Abstract
Survivin is highly expressed in most cancers, including glioblastoma, and it plays a significant role in inhibiting apoptosis and promoting tumor growth. Treatment of cancer cells with N-(4-hydroxyphenyl) retinamide (4-HPR) induces apoptosis through destabilization of mitochondrial membrane and activation of caspase-mediated apoptotic pathways. We studied the efficacy of a combination of survivin knockdown and 4-HPR treatment to induce apoptosis and inhibit invasion, angiogenesis, and growth of human glioblastomas in vitro and in vivo. Using a plasmid encoding survivin shRNA, we downregulated survivin in glioblastoma U251MG and U118MG cells and simultaneously treated with 1 µM 4-HPR for 48 hours. Cells following treatments were subjected to the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) and invasion assays. In vivo angiogenesis and tumor regression studies were performed in nude mice. TUNEL assay demonstrated apoptosis in more than 80% of cells after survivin knockdown and 4-HPR treatment. Matrigel invasion assays demonstrated marked decreases in tumor cell invasion. In vivo angiogenesis studies depicted a remarkable inhibition of neovascularization due to the knockdown of survivin and 4-HPR treatment. Imaging of intracerebral tumorigenesis and longitudinal studies on subcutaneous solid tumor formation showed dramatic decreases in tumorigenesis and solid tumor progression, respectively, after treatment with the combination. Studies to elucidate the molecular mechanisms of the inhibition of angiogenesis and tumor regression demonstrated marked decreases in proliferating cell nuclear antigen, metalloproteinase-9, vascular endothelial growth factor, basic fibroblast growth factor, and CD31 in solid tumors. Our data demonstrated that survivin knockdown and concurrent 4-HPR treatment could be a novel therapeutic strategy for controlling growth of human glioblastomas.
Keywords: 4-HPR, glioblastoma, intracerebral tumors, survivin, U118MG, U251MG
Glioblastomas are very heterogenous and the most common form of malignant primary brain tumors.1 A major challenge in patients with glioblastomas is the propensity of the tumor cells to invade rapidly deep into the surrounding tissues. Invasive tumor cells escape surgical removal and, because of their increased resistance to apoptosis, they are relatively resistant to radiation and chemotherapy.2 It is necessary to develop explicit treatment strategies targeting the specific molecular aberrations that underlie the pathogenesis of glioblastomas. The development of appropriate combination therapeutic strategies, including gene therapy, could control the aggressive growth of this dreaded tumor.3,4
Survivin is a member of the family of inhibitor-of-apoptosis (IAP) proteins, and it functions as a key regulator of mitosis and programmed cell death or apoptosis.5 The role of survivin in the pathogenesis of cancer is not limited to the inhibition of apoptosis but also involves the regulation of the mitotic spindle checkpoint and the promotion of angiogenesis and chemoresistance.5 Survivin is highly expressed in most human tumors and fetal tissue, but it is completely absent in terminally differentiated cells.6 Tumors that highly express survivin generally bear a poor prognosis and are associated with resistance to radiation and chemotherapy.7 Survivin gene expression is transcriptionally repressed by wild-type p53 and can be deregulated in cancers by several mechanisms, including gene amplification, hypomethylation, increased promoter activity, and loss of p53 function.8,9 Therefore, survivin is an ideal target for cancer therapy for killing tumor cells alone and leaving the normal cells unaffected.
The synthetic retinoid N-(4-hydroxyphenyl) retinamide (4-HPR) has a low pharmacological toxicity and is a powerful agent to induce apoptosis in various cancers. Many animal and clinical studies have shown that 4-HPR directly affects cell proliferation and growth, inhibiting angiogenesis and malignant tumor growth.10–12 Treatment of tumor cells with 4-HPR results in the induction of apoptosis through destabilization of mitochondrial membrane, release of mitochondrial cytochrome c, and activation of intrinsic caspase-mediated pathway leading to apoptosis.13,14 However, treatment with 4-HPR may induce apoptosis in normal cells also. This problem could be solved by using a low dose of 4-HPR and simultaneously adopting a mechanism to induce apoptosis exclusively in tumor cells.
Small interfering RNAs (siRNAs) are synthetic antisense oligonucleotides that silence the expression of a particular gene by its complementary binding and cleavage, resulting in the disruption of translation of that particular gene.15,16 The purpose of this investigation was to induce apoptosis via the knockdown of survivin using cognate siRNA and simultaneous 4-HPR treatment in two highly invasive human glioblastoma cell lines, U251MG and U118MG, and to examine whether such a combination therapy could inhibit cell invasion, angiogenesis, and tumor growth in nude mice. Our study was also aimed at elucidating the mechanisms of inhibition of angiogenesis and tumor progression in vivo after the treatment with a combination of survivin siRNA and 4-HPR.
Materials and Methods
Cell Culture Conditions
The human glioblastoma U251MG cell line was procured from the National Cancer Institute (Frederick, MD, USA). The other human glioblastoma U118MG cell line was purchased from the American Type Culture Collection. We have selected U251MG and U118MG cells for this study because U251MG is the most aggressive cell line among glioblastomas and the U118MG cell line produces a moderate growth of subcutaneous tumors. The U251MG and U118MG cell lines were propagated in RPMI 1640 and DMEM (Mediatech), respectively, supplemented with 10% fetal bovine serum (Invitrogen) and antibiotics in a fully humidified incubator containing 5% CO2 in air at 37°C.
Construction of Survivin siRNA Expression Vector
The survivin siRNA cDNA with sense and antisense strands was constructed into the mammalian expression vector pRNAT-CMV3.2/Neo (GenScript), between the BamHI and XhoI sites. We prepared 3 siRNA sequences, and the most effective one was selected on the basis of percent knockdown of survivin. The selected siRNA sequence began at nucleotide 345 (AY889741), 5′-GGA AAC CAA CAA TAA GAA GTT-3′ (sense) and 3′-CTT CTT ATT GTT GGT TTC C-5′ (antisense). The scrambled siRNA sequence contained 5′-ACA AGA GAG ACA GAA CAT ATT-3′ (sense) and 3′-TTC TTG CTT CTG TGT CTT A-5′ (antisense). The linear siRNA construct was annealed with the complementary strand and ligated into the plasmid vector between the BamHI and XhoI sites. In this vector, the powerful cytomegalovirus (CMV) promoter drives the expression of siRNA, and the simian virus 40 promoter drives the expression of the resistance gene neomycin. This vector also carries coral green fluorescein protein (cGFP) for tracking of transfection efficiency in cell cultures. The siRNA sequence was confirmed by DNA sequencing using a forward sequencing primer for the CMV (5′-GTA CGG TGG GAG GTC TAT AT-3′) and a reverse sequencing primer for pRNA (5′-TAG AAG GCA CAG TCG AGG-3′). The plasmid encoding for survivin shRNA was transformed into the JM109 competent cells (Promega). The positive colonies were screened using a miniprep plasmid DNA purification kit (Qiagen) and further propagated.
Treatment of U251MG and U118MG Cells with Survivin siRNA Plasmid Vector and 4-HPR
About 80% confluent cultures of U251MG and U118MG cells were transfected with the plasmid encoding for survivin shRNA, treated with 1 µM 4-HPR, or both in combination in a serum-free medium. The plasmid vector was transfected with Fugene HD (Roche Diagnostics). Transfection efficiency was monitored using an inverted fluorescent microscope (Olympus IX71) for monitoring the expression of cGFP. A set of cultures was also transfected with the plasmid encoding for survivin scrambled shRNA. After 24 hours, the medium was replaced with a regular serum medium, and the cultures were incubated for another 24 hours. A dose of 1 µM 4-HPR was selected based on a dose-response study for the detection of apoptosis using the TUNEL assay. Higher concentrations of 4-HPR did not significantly increase apoptosis, whereas lower concentrations were found to be less effective.
Real-Time Reverse Transcription–Polymerase Chain Reaction for Examination of Survivin mRNA
Real-time reverse transcription–polymerase chain reaction (RT–PCR) was carried out to determine the knockdown of survivin mRNA after transfection with a recombinant plasmid vector for producing survivin siRNA, treatment with 4-HPR, or both agents together. Total cellular RNA was isolated using the Aurum kit (Bio-Rad). We used the following primer sequences for PCR amplification of the survivin gene: 5′-GGA CCA CCG CAT CTC TAC AT-3′ (forward) and 5′-GAC AGA AAG GAA AGC GCA AC-3′ (reverse). Real-time RT–PCR was carried out with 100 ng of total RNA using a 1-step RT–PCR kit containing SYBR green (Bio-Rad) in a real-time PCR machine (iCycler iQ5, Bio-Rad) with the following reaction conditions: cDNA synthesis for 10 min at 50°C; reverse transcriptase inactivation at 95°C for 5 min; PCR cycling and detection at 95°C for 10 seconds; and data collection at 56°C for 30 seconds.
Western Blotting for Survivin and Other Proteins Involved in Caspase-Mediated Apoptosis
Western blotting for survivin was carried out to examine the downregulation of survivin protein levels after transfection with survivin siRNA, treatment with 4-HPR, and both in combination for 48 hours. Western blotting was also performed for the pivotal molecules involved in caspase-mediated pathway leading to apoptosis. The proteins prepared from the cell lysate of control, and treated cells were resolved on 4%–20% polyacrylamide gradient gels (Bio-Rad) and electroblotted to the activated PVDF membranes (Millipore). Nonspecific binding sites were blocked with 10% nonfat milk, and the membranes were incubated overnight on a rocker at 4°C with specific antibodies. The primary IgG antibodies for caspase-7, caspase-3, caspase-activated DNase (CAD), and poly(ADP-ribose) polymerase (PARP) were purchased from Santa Cruz Biotechnology, and antibodies for caspase-9 and cytochrome c were obtained from BD Biosciences. After incubation, the blots were washed and treated with horseradish peroxidase conjugated respective secondary IgG antibodies (Biomeda) at room temperature for 2 hours. The blots were washed again, treated with an enhanced chemiluminescence reagent (GE Healthcare), exposed to Kodak autoradiography film (BioMax XAR), and developed. The blots were reprobed using the Western reprobe buffer (Gbiosciences) for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) content with a monoclonal primary IgG antibody (Novus Biologicals) to demonstrate that equal amounts of protein were loaded in all lanes. The Western blots were digitally imaged, and band intensities were quantified using the Gel-Pro analyzer software (Media Cybernetics).
TUNEL Assay for Detection of Apoptosis
Both U251MG and U118MG cells were transfected with survivin siRNA, treated with 4-HPR, or both agents together in serum-free media for the first 24 hours and then with serum-containing media for the next 24 hours. The culture media were removed at 48 hours, cells were washed twice with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS for 15 minutes. The TUNEL assay was carried out using a fluorometric TUNEL kit (Millipore). Briefly, the fixed cells were washed thrice in PBS and permeabilized with 0.2% Triton X-100 for 5 minutes. The cells were washed and incubated with 50 µL of recombinant terminal deoxynucleotidyl transferase fluorescein-12-dUTP cocktail for 1 hour at 37°C in a fully humidified chamber. The reaction was terminated, and the slides were washed thrice in PBS and mounted with antifading agent (Vector Laboratories). Slides were dried in the dark, examined under a fluorescent microscope (Olympus), and photographed. The apoptotic cells were quantified using the Image-Pro Discovery software (Media Cybernetics).
Matrigel Invasion Assay
The effects of survivin siRNA and 4-HPR alone and in combination on the invasive properties of U251MG and U118MG cells were measured by the invasion of cells through a Matrigel-coated membrane.17 Transwell inserts of 12 wells with 12.0 µm pore size (Corning Life Sciences) were coated with a final concentration of 1.0 mg/mL Matrigel (BD Biosciences) in an ice-cold serum-free medium and allowed to dry at 37°C. Cells were transfected with the plasmid encoding for survivin shRNA, treated with 4-HPR, or a combination of both for 48 hours. Following treatments, cells were trypsinized and 200 µL of cell suspension (2 × 105 viable cells) from each sample was added to the top of the Matrigel in triplicate. After 48-hour incubation in a CO2 incubator, the membranes were collected and stained with HEMA stain (Fisher Scientific). The cells that migrated to the undersurface of the membrane were examined under a microscope, counted in 10 randomly selected microscopic fields, and photographed.
3-(4,5-Dimethylthiazolyl-2)-2,5-Diphenyltetrazolium Bromide Assay for Cell Viability
The 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay18 was performed to determine cell viability after transfection with survivin siRNA and/or treatment with 4-HPR. Approximately 104 cells were seeded into each well of a 96-well plate and treated for 48 hours. We performed the MTT assay as per the manufacturer's instructions (Chemicon International). The purple formazan was dissolved with the addition of isopropanol, and the absorbance of the final color solution was measured using an ELISA reader (BioTek).
Western Blotting for Molecules Involved in Cell Proliferation and Invasion
Western blotting was performed for the prominent molecules involved in cell proliferation and invasion after transfection with survivin siRNA and/or treatment with 4-HPR as described above.
The primary IgG antibody for proliferating cell nuclear antigen (PCNA) was procured from BD Biosciences, focal adhesion kinase (FAK) was purchased from Santa Cruz Biotechnology, and matrix metalloproteinase-9 (MMP-9) was procured from Cell Signaling Technology.
In Vivo Angiogenesis Assay
The antiangiogenic effects of survivin siRNA and/or 4-HPR on U251MG and U118MG cells in vivo were studied using the dorsal skinfold chamber model.19 The diffusion chamber consisting of a ring (Millipore) covered with Millipore membrane filters (0.45 µm) on both sides was sterilized by UV irradiation. The cells transfected with either survivin siRNA or treated 4-HPR or both agents together in 200 µL of suspension (2 × 105 cells) were injected into the chamber. The opening of the chamber was subsequently sealed with sterile bone wax, and the chambers were surgically implanted under the dorsal skin of nude mice. After 10 days, the chambers were removed surgically and the superficial fascia exposed to the chamber was harvested. The formation of new blood vessels (neovascularization) was distinguished from pre-existing vessels as curved thin structures in a zigzag pattern using a stereomicroscope (Olympus SZX12) equipped with a Spot RT Slider digital camera (Meyer Instruments) and photographed. The branching of tumor-induced neovasculature was counted, the length of each neovasculature was measured using an ocular micrometer, and the total length was quantified in each treatment group.
Orthotopic Tumorigenesis in Nude Mice
Both U251MG and U118MG cells were stably transfected with a plasmid vector carrying the luciferase gene (phCMV-FSR; Genlantis) and propagated in media containing G-418 (500 µg/mL). The cells were highly homogeneous and propagated from a single-cell colony with a strong emission of photons. The cells were harvested, counted, and 1 × 106 cells suspended in 10 µL of the serum-free medium to inject into the intracerebrum of nude mice, using a 25-µL Hamilton syringe with the help of a digital stereotaxic apparatus (Stoelting) after drilling a small hole on the skull. The animals were left for 3 days without any treatment. Afterwards, the mice received intracerebral injection with either the survivin siRNA plasmid vector (5 µg DNA/injection/mouse) or 4-HPR (1 µg/injection/mouse) or both agents for another 20 days on alternate days. The plasmid vector carrying survivin siRNA cDNA was suspended in RNAse-free sterile water (25 µg DNA/10 µL) and mixed (1:4, v/v) with an i-Fect transfection reagent (Neuromics) to obtain 5 µg DNA/10 µL of injection volume. The injections were given using a Hamilton syringe with the help of a stereotaxic apparatus at the site of tumor cell plantation. In the case of combination treatment, 4-HPR was injected first followed by the siRNA plasmid vector with an interval of 10 minutes. One set of mice received similar injections with survivin scrambled siRNA vector, whereas another set was left untreated. Furthermore, one set of normal mice without tumor implantation was injected with i-Fect transfection reagent and scrambled siRNA in order to study any nonspecific toxicity. On day 21, the mice were injected intraperitoneally with 100 µL (50 mg/mL) of luciferin (Genlantis). After 10 minutes, the mice were visualized for luciferase activity using the Xenogen IVIS-200 (Hopkinton, MA) imaging system. After imaging, the animals were sacrificed, brain tissue was extracted, and survivin protein levels were determined in the orthotopic tumors by Western blotting as described above. A total of 6 mice were used in each group. All animal experiments were performed in compliance with our Institutional Animal Care and Use Committee (IACUC).
Solid Tumor Development in Nude Mice
Since the solid tumor formation of U251MG cells is very slow in the subcutaneous area, we used only the U118MG cells to induce tumors in the subcutaneous region of nude mice. About 80% confluent cultures of U118MG cells were harvested, counted, and suspended in an equal volume of high-concentrated Matrigel (BD Biosciences). We used 100 µL of this cell suspension (5 × 106 cells) in Matrigel to inject under the dorsal skin of nude mice. The animals were left for 3 weeks without any treatment for the uniform development of visible tumors with approximate volume of 150–250 mm3. The animals were then divided into 5 groups of 6 mice each. Afterwards, the mice were injected at the tumor site with either the survivin siRNA plasmid vectors (50 µg DNA/injection/mouse), 4-HPR (10 µg/injection/mouse), or both agents together on alternate days for 5 weeks. One group of mice received survivin scrambled siRNA vector and was considered as the treated controls. Tumor volume was measured beginning from week 3 using a digital vernier caliper. Tumor volume was calculated using the formula [(smallest diameter2 × widest diameter)/2], and the growth curves were plotted for each treatment group.20 At the end of the 8th week, the animals were anesthetized with a mixture of ketamine and xylazine and photographed. The solid tumors were surgically removed, tumor weight was recorded, and the tumors were photographed. Tumor samples were stored at −80°C for further analysis.
Western Blotting for Molecules Involved in Cell Proliferation, Angiogenesis, and Tumor Progression in Solid Tumors
Western blotting was carried out using the U118MG solid tumor samples for molecules involved in cell proliferation, angiogenesis, and tumor progression after treatment with survivin siRNA and/or 4-HPR. The tumor samples were weighed, cut into small pieces, and homogenized using an Omni Ruptor 400 Ultrasonic homogenizer (Omni International). The homogenized tumor samples were centrifuged at 15 000 g for 10 minutes at 4°C, and the supernatants were collected. Protein concentration in the supernatant was determined using the Coomassie-Plus protein assay (Pierce Biotechnology), and the samples were stored at −20°C until used. Western blotting was performed as described above. The primary IgG antibodies for PCNA and CD31 were obtained from BD Biosciences, vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) were purchased from Santa Cruz Biotechnology, and the antibody for MMP-9 was procured from Cell Signaling Technology.
Statistical Analysis
Arithmetic mean and standard deviation (SD) were calculated for all quantitative data. The results were statistically evaluated using one-way analysis of variance (ANOVA). The least significant difference method was used to compare the mean values of control or scrambled siRNA-treated groups with those of survivin siRNA- or 4-HPR-treated groups. The individual survivin siRNA or 4-HPR mean values were also compared with the combination treatment mean values. A value of P < .05 was considered statistically significant.
Results
Downregulation of Survivin mRNA and Protein Levels in U251MG and U118MG Cells
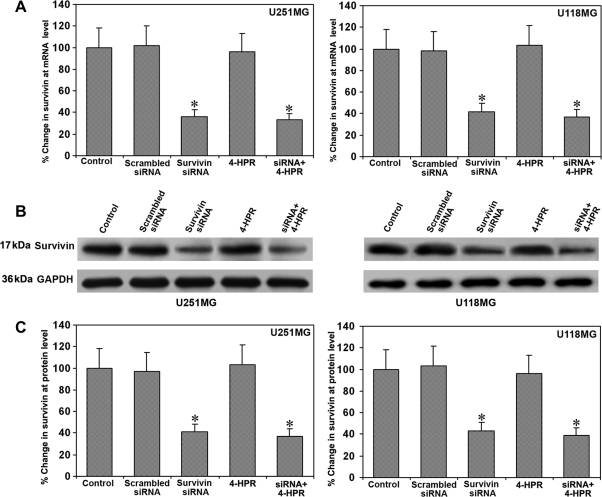
We examined the downregulation of survivin mRNA and protein levels in glioblastoma U251MG and U118MG cells after the treatments (Fig. 1). In vitro transfection of U251MG and U118MG cells with a plasmid encoding for survivin shRNA resulted in a marked downregulation of cognate mRNA (Fig. 1A) and protein (Fig. 1B) levels in both cell lines. There was no alteration in survivin mRNA or protein levels after transfection with scrambled siRNA or treatment with 4-HPR. We used GAPDH as an internal control for protein loading. Quantification of Western blot images using Gel-Pro analyzer software demonstrated more than 60% knockdown of survivin protein levels in both glioblastoma cell lines after treatment with a combination of survivin siRNA and 4-HPR (Fig. 1C).
Fig. 1.
Survivin mRNA and protein levels in U251MG and U118MG cells. Treatments (48 hours): control, scrambled siRNA, survivin siRNA, 1 µM 4-HPR, and survivin siRNA + 1 µM 4-HPR. (A) Real-time RT–PCR analysis for survivin mRNA. Values are mean ± SD of 6 assays in each group (*P < .001 compared with the control mean values). (B) Western blotting for survivin. The blots were reprobed for GAPDH content to demonstrate that an equal amount of protein was loaded in each lane. The data are representative of 6 independent experiments. (C) Quantitative evaluation of Western blots. Values are mean ± SD of 6 assays in each group (*P < .001 compared with the control mean values).
Survivin siRNA and 4-HPR Increased Release of Cytochrome c from Mitochondria and Activation of Caspases for Apoptosis
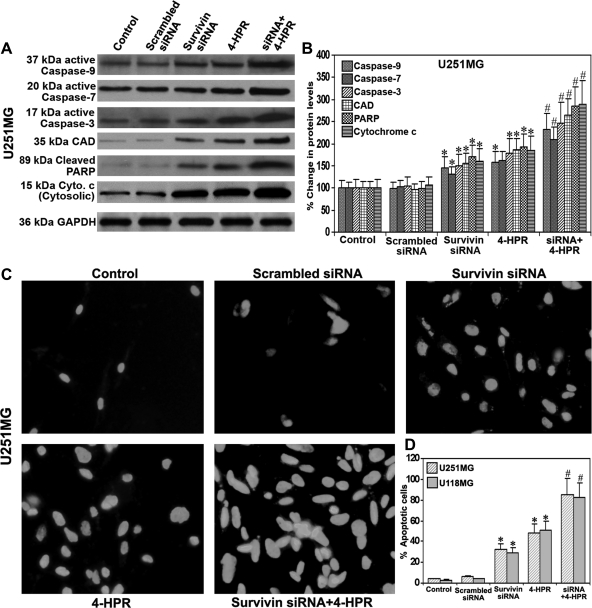
We performed Western blotting to determine the effect of survivin siRNA and/or 4-HPR on the mitochondrial membrane for releasing of cytochrome c from mitochondria, cleavage of caspases to generate their active subunits, and molecules involved in the execution of apoptosis in U251MG cells after the treatments (Fig. 2). The cytochrome c content in the cytosolic fraction was markedly increased after the knockdown of survivin and/or treatment with 4-HPR (Fig. 2A). The active subunits of caspase-9, caspase-7, and caspase-3 were increased significantly after the treatment with a combination of both agents (Fig. 2A). The 2 prominent apoptotic markers, CAD and cleaved PARP, due to the activation of the final executioner caspase-3 were remarkably increased after the knockdown of survivin and/or treatment with 4-HPR (Fig. 2A). The cells transfected with scrambled siRNA did not show a significant alteration in any of the molecules studied. Further, we quantitated the band intensities of the Western blots of caspases-9, caspase-7, caspase-3, CAD, PARP, and cytochrome c (Fig. 2B). The highest increases in cytosolic cytochome c and cleaved PARP occurred after the treatment of cells with the combination of both agents.
Fig. 2.
Activation of caspases and induction of apoptosis in glioblastoma cells. Treatments (48 hours): control, scrambled siRNA, survivin siRNA, 1 µM 4-HPR, and survivin siRNA + 1 µM 4-HPR. (A) Representative Western blots for active subunits of caspase-9, caspase-7, caspase-3, CAD, cleaved PARP, and cytosolic cytochrome c in the cell lysates of U251MG cells. Cleaved PARP was determined in the nuclear fraction. Mitochondria were isolated from the total cell lysate, and cytochrome c levels were determined in the cytosolic fraction. Western blots were reprobed for GAPDH to demonstrate that equal amounts of proteins were loaded in all lanes. (B) Quantitative evaluation of percent changes in the protein levels. Western blot images were quantified using Gel-Pro analyzer software. Data are mean ± SD of 6 independent experiments (*P < .001 compared with the mean values of control and #P < .001 compared with the mean values of survivin siRNA- or 4-HPR-treated samples). (C) TUNEL staining for the detection of apoptotic cells. (D) Quantitation of TUNEL positive cells using Image-Pro Discovery software. Data are mean ± SD of 6 independent experiments in each group (*P < .001 compared with the control mean values and #P < .001 compared with the survivin siRNA or 4-HPR mean values).
Survivin Knockdown and Concurrent 4-HPR Treatment Increased Apoptosis
TUNEL staining showed the induction of apoptosis in U251MG cells after survivin knockdown or 4-HPR treatment (Fig. 2C). However, 4-HPR alone was more effective to induce apoptosis than survivin knockdown. Treatment with a combination of both agents resulted in more than doubling of apoptotic cells, compared with either treatment alone. TUNEL staining was not prominent in untreated control cells and cells transfected with scrambled siRNA. Quantitation of the TUNEL positive cells showed 32%, 48%, and 85% apoptosis in U251MG cells and 29%, 51%, and 82% in U118MG cells after treatments with survivin siRNA, 4-HPR, and a combination of both agents, respectively (Fig. 2D). The mean value of the combination treatment was significantly different (P < .001) from that of the individual mean values of survivin siRNA or 4-HPR treatment.
Survivin Knockdown and Concurrent 4-HPR Treatment Resulted in Remarkable Reduction in Glioblastoma Cell Invasion
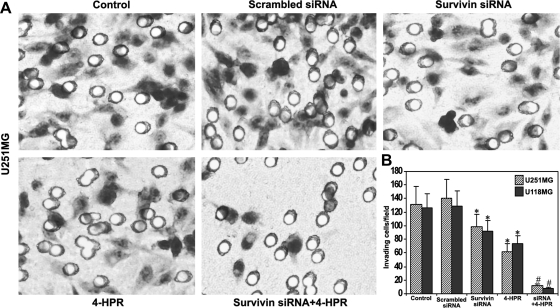
We examined the effects of survivin knockdown and/or 4-HPR treatment on the capability of glioblastoma cell invasion (Fig. 3). Matrigel invasion assays demonstrated remarkable reduction in the invasive property of U251MG cells after survivin knockdown and/or 4-HPR treatment (Fig. 3A). The staining of cells that invaded through the transwell membrane demonstrated that the capability of invasion was reduced due to treatment with survivin siRNA or 4-HPR, compared with control cells or cells transfected with scrambled siRNA. Treatment with the combination of survivin siRNA and 4-HPR resulted in a remarkable reduction in tumor cell invasion, compared with either treatment alone. We presented the quantitative data showing the percentage of tumor cells that invaded through the transwell membrane after treatment with survivin siRNA, 4-HPR, and both agents together (Fig. 3B). We found decreases in cell invasion to 74%, 47%, and 9% in U251MG cells and 73%, 58%, and 6% in U118MG cells after the treatments with survivin siRNA, 4-HPR, respectively, when compared with the untreated control cells.
Fig. 3.
Changes in ability of cell invasion in glioblastoma cells. Treatments (48 hours): control, scrambled siRNA, survivin siRNA, 1 µM 4-HPR, and survivin siRNA + 1 µM 4-HPR. (A) Representative Matrigel invasion assay for U251MG cells. A significant reduction in the number of invaded cells indicated the decrease in invasive potency of the treated cells. (B) Quantitative evaluation of Matrigel invasion assay. The data are mean ± SD of 10 randomly selected microscopic fields from 3 independent wells in each group (*P < .001 compared with the control mean values and #P < .001 compared with the survivin siRNA or 4-HPR mean values).
Survivin Knockdown and 4-HPR Treatment Resulted in Marked Decreases in Cell Viability and the Molecules Involved in Proliferation and Invasion
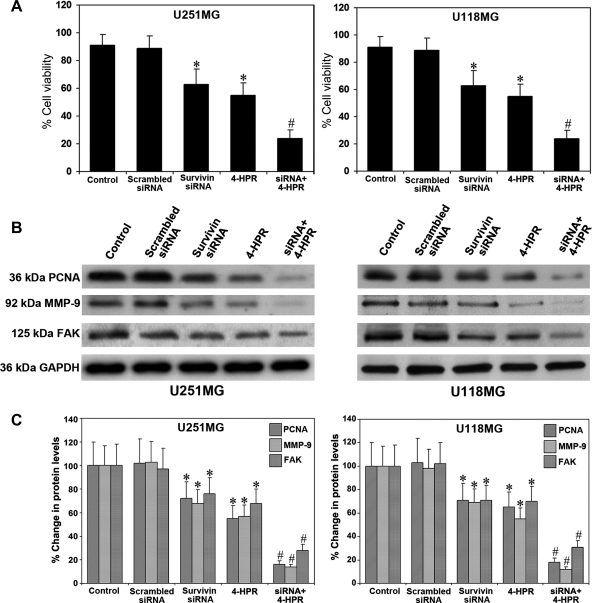
We examined the consequences of treatments on cell viability and expression of molecules involved in proliferation and invasion (Fig. 4). Significant decreases (P < .001) in cell viability occurred in both U251MG and U118MG cells after survivin knockdown or 4-HPR treatment (Fig. 4A). The effect of the combination of survivin siRNA and 4-HPR resulted in a reduction in mean cell viability to 24% and 26% in U251MG and U118MG cells, respectively. To elucidate the mechanisms responsible for the marked reduction in cell invasion after survivin knockdown and 4-HPR treatment, we performed Western blotting to examine the protein levels of prominent molecules involved in cell proliferation and cell invasion in both U251MG and U118MG cells (Fig. 4B). We observed highly significant decreases in the protein levels of PCNA, MMP-9, and FAK in both cell lines due to the treatment with a combination of survivin siRNA and 4-HPR (Fig. 4C). The dramatic decreases in the expression of these molecules following survivin knockdown and 4-HPR treatment caused the inhibition of cell proliferation and cell invasion.
Fig. 4.
Determination of percent changes in cell viability and molecules responsible for cell proliferation and invasion. Treatments (48 hours): control, scrambled siRNA, survivin siRNA, 1 µM 4-HPR, and survivin siRNA + 1 µM 4-HPR. (A) MTT assay for determination of cell viability in U251MG and U118MG cells. Data are mean ± SD of 6 independent experiments in duplicate (*P < .001 compared with the control mean values and #P < .001 compared with the survivin siRNA or 4-HPR mean values). (B) Representative Western blots for PCNA, MMP-9, and FAK in the cell lysates of U251MG and U118MG cells. The blots were reprobed for GAPDH content to demonstrate that the same amount of protein was loaded in each lane. The data are representative of 4 independent experiments in each group. (C) Quantitative measurement of Western blots. The Western blot images were quantified using the Gel-Pro analyzer software. Data are mean ± SD of 4 independent experiments (*P < .001 compared with the control mean values and #P < .001 compared with the survivin siRNA or 4-HPR mean values).
Combination of Survivin Knockdown and 4-HPR Treatment Inhibited In Vivo Angiogenesis
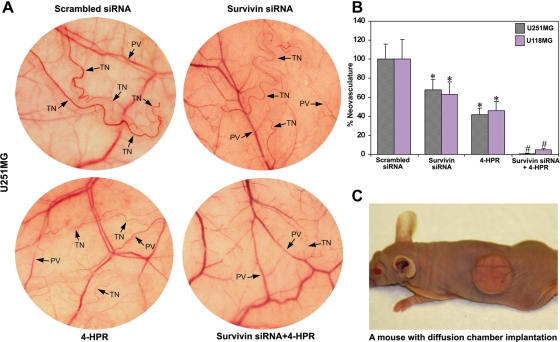
A dorsal skinfold chamber model was used to study the effect of survivin knockdown and/or 4-HPR treatment on in vivo angiogenesis in the superficial fascia of nude mice (Fig. 5). The implantation of diffusion chambers containing U251MG and U118MG control cells and cells transfected with scrambled siRNA resulted in the development of microvessels as indicated by the thin and curved structures arising from the pre-existing vessels in a zigzag manner (Fig. 5A). The ability of formation of such microvessels was remarkably reduced in the cells treated with survivin siRNA or 4-HPR and almost completely inhibited in the cells treated with a combination of both agents (Fig. 5A). Quantitative measurement revealed 32%, 58%, and 99% inhibition of the tumor-induced neovasculature after treatments with survivin siRNA, 4-HPR, and a combination of both agents, respectively, in U251MG cells (Fig. 5B). Similarly, we observed 37%, 54%, and 95% inhibition of the tumor-induced neovasculature after treatments with survivin siRNA, 4-HPR, and a combination of both agents, respectively, in U118MG cells. The scrambled siRNA results were considered as the treated controls. We showed a mouse bearing surgically implanted diffusion chamber for an in vivo angiogenesis assay (Fig. 5C). The diffusion of angiogenic factors through the pores (0.45 µm) of the chamber membrane induces neovascularization in the superficial fascia of nude mice. Treatment of cells with a combination of survivin siRNA and 4-HPR markedly averted the process of diffusion of angiogenic factors, resulting in the inhibition of neovascularization.
Fig. 5.
In vivo angiogenesis assay. Treatments (48 hours): scrambled siRNA, survivin siRNA, 1 µM 4-HPR, and survivin siRNA + 1 µM 4-HPR. (A) In vivo angiogenesis assay using U251MG cells for implantation. The cells were treated for 48 hours, suspended in 200 µL of serum-free medium, injected into a diffusion chamber, implanted under the dorsal skin of nude mice, and left for 10 days. Strong development of tumor-induced neovasculature (TN) arising from pre-existing vessels (PV) as curved thin structures in zigzag pattern was observed in U251MG control (not shown) and scrambled siRNA transfected cells. The formation of such microvasculature was considerably reduced in both survivin siRNA- and 4-HPR-treated cells and almost completely inhibited after treatment with a combination of both agents. (B) Quantitative presentation of in vivo angiogenesis. The TN was measured with the help of an ocular micrometer. Values are mean ± SD of 6 samples from each group (*P < .001 compared with the control mean values and #P < .001 compared with the survivin siRNA or 4-HPR mean values). (C) A nude mouse bearing a diffusion chamber implanted under the dorsal skin for the in vivo angiogenesis assay.
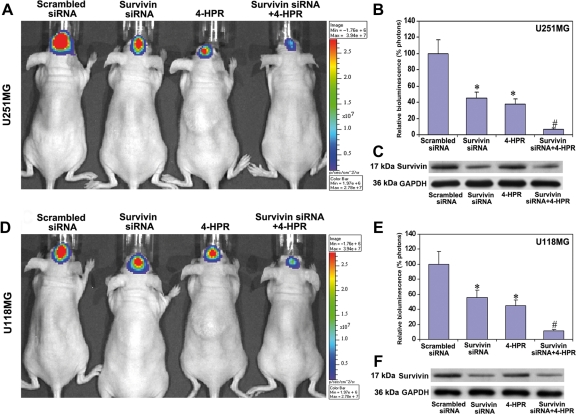
Combination of Survivin Knockdown and 4-HPR Treatment Repressed Orthotopic Tumorigenesis in Nude Mice
We studied the effect of survivin knockdown and/or 4-HPR treatment on the inhibition of orthotopic tumorigenesis in nude mice (Fig. 6). Both U251MG and U118MG cells were stably transfected with a luciferase gene and injected into the intracerebrum of nude mice, allowing the cells to multiply for 3 weeks during treatment. The mice that left untreated and those that received injections of scrambled siRNA showed tumorigenesis as indicated by a large bioluminescent image produced by the tumor cells carrying the luciferase gene (Fig. 6A and D). The production of such a bioluminescent image was partially inhibited in animals injected with survivin siRNA vector or treated with 4-HPR and was almost completely inhibited in animals treated with both agents together, indicating inhibition of orthotopic tumorigenesis (Fig. 6A and D). The control animals that received the i-Fect transfection reagent or scrambled siRNA did not show any nonspecific toxicity, and all the animals were completely normal after the treatment. Quantitation of the bioluminescent images demonstrated the inhibition of the ability of orthotopic tumorigenesis by 55%, 62%, and 93.2% in U251MG cells and 44%, 55%, and 88.8% in U118MG cells, respectively, after the treatments with survivin siRNA, 4-HPR, and a combination of both agents (Fig. 6B and E). Furthermore, we performed Western blotting for survivin protein levels in the brain tumor tissues and found a clear reduction in survivin protein levels due to survivin siRNA (Fig. 6C and F), implying the marked knockdown of survivin mRNA levels after treatment with a plasmid encoding for survivin shRNA. The scrambled siRNA-treated nude mice were considered as the treated controls.
Fig. 6.
Inhibition of intracerebral tumorigenesis in nude mice after the treatments. (A and D) In vivo imaging of intracerebral tumorigenesis in nude mice. U251MG or U118MG cells (1 × 106 cells) stably transfected with luciferase gene were injected into the intracerebrum of nude mice. Beginning from day 3, the mice were injected either with survivin siRNA plasmid vector (5 µg DNA/injection/mouse), 4-HPR (1 µg/injection/mouse), or both agents together for 20 days on alternate days at the site of tumor cell implantation. Then, the mice were injected intraperitoneally with 100 µL (50 mg/mL) of luciferin and visualized for the effect of treatments using Xenogen IVIS-200 imaging system. The data are representative of 6 sets of mice in each group. The background bioluminescence signal from an untreated mouse is about 1.5e + 05 photons on Xenogen IVIS-200 imaging machine. (B and E) Quantitative presentation of relative bioluminescence as percentage of photons in both U251MG and U118MG tumors. Data are mean ± SD of 6 animals in each group (*P < .001 compared with the scrambled siRNA mean values and #P < .001 compared with the survivin siRNA or 4-HPR mean values). (C and F) Western blots for survivin protein levels in the brain tumor tissues after the treatments. The blots were reprobed for GAPDH content to demonstrate that same amount of protein was loaded in each lane. The data are representative of 4 independent experiments.
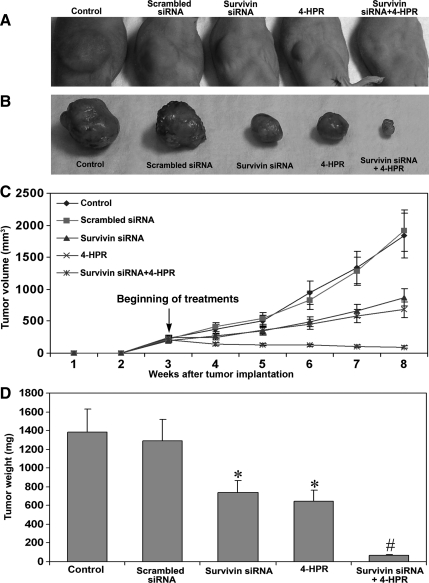
Combination of Survivin siRNA and 4-HPR Treatment Inhibited Subcutaneous Solid Tumor Formation in Nude Mice
We examined the effect of survivin knockdown and/or 4-HPR treatment on the capability of U118MG cells for subcutaneous solid tumor formation in nude mice (Fig. 7). The mice that left untreated and those that received scrambled siRNA injections produced large tumors (Fig. 7A and B). The treatment with a combination of survivin siRNA and 4-HPR resulted in a remarkable reduction in tumor size (Fig. 7A and B). There was no visible tumor at the end of the 8th week after the treatment with a combination of both agents. The longitudinal measurement of tumor volume showed a steady-state increase in control mice and mice injected with scrambled siRNA (Fig. 7C). The tumor size was significantly reduced (P < .001) in the mice injected with survivin siRNA or 4-HPR. The tumor growth curve was significantly switched downward after the treatment with survivin siRNA or 4-HPR and was running straight after the treatment with the combination of both agents (Fig. 7C). Measurement of tumor weight demonstrated 46% and 52% reduction after injections with survivin siRNA and 4-HPR alone, respectively (Fig. 7D). The treatment with the combination of both agents resulted in 96% reduction in tumor weight.
Fig. 7.
Inhibition of subcutaneous solid tumor development in nude mice after the treatments. (A) Subcutaneous solid tumors in nude mice. U118MG cells were harvested, counted, and suspended in an equal volume of high-concentrated Matrigel and then 100 µL of the suspension (5 × 106 cells) was injected under the dorsal skin of nude mice. The animals were left for 3 weeks without any treatment for uniform development of visible tumors. Afterwards, the mice were injected at the tumor site with either survivin siRNA plasmid vector (50 µg DNA/injection/mouse) or 4-HPR (10 µg/injection/mouse) or both agents together on alternate days for 5 weeks. At the end of 8th week, the animals were anesthetized with ketamine and xylazine and then photographed. (B) Subcutaneous solid tumors surgically removed and photographed. The data are representative of 6 mice in each group. (C) Longitudinal measurement of tumor volume using a digital vernier caliper in nude mice. Data are mean ± SD of 6 animals in each group. (D) Quantitative presentation of tumor weight. Data are mean ± SD of 6 animals in each group (*P < .001 compared with the control mean values and #P < .001 compared with the survivin siRNA or 4-HPR mean values).
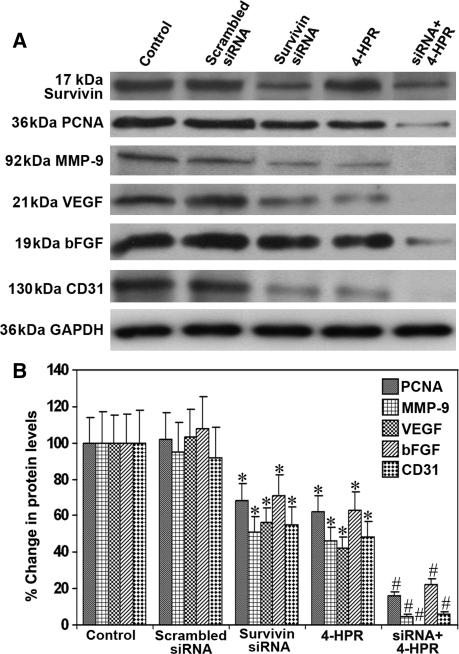
Combination of Survivin siRNA and 4-HPR Treatment Downregulated Molecules Involved in Cell Invasion and Angiogenesis in Subcutaneous Solid Tumors
In an attempt to unravel the mechanisms of inhibition of cell invasion, angiogenesis, and tumor progression after survivin knockdown and 4-HPR treatment, we examined the protein levels of the prominent molecules involved in these processes in the U118MG subcutaneous solid tumors (Fig. 8). We used uniform expression of GAPDH as a loading control in the Western blotting (Fig. 8A). Measurement of the survivin protein levels demonstrated more than 60% decrease after injection with survivin siRNA vectors (Fig. 8A and B). Treatment with 4-HPR did not alter the survivin protein levels in the solid tumors. PCNA and MMP-9, the molecules involved in tumor cell proliferation and invasion, were significantly decreased after the treatment with a combination of survivin siRNA and 4-HPR (Fig. 8A and B). The key angiogenic stimulators VEGF and bFGF as well as the endothelial cell and angiogenesis marker CD31 were also markedly reduced after the injection with survivin siRNA and/or 4-HPR (Fig. 8A and B). There was no significant alteration in the protein levels of all these molecules in the tumors due to treatment with the scrambled siRNA when compared with the untreated controls.
Fig. 8.
Western blotting using subcutaneous solid tumors. (A) Representative Western blots for survivin, PCNA, MMP-9, VEGF, bFGF, and CD31 in the subcutaneous solid tumors of U118MG cells treated with a plasmid encoding survivin shRNA, 4-HPR, or both agents together. The blots were reprobed for GAPDH content to demonstrate that the same amount of protein was loaded in each lane. The data are representative of 4 independent experiments. (B) Quantitative measurement of Western blots. The Western blot images were quantified using the Gel-Pro analyzer software. Data are mean ± SD of 4 independent experiments (*P < .001 compared with the control mean values and #P < .001 compared with the survivin siRNA or 4-HPR mean values).
Discussion
Our study demonstrated that the combination of survivin knockdown and 4-HPR treatment successfully induced apoptosis and inhibited cell invasion, angiogenesis, and tumorigenesis in human glioblastomas. The powerful synthetic retinoid 4-HPR possesses antiproliferative activity and induces apoptosis by altering the mitochondrial membrane permeability transition pore and stimulates the release of cytochrome c from mitochondria triggering the caspase-mediated pathway for apoptosis.13,21 Survivin is the smallest member of the IAP family in mammalian cells. The survivin gene is alternatively spliced extensively to generate several protein isoforms22 and is transcriptionally controlled in a sharp cell cycle-dependent manner, with peak expression at mitosis.23 Survivin blocks apoptosis by inhibiting several members of the caspases, such as caspase-9, caspase-3, and caspase-7.24,25 The powerful synthetic retinoid 4-HPR induces nitric oxide production leading to an increase in reactive oxygen species, which can subsequently destabilize the mitochondrial membrane.26 This facilitates the release of cytochrome c from mitochondria that eventually associates with apoptotic protease activating factor-1. This complex then binds to pro-caspase-9 and processes to generate active 37 kDa caspase-9. Active caspase-9 processes other caspases, including caspase-3 and caspase-7 leading to apoptosis.27 In the present study, we have observed significant increases in active forms of all these caspases for induction of apoptosis after treatment with 4-HPR. However, in the absence of survivin suppression or survivin knockdown, survivin blocks all active caspases and prevents apoptosis.24,25 Therefore, we knocked down survivin expression using siRNA and concurrently used 4-HPR treatment, which resulted in doubling the amount of apoptosis in human glioblastoma U251MG and U118MG cells.
Tumor cells adapt different prosurvival mechanisms including overexpression of antiapoptotic molecules such as Bcl-2 and survivin to grow indefinitely, thus circumventing normal cellular senescence or apoptosis so as to promote tumor growth.5,15 Targeting the anti-apoptotic factors for cancer treatment is supported by several findings, emphasizing the role of aberrant apoptosis in tumorigenesis and also resistance to cancer therapy. Evasion from apoptosis is critical for tumor growth and a hallmark of all tumor cells.28 Survivin is selectively expressed in transformed cells and in most human cancers, including glioblastomas.29 Increased expression of survivin in cancer patients is a dismal prognostic marker correlating with decreased overall survival. Survivin expression can be downregulated in cancer by several mechanisms, including the prevention of amplification of the survivin locus on chromosome 17q25,30 the use of survivin inhibitor such as YM155,31 and knockdown of survivin mRNA using cognate siRNA. In the present study, we successfully employed survivin siRNA to knockdown survivin mRNA and thereby its protein levels in 2 human glioblastoma cell lines in cultures as well as in tumors in nude mice.
The distinct ability of brain tumor cells to infiltrate the extracellular matrix of normal brain tissue makes it impossible to treat the highly invasive glioblastomas using surgery and radiation. The dislodgment of tumor cells from the primary site and their subsequent invasion of normal adjacent tissues is a characteristic feature of glioblastoma. MMPs, especially MMP-9, can play a significant role in tumor cell invasion through proteolytic degradation of the extracellular matrix. Tumor cell invasion through Matrigel and subsequently through a polycarbonate membrane with a particular pore size is an excellent technique to measure any change in the invasive potency of tumor cells after exposure to radiation or chemotherapy. Not all sorts of tumor cells have the ability to penetrate Matrigel and pass through a polycarbonate membrane with a particular pore size. However, almost all glioblastoma cells are highly invasive and posses this ability. In the present study, we used the technique of a Matrigel invasion assay to measure the ability of 2 highly invasive glioblastoma cell lines that invade Matrigel and then pass through a polycarbonate membrane of 12-µm pore size after survivin knockdown and/or 4-HPR treatment. The combination of survivin knockdown and 4-HPR treatment resulted in up to 90% inhibition in the ability of brain tumor cells to penetrate Matrigel and the polycarbonate membrane. We did not use any chemoattractant in the medium placed underneath the membrane. This indicated that the treated tumor cells lost the ability to secrete the required amount of proteolytic enzymes such as MMP-9 to degrade the matrix and pass through the membrane. We observed that survivin knockdown and 4-HPR treatment resulted in remarkable decreases in the expression of molecules such as PCNA, MMP-9, and FAK that could otherwise promote tumor cell proliferation and invasion.
Angiogenesis, the formation of new blood vessels from pre-existing ones, plays an important role in tumor progression and metastasis. Angiogenesis is a key regulatory factor in the progression and growth of malignant brain tumors, including anaplastic astrocytomas and glioblastomas. The development of an appropriate targeted molecular strategy to prevent angiogenesis is an important milestone in the therapeutic intervention against glioblastomas. The mechanism of angiogenesis is primarily mediated by hypoxia through chronic activation of the hypoxia-inducible factor pathway leading to the production of VEGF and bFGF.32,33 Angiogenesis in glioblastomas is regulated by a paracrine mechanism involving VEGF and Flt-1 (VEGF receptor 1). The endothelial-cell-specific mitogen VEGF is abundantly expressed in glioblastoma cells, which reside along necrotic areas, whereas Flt-1, a tyrosine kinase receptor for VEGF, is expressed in tumor endothelial cells but not in endothelial cells in normal adult brain.33 In the present study, we observed a remarkable decrease in neovascularization under the dorsal of the skin of nude mice after survivin knockdown and 4-HPR treatment. This suggested that the cells treated with the combination of both agents failed to secrete potent angiogenic factors such as VEGF and bFGF. Furthermore, we observed dramatic decreases in VEGF and bFGF and the angiogenesis marker CD31 in subcutaneous solid tumors after survivin knockdown and treatment with 4-HPR. Thus, the present combination therapy inhibited the secretion of potent angiogenic stimulants and subsequently prevented tumor progression.
In glioblastoma, the degree of survivin expression is a survival predictor and its overexpression is correlated with a poor survival rate.29 Survivin expression is often linked to resistance to chemotherapy, aggressive tumor behavior, and shortened survival.34 Suppression of survivin expression has enormous therapeutic potential in various cancers, including glioblastomas, since this molecule plays a key role in promotion of mitosis and prevention of programmed cell death.35 Survivin antisense oligonucleotides, siRNA, and dominant-negative constructs have shown efficacy on tumor cells, shutting off cell proliferation, causing mitotic defects often incompatible with cell cycle re-entry, and triggering spontaneous apoptosis. In contrast, these agents are for the most part harmless to normal cells and relatively well tolerated when systemically given as experimental anticancer regimens to mice.36 It should be verified via in vivo bioluminescent imaging whether the tumors are established at 3 days post-tumor cell injection into the brain before beginning the treatments. In the present study, we observed over 90% inhibition of intracerebral tumorigenesis and subcutaneous solid formation after the combination therapy with survivin knockdown and 4-HPR treatment. This suggested that the suppression of survivin during treatment with a powerful anticancer agent could pave the path to an effective treatment modality for glioblastomas.
In conclusion, the present study demonstrated that survivin knockdown and concurrent 4-HPR treatment effectively induced apoptosis and inhibited invasion, angiogenesis, and growth of glioblastomas through downregulation of molecules involved in these processes. Therefore, the combination of survivin knockdown and 4-HPR treatment can offer a novel therapeutic strategy for controlling the growth of human glioblastomas.
Conflict of interest statement. None declared.
Funding
This work was supported in part by R01 grants (CA-91460 and NS-57811 to S.K.R.) from the National Institutes of Health (Bethesda, MD, USA).
References
- 1.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. doi:10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 2.Drappatz J, Norden AD, Wen PY. Therapeutic strategies for inhibiting invasion in glioblastoma. Expert Rev Neurother. 2009;9:519–534. doi: 10.1586/ern.09.10. doi:10.1586/ern.09.10. [DOI] [PubMed] [Google Scholar]
- 3.George J, Banik NL, Ray SK. Bcl-2 siRNA augments taxol mediated apoptotic death in human glioblastoma U138MG and U251MG cells. Neurochem Res. 2009;34:66–78. doi: 10.1007/s11064-008-9659-z. doi:10.1007/s11064-008-9659-z. [DOI] [PubMed] [Google Scholar]
- 4.George J, Gondi CS, Dinh DH, Gujrati M, Rao JS. Restoration of tissue factor pathway inhibitor-2 in a human glioblastoma cell line triggers caspase-mediated pathway and apoptosis. Clin Cancer Res. 2007;13:3507–3517. doi: 10.1158/1078-0432.CCR-06-3023. doi:10.1158/1078-0432.CCR-06-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. doi:10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 6.Sah NK, Khan Z, Khan GJ, Bisen PS. Therapeutic strategies for inhibiting invasion in glioblastoma. Cancer Lett. 2006;244:164–171. doi: 10.1016/j.canlet.2006.03.007. doi:10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto H, Ngan CY, Monden M. Cancer cells survive with survivin. Cancer Sci. 2008;99:1709–1714. doi: 10.1111/j.1349-7006.2008.00870.x. doi:10.1111/j.1349-7006.2008.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247–3257. doi: 10.1074/jbc.M106643200. doi:10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- 9.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy. Expert Opin Ther Targets. 2008;12:463–476. doi: 10.1517/14728222.12.4.463. doi:10.1517/14728222.12.4.463. [DOI] [PubMed] [Google Scholar]
- 10.Sogno I, Venè R, Sapienza C, Ferrari N, Tosetti F, Albini A. Anti-angiogenic properties of chemopreventive drugs: fenretinide as a prototype. Recent Results Cancer Res. 2009;181:71–76. doi: 10.1007/978-3-540-69297-3_8. doi:10.1007/978-3-540-69297-3_8. [DOI] [PubMed] [Google Scholar]
- 11.Damodar Reddy C, Guttapalli A, Adamson PC, Vemuri MC, O'Rourke D, Sutton LN, et al. Anticancer effects of fenretinide in human medulloblastoma. Cancer Lett. 2006;231:262–269. doi: 10.1016/j.canlet.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Simeone AM, Tari AM. How retinoids regulate breast cancer cell proliferation and apoptosis. Cell Mol Life Sci. 2004;61:1475–1484. doi: 10.1007/s00018-004-4002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiwari M, Kumar A, Sinha RA, Shrivastava A, Balapure AK, Sharma R, et al. Mechanism of 4-HPR-induced apoptosis in glioma cells: evidences suggesting role of mitochondrial-mediated pathway and endoplasmic reticulum stress. Carcinogenesis. 2006;27:2047–2058. doi: 10.1093/carcin/bgl051. doi:10.1093/carcin/bgl051. [DOI] [PubMed] [Google Scholar]
- 14.Lovat PE, Corazzari M, Goranov B, Piacentini M, Redfern CP. Molecular mechanisms of fenretinide-induced apoptosis of neuroblastoma cells. Ann N Y Acad Sci. 2004;1028:81–89. doi: 10.1196/annals.1322.009. doi:10.1196/annals.1322.009. [DOI] [PubMed] [Google Scholar]
- 15.George J, Banik NL, Ray SK. Combination of taxol and Bcl-2 siRNA induces apoptosis in human glioblastoma cells and inhibits invasion, angiogenesis, and tumour growth. J Cell Mol Med. 2009;13:4205–4218. doi: 10.1111/j.1582-4934.2008.00539.x. doi:10.1111/j.1582-4934.2008.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George J, Tsutsumi M. siRNA-mediated knockdown of connective tissue growth factor prevents N-nitrosodimethylamine-induced hepatic fibrosis in rats. Gene Ther. 2007;14:790–803. doi: 10.1038/sj.gt.3302929. doi:10.1038/sj.gt.3302929. [DOI] [PubMed] [Google Scholar]
- 17.Tolboom TC, Huizinga TW. In vitro matrigel fibroblast invasion assay. Methods Mol Med. 2007;135:413–422. doi: 10.1007/978-1-59745-401-8_27. doi:10.1007/978-1-59745-401-8_27. [DOI] [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. doi:10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Sckell A, Leunig M. Dorsal skinfold chamber preparation in mice. In: Murray JC, editor. Angiogenesis Protocols: Methods in Molecular Medicine. Vol 46. Totowa, NJ: Humana Press; 2001. pp. 95–105. [DOI] [PubMed] [Google Scholar]
- 20.Wachsberger PR, Burd R, Marero N, Daskalakis C, Ryan A, McCue P, et al. Effect of the tumor vascular-damaging agent, ZD6126, on the radioresponse of U87 glioblastoma. Clin Cancer Res. 2005;11:835–842. [PubMed] [Google Scholar]
- 21.Wang H, Maurer BJ, Liu YY, Wang E, Allegood JC, Kelly S, et al. N-(4-hydroxyphenyl)retinamide increases dihydroceramide and synergizes with dimethylsphingosine to enhance cancer cell killing. Mol Cancer Ther. 2008;7:2967–2976. doi: 10.1158/1535-7163.MCT-08-0549. doi:10.1158/1535-7163.MCT-08-0549. [DOI] [PubMed] [Google Scholar]
- 22.Sampath J, Pelus LM. Alