only in the 2 small regions at the ventral surface of the medulla, presumably where the future medullary pyramids are formed. (D–F) Show sections of caudal medulla at the level of the decussation, at 11 PCW. Small numbers of fibers crossing over the midline in the medulla were immunoreactive for GAP43 (D) but negative for ROBO1 (E) and SRGAP1 (F). (G–J) Show caudal medulla sections at the level of decussation at 14 and 17 PCW. Both ROBO1 (G, I) and SRGAP1 (H, J) were expressed in fibers crossing over at the decussation. Scale bars represent 500 μm.
ROBO1 (Fig. 6G) and SRGAP1 (Fig. 6H) -immunoreactive pyramidal fibers were first detected at the decussation at around 14 PCW. By 17 PCW, prominent pyramids were present on the ventral surface of the medulla, and these were strongly immunoreactive for both ROBO1 (Fig. 6I) and SRGAP1 (Fig. 6J) as fibers have extended to the decussation and beyond.
Discussion
In order to understand the origins and formation of the human CST, we have examined the expression patterns of 3 CST-related genes ROBO1, SRGAP1, and CTIP2 using rtPCR, ISH, and IHC during the early stages of corticogenesis. Prominent ROBO1 and SRGAP1 immunoreactivity was seen in pyramidal fibers in the medulla from 11 to 17 PCW, confirming their role in CST development. All 3 genes showed regionalized expression at the early stages of CP formation to the anterior pole of the neocortex, providing further evidence for a protomap in the neocortex based on gene expression patterns prior to the establishment of connectivity. Furthermore, 2 transcription factors deemed key to the development of Layer V subcerebral projection neurons, FEZF2 (Chen, Rašin, et al. 2005; Chen, Schaevitz, and McConnell 2005; Molyneaux et al. 2005; Chen et al. 2008) and SOX5 (Kwan et al. 2008; Lai et al. 2008), showed no gradients or small posterior to anterior gradients depending on age and method of study, indicating that anterior to posterior expression gradients are not universal to Layer V-related genes at this stage of development. ROBO1, SRGAP1, and CTIP2 were all persistently expressed in both the SVZ and CP of the neocortex from 8 to 15 PCW. However, expression in the SVZ was in postmitotic neurons only and not in INPs. By 15 PCW, expression of ROBO1 and SRGAP1 was concentrated in Layer V of the CP, the origin of subcerebral projection neurons.
Ontogeny of CST Development Revealed by Expression of ROBO1 and SRGAP1 in Corticospinal Axons
The CST is the major descending sensorimotor pathway and predominantly originates from Layer V of the frontal motor areas in primates (Lemon 2008). This long distance projection requires proper guidance for correct axonal pathfinding and target selection at each point from its origin to its destination, potentially through cell adhesion molecules (Faulkner et al. 2008; Runker et al. 2008). Here, we show that the cell adhesion/axon guidance molecule ROBO1 and its downstream signaling molecule SRGAP1 were expressed in corticospinal axons at various fetal stages during human CST formation, potentially playing a role in guiding axons toward the spinal cord.
Although multiple GAP43-positive axon pathways were present in the medulla at 11 PCW, ROBO1 and SRGAP1 were solely expressed by fibers located at the future sites of the CST pyramids on the ventral surface of the medulla. Thus, these ROBO1- and SRGAP1-positive fibers probably represent the earliest arriving corticospinal axons at the level of the olives. In more caudal sections, small numbers of GAP43-positive but ROBO1- and SRGAP1-negative fibers crossed the decussation. Perhaps, these decussating fibers are not corticospinal axons but pioneer fibers in the CST serving as scaffolds or temporary targets for the developing corticospinal axons, in the same way as the establishment of subcortical projections is dependent on the guidance of pioneer axons from SP cells projecting toward the internal capsule (McConnell et al. 1989, 1994; Kim et al. 1991). Alternatively, CST growth cones may not express the ROBO1 receptor complex as they cross the decussation to avoid repulsive interaction with SLIT expressed in the extracellular matrix at the midline (Kidd et al. 1998; Long et al. 2004). However, in mice, Robo (1 and 2) are strongly expressed in corticospinal fibers before, during, and after crossing the midline in the caudal medulla from P2 onward and may be involved in axon fasciculation as well as guidance (Sundaresan et al. 2004). By 14–17 PCW, prominent pyramids were present which were strongly immunoreactive for both ROBO1 and SRGAP1, as were fibers extending to the decussation and beyond. The decussation of ROBO1- and SRGAP1-positive fibers was observed around the time when a distinct ROBO1- and SRGAP1-positive Layer V emerges in the neocortex.
Expression of Corticofugal Markers in the SVZ and IZ
The expression of CTIP2/CTIP2 in the human SVZ is an intriguing discovery since its expression is absent in the mouse VZ or SVZ from E12 to P6 (Arlotta et al. 2005). The human SVZ, however, is relatively larger and divided into 2 layers, the inner and outer SVZ, by an inner fibrous layer from 11 PCW onward and contains postmitiotic markers, such as TBR1 (Bayatti, Moss, et al. 2008). By comparison, the thin rodent SVZ constitutes only a few layers of cells at a comparable developmental stage (Martinez-Cerdeno et al. 2006) and is Tbr1-negative (Hevner et al. 2003). In the present study, CTIP2-positive cells were not found to express the INPs marker TBR2 (Hevner et al. 2003) and thus are very likely to be postmitotic neurons. Therefore, the previous proposal (Arlotta et al. 2005) that Ctip2 is not involved in early specification of cortical progenitors in mice due to the lack of its expression in the VZ or SVZ is likely to hold true in humans also, despite its expression in the SVZ. Instead, CTIP2 may control the expression of corticofugal axon markers, such as ROBO1 and SRGAP1, which could be required from the earliest stages of development, for instance, in guiding the outgrowth of their axons toward the internal capsule while the immature neurons are still migrating toward the CP.
Our observation of ROBO1 and SRGAP1 expression in the human SVZ and IZ was largely in agreement with the expression patterns in mice. Robo1 protein expression was detected in the axons transversing the lower IZ/SVZ from E15.5 while srGAP1 mRNA expression was observed in the proliferative zone at E16.5 in mice (Andrews et al. 2006, 2007, 2008; Bacon et al. 2009). The observation of a gradual loss of ROBO1 and CTIP2 mRNA expression within SP/IZ from 10 PCW onward suggests that the occurrence of radial somal translocation of ROBO1+/CTIP2+ subcerebral projecting neurons through the SP/IZ toward the CP is potentially guided by ROBO1/SRGAP1 receptor complexes.
Emergence of Laminar-Specific Expression of Corticofugal Markers in the CP
Expression of ROBO1 mRNA, SRGAP1 protein, and CTIP2/CTIP2 mRNA and protein within the human CP was largely consistent with the expression patterns observed in rodents (Bagri et al. 2002; Whitford et al. 2002; Arlotta et al. 2005; López-Bendito et al. 2007; Bacon et al. 2009). An exception is Robo1 protein, which is only weakly expressed in the CP of mice. The expression of these genes and proteins in the human CP, which constitutes postmitotic neurons, suggest that ROBO1/ROBO1, SRGAP1, and CTIP2/CTIP2 are likely to play a role in mediating processes involved in the differentiation of subcerebral projecting neurons, including corticospinal motor neurons, which includes axonal projection fasciculation, outgrowth, and pathfinding. Note that SRGAP1 cytoplasmic expression was observed at all stages investigated in our study which differs from the reported nucleus–cytoplasm shuttling phenomenon occurring from P1 to adult in mice (Yao et al. 2008). Nuclear expression of SRGAP1 might be observed at later stages in human cortical development not studied here.
In humans, previous work has shown both Layer V and VI can be distinguished within the CP by 16 PCW (Bayatti, Moss, et al. 2008). Our new observations suggest that Layer V is probably formed slightly earlier, from 12 to 15 PCW. Layer V was easily distinguishable by the restricted expression of SATB2 and concentrated expression of ER81 in this layer and the absence of expression of NURR1. However, ROBO1 and SRGAP1 expression was not restricted to the putative Layer V at 12 PCW but only at 15 PCW. Thus, the present study in humans only finds ROBO1+/SRGAP1+/CTIP2+ positive neurons to be restricted to layer V by the time that presumptive ROBO1/SRGAP1-positive corticospinal fibers are crossing the decussation in large numbers.
The expression of ROBO1 and SRGAP1 in Layer V of the CP is consistent with the findings in rodents, in which Robo1 was expressed at Layer V in P7 mice (Sundaresan et al. 2004) and Layer II and V in P10 rats (Whitford et al. 2002), while srGAP1 was primarily expressed at Layer II–V in P7 mice (Bacon et al. 2009). Expression of ROBO1 and SRGAP1 in superficial layers of the CP might also be observed at later stages in human cortical development not studied here. In addition to guiding subcerebral projection neurons, Robo protein was found to be responsible for establishing various forebrain commissures in rodents (Sundaresan et al. 2004). That a subpopulation of SATB2+ callosal projecting neurons (Alcamo et al. 2008; Britanova et al. 2008) coexpressed ROBO1 might suggest its involvement in directing axon outgrowth from this neuronal subtype in layer V.
Although CTIP2 expression is seen in both Layer V and VI at 15 PCW, it has been shown to be predominantly expressed in Layer V from 19 PCW in human (Johnson et al. 2009). The change in laminar expression pattern is likely to be under the control of 2 genes; Fezf2, an upstream gene that promotes Ctip2 expression (Chen, Schaevitz, and McConnell 2005; Chen et al. 2008; Leone et al. 2008) and Sox5, which was found to regulate and restrict the expression of Fezf2 and Ctip2 from a domain spanning the SP, Layer VI, and V at E14.5 to only Layer V by P0 in mice (Kwan et al. 2008). Similarly, FEZF2 expression is restricted to Layer V in the human neocortex at 22 PCW (Kwan et al. 2008) but was shown to be expressed throughout the CP in our study at 12 PCW. Despite the lack of CTIP2/FEZF2 enrichment in Layer V at this stage, the molecular identities of neurons within this layer are already specified, including presumptive SATB2+/ROBO1+/CTIP2- or SATB2+/ROBO1−/CTIP2-callosal projecting neurons and CTIP2+/ROBO1+/SATB2-subcerebral projecting neurons.
Is the Anterior Neocortex the Potential site of Origin of the Motor Cortex during the Early Stages of Human CP Development?
This study provides extensive evidence for high anterior to low posterior expression gradients for ROBO1, SRGAP1, and CTIP2, in addition to our previously published evidence for similar gradients for other genes, including ER81 and S100A10 (Ip et al. 2010) associated with the corticospinal neuron development but also with all subcerebral projection neurons (Hevner et al. 2003; Arlotta et al. 2005; Yoneshima et al. 2006). SOX5 and FEZF2, acting upstream to promote expression of transcription factors such as CTIP2 in all subcerebral projection neurons (Chen, Schaevitz, and McConnell 2005; Chen et al. 2008; Kwan et al. 2008; Leone et al. 2008), were not expressed in this gradient. Why should it be that, as development unfolds, gene expression associated with corticofugal axon projections is relatively higher at the anterior pole of the neocortex?
It may be that a much higher number of layer V neurons from frontal cortex are corticofugal neurons compared with layer V neurons at the occipital pole. Retrograde tracing has demonstrated that the various motor areas of the frontal lobe make the predominant contribution to both corticospinal (Galea and Darian-Smith 1994) and corticopontine (Glickstein et al. 1985) pathways in the adult macaque, whereas retrograde labeling from the superior collicullus results in a patchy distribution of labeled neurons in the visual cortex sometimes present at a relatively low density (Lock et al. 2003). This disparity could be reflected in relatively higher expression of some corticofugal marker genes anteriorly compared with posteriorly. The motor cortex is not found predominantly toward the anterior pole of the neocortex in the adult human brain as it is in the rodent. This may be because the prefrontal cortex, a much larger structure in the human compared with rodent brain, starts to expand during later stages of development (Fuster 2002) such that the motor cortex location would then begin to shift posteriorly to its adult position anterior to the central sulcus.
In order to ensure that the required larger numbers of corticofugal neurons are initially specified at anterior pole of the neocortex, arealisation mechanisms may interact with the specification of corticofugal identity by genes such as SOX5 and FEZF2, accounting for the observed anterior to posterior expression gradients of ROBO1/SRGAP1 and CTIP2. It is known that the conditional knockout of the transcription factor Sp8, expressed in a high anteriomedial to low posteriolateral gradient across the VZ in mice (Sahara et al. 2007) results in a reduction of Robo1 expression in deep layers of the CP at E18.5 (Zembrzycki et al. 2007). Another transcription factor, PAX6, is expressed in a high anteriolateral to low posteriomedial gradient in humans at 8 PCW (Bayatti, Sarma, et al. 2008) and in mice throughout cortical development (Bishop et al. 2000). Similar phenotypes, in which thalamocortical and corticofugal fibers were ventrally misprojected at the diencephalic level in mice at E18.5, have been observed in both Pax6−/−- mutant mice (Jones et al. 2002) and robo1−/−robo2−/− double mutants (López-Bendito et al. 2007). In addition, Robo2 expression disappears from the cortical neurepithelium in the homozygous Pax6 mutant at E17 (Jimenez et al. 2002). In humans, the PAX6 gradient has disappeared by 9 PCW, so although the ROBO1 expression may also be under the regulation of PAX6, this may be indirect or under the control of genes downstream of PAX6 which exhibit gradients at later stages (Bayatti, Sarma, et al. 2008). It is noteworthy that an isoform of ROBO1 (DUTT1/ROBO1b) was also found to be highly enriched in the prefrontal region of the neocortex during late midfetal stages in humans (Johnson et al. 2009).
A recent study in knockout mice (Tomassy et al. 2010) has revealed that the transcription factor COUP-TFI, expressed in a high posterior to low anterior gradient across the neocortex in both mice (Armentano et al. 2007) and humans (Ip et al. 2010) represses the differentiation of Layer VI corticothalamic neurons in sensory areas into Layer V corticofugal neurons by inhibiting expression of Ctip2 and Fezf2, such that, in the absence of COUP-TFI function, presumptive corticothalamic neurons abnormally display the molecular features and projection patterns of corticospinal neurons. At the same time, Layer V neurons in the sensorimotor cortex fail to develop into corticospinal neurons. Therefore, the observation of a high anterior to low posterior gradient in CTIP2 expression between 8 and 12 PCW may reflect necessary suppression of CTIP2 expression by COUP-TFI in layer VI of the posterior, sensory cortex.
Elevated expression of ROBO1, SRGAP1, and CTIP2 by corticospinal neurons compared with other corticofugal projection neurons may be required for growth and maintenance of the CST beyond the pons and across the medullary decussation (Arlotta et al. 2005; López-Bendito et al. 2007), whereas the other major subcerebral cortical projections terminate, in maturity, predominantly ipsilaterally in the midbrain and pons. In rodent early cortical development, all the Layer V neurons of the neocortex project to the spinal cord but subsequently selective axon pruning reduces the origins of the CST to the sensorimotor cortex (Oudega et al. 1994). There is no direct evidence that this is also the case in humans, however, a recent study found that an early lesion to the frontal and parietal cortex resulted in the formation of direct connections from occipital cortex to spinal cord motor neurons from the lesioned hemisphere (Basu et al. 2010). This could represent retention of a pathway normally removed during development. Regionalized expression of specific cell–cell signaling molecules such as ROBO1/SRGAP1 may protect corticospinal axons from retraction from the spinal cord and promote their removal from inappropriate target areas, in the same way as arealized expression of plexins 3A and 4A in the rodent visual cortex promotes pruning of their corticospinal projections and protection of appropriate projections to the superior colliculus (Low et al. 2008). However, the plasticity of corticospinal development under pathological or experimental conditions (Sharkey et al. 1986; Li et al. 1995; Basu et al. 2010) suggests that genetically specified regionalization mechanisms can be overridden by epigenetic influences.
Defining the temporal and spatial expression patterns of genes that specify the phenotype of corticospinal neurons will be invaluable in understanding how lesions and infections can perturb the protomap during cortical development and how to produce corticospinal neurons from stem cells for the repair of brain following neonatal hypoxia or adult stroke. The present study provides evidence for the localized expression of genes associated with corticofugal neurons in the early stages of human cortical development. The next step will be to probe the mechanisms that regulate the expression of these genes in vitro in human cortical cell cultures.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
B.K.I. was supported by a studentship awarded by the Anatomical Society of Great Britain and Ireland; Wellcome Trust (WT0655577MA).
Supplementary Material
Acknowledgments
The human embryonic and fetal material was provided by the Joint MRC-Wellcome Trust Human Developmental Biology Resource (http://www.hdbr.org) at the Institute of Human Genetics, Newcastle-upon-Tyne, UK. We thank the consenting women who made this study possible and A. Farnworth, T. Kelly, and C. Collins who gained consent on our behalf, also Prof J. Eyre for advice and support. Conflict of Interest: None declared.
References
- Abrahams BS, Tentler D, Perederiy JV, Oldham MC, Coppola G, Geschwind DH. Genome-wide analyses of human perisylvian cerebral cortical patterning. Proc Natl Acad Sci U S A. 2007;104:17849–17854. doi: 10.1073/pnas.0706128104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Farinas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Andrews W, Barber M, Hernadez-Miranda LR, Xian J, Rakic S, Sundaresan V, Rabbitts TH, Pannell R, Rabbitts P, Thompson H, et al. The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons. Dev Biol. 2008;313:648–658. doi: 10.1016/j.ydbio.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Andrews WD, Barber M, Parnavelas JG. Slit-Robo interactions during cortical development. J Anat. 2007;211:188–198. doi: 10.1111/j.1469-7580.2007.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Armentano M, Chou SJ, Tomassy GS, Leingartner A, O'Leary DD, Studer M. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. [DOI] [PubMed] [Google Scholar]
- Bacon C, Endris V, Rappold G. Dynamic expression of the Slit-Robo GTPase activating protein genes during development of the murine nervous system. J Comp Neurol. 2009;513:224–236. doi: 10.1002/cne.21955. [DOI] [PubMed] [Google Scholar]
- Bagri A, Márin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Basu A, Graziadio S, Smith M, Clowry GJ, Cioni G, Eyre JA. Developmental plasticity connects visual cortex to motoneurons after stroke. Ann Neurol. 2010;67:132–136. doi: 10.1002/ana.21827. [DOI] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, Ambrose P, Ward JF, Lindsay S, Clowry GJ. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 2008;18:1536–1548. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayatti N, Sarma S, Shaw C, Eyre JA, Vouyiouklis DA, Lindsay S, Clowry GJ. Progressive loss of PAX6, TBR2, NEUROD and TBR1 mRNA gradients correlates with translocation of EMX2 to the cortical plate during human cortical development. Eur J Neurosci. 2008;28:1449–1456. doi: 10.1111/j.1460-9568.2008.06475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O'Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Rubenstein JL, O'Leary DD. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Wang SS, Hattox AM, Rayburn H, Nelson SB, McConnell SK. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci U S A. 2008;105:11382–11387. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Rašin MR, Kwan KY, Šestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. 2007;31:1136–1149. doi: 10.1016/j.neubiorev.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Faulkner RL, Low LK, Liu XB, Coble J, Jones EG, Cheng HJ. Dorsal turning of motor corticospinal axons at the pyramidal decussation requires plexin signaling. Neural Dev. 2008;3:21. doi: 10.1186/1749-8104-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Galea MP, Darian-Smith I. Multiple corticospinal neuron populations in the macaque monkey are specified by their unique cortical origins, spinal terminations, and connections. Cereb Cortex. 1994;4:166–194. doi: 10.1093/cercor/4.2.166. [DOI] [PubMed] [Google Scholar]
- Glickstein M, May JG, III, Mercier BE. Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol. 1985;235:343–359. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Molnár Z, Tarabykin V, Stoykova A. Molecular mechanisms of cortical differentiation. Eur J Neurosci. 2006;23:857–868. doi: 10.1111/j.1460-9568.2006.04626.x. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PR, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hern WM. Correlation of fetal age and measurements between 10 and 26 weeks of gestation. Obstet Gynecol. 1984;63:26–32. [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Rubenstein JL, Stunnenberg H, Olavarria JF, Englund C. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev Neurosci. 2003;25:139–151. doi: 10.1159/000072263. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Wang WZ, Lee S, Davies KE, Goffinet AM, Rakic S, Parnavelas J, Reim K, Nicolic M, Paulsen O, et al. Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex. 2009;19:1738–1750. doi: 10.1093/cercor/bhn195. [DOI] [PubMed] [Google Scholar]
- Ip BK, Wappler I, Peters H, Lindsay S, Clowry GJ, Bayatti N. Investigating gradients of gene expression involved in early human cortical development. J Anat. 2010;217:300–312. doi: 10.1111/j.1469-7580.2010.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen JC, Chan WM, Bosley TM, Wan J, Carr JR, Rub U, Shattuck D, Salamon G, Kudo LC, Ou J, et al. Mutations in a human ROBO gene disrupt hindbrain axon pathway crossing and morphogenesis. Science. 2004;304:1509–1513. doi: 10.1126/science.1096437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez D, Lopez-Mascaraque L, de Carlos JA, Valverde F. Further studies on cortical tangential migration in wild type and Pax-6 mutant mice. J Neurocytol. 2002;31:719–728. doi: 10.1023/a:1025751914372. [DOI] [PubMed] [Google Scholar]
- Johnson MB, Kawasawa YI, Mason CE, Krsnik Z, Coppola G, Bogdanovic D, Geschwind DH, Mane SM, State MW, Šestan N. Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron. 2009;62:494–509. doi: 10.1016/j.neuron.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, López-Bendito G, Gruss P, Stoykova A, Molnár Z. Pax6 is required for the normal development of the forebrain axonal connections. Development. 2002;129:5041–5052. doi: 10.1242/dev.129.21.5041. [DOI] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Kim GJ, Shatz CJ, McConnell SK. Morphology of pioneer and follower growth cones in the developing cerebral cortex. J Neurobiol. 1991;22:629–642. doi: 10.1002/neu.480220608. [DOI] [PubMed] [Google Scholar]
- Kim SY, Chung HS, Sun W, Kim H. Spatiotemporal expression pattern of non-clustered protocadherin family members in the developing rat brain. Neuroscience. 2007;147:996–1021. doi: 10.1016/j.neuroscience.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Kostović I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Lam MM, Krsnik Z, Kawasawa YI, Lefebvre V, Šestan N. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci U S A. 2008;105:16021–16026. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T, Jabaudon D, Molyneaux BJ, Azim E, Arlotta P, Menezes JR, Macklis JD. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CP, Olavarria JF, Greger BE. Occipital cortico-pyramidal projection in hypothyroid rats. Brain Res. 1995;89:227–234. doi: 10.1016/0165-3806(95)00119-x. [DOI] [PubMed] [Google Scholar]
- Lock TM, Baizer JS, Bender DB. Distribution of corticotectal cells in the macaque. Exp Brain Res. 2003;151:455–470. doi: 10.1007/s00221-003-1500-y. [DOI] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- López-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Márin O. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci. 2007;27:3395–3407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc Natl Acad Sci U S A. 2008;105:8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16(Suppl 1):i152–i161. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 1989;245:978–982. doi: 10.1126/science.2475909. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. Subplate pioneers and the formation of descending connections from cerebral cortex. J Neurosci. 1994;14:1892–1907. doi: 10.1523/JNEUROSCI.14-04-01892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Schaaps JP, Moreau L, Goffinet AM. Embryonic and early fetal development of the human neocortex. J Neurosci. 2000;20:1858–1868. doi: 10.1523/JNEUROSCI.20-05-01858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- O'Leary DD, Chou SJ, Sahara S. Area patterning of the mammalian cortex. Neuron. 2007;56:252–269. doi: 10.1016/j.neuron.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Oudega M, Varon S, Hagg T. Distribution of corticospinal motor neurons in the postnatal rat: quantitative evidence for massive collateral elimination and modest cell death. J Comp Neurol. 1994;347:115–126. doi: 10.1002/cne.903470109. [DOI] [PubMed] [Google Scholar]
- Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runker AE, Little GE, Suto F, Fujisawa H, Mitchell KJ. Semaphorin-6A controls guidance of corticospinal tract axons at multiple choice points. Neural Dev. 2008;3:34. doi: 10.1186/1749-8104-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara S, Kawakami Y, Izpisua Belmonte JC, O'Leary DD. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- Sharkey MA, Lund RD, Dom RM. Maintenance of transient occipitospinal axons in the rat. Brain Res. 1986;395:257–261. doi: 10.1016/s0006-8993(86)80204-9. [DOI] [PubMed] [Google Scholar]
- Shoemaker LD, Arlotta P. Untangling the cortex: advances in understanding specification and differentiation of corticospinal motor neurons. Bioessays. 2010;32:197–206. doi: 10.1002/bies.200900114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan V, Mambetisaeva E, Andrews W, Annan A, Knoll B, Tear G, Bannister L. Dynamic expression patterns of Robo (Robo1 and Robo2) in the developing murine central nervous system. J Comp Neurol. 2004;468:467–481. doi: 10.1002/cne.10984. [DOI] [PubMed] [Google Scholar]
- Tomassy GS, De Leonibus E, Jabaudon D, Lodato S, Alfano C, Mele A, Macklis JD, Studer M. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci U S A. 2010;107:3576–3581. doi: 10.1073/pnas.0911792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WZ, Hoerder-Suabedissen A, Oeschger FM, Bayatti N, Ip BK, Lindsay S, Supramaniam V, Srinivasan L, Rutherford M, Møllgård K, et al. Subplate in the developing cortex of mouse and human. J Anat. 2010;217:368–381. doi: 10.1111/j.1469-7580.2010.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M, Chedotal A, Ghosh A. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002;33:47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, et al. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- Yao Q, Jin WL, Wang Y, Ju G. Regulated shuttling of Slit-Robo-GTPase activating proteins between nucleus and cytoplasm during brain development. Cell Mol Neurobiol. 2008;28:205–221. doi: 10.1007/s10571-007-9187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshima H, Yamasaki S, Voelker CC, Molnár Z, Christophe E, Audinat E, Takemoto M, Nishiwaki M, Tsuji S, Fujita I, et al. Er81 is expressed in a subpopulation of layer 5 neurons in rodent and primate neocortices. Neuroscience. 2006;137:401–412. doi: 10.1016/j.neuroscience.2005.08.075. [DOI] [PubMed] [Google Scholar]
- Zembrzycki A, Griesel G, Stoykova A, Mansouri A. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.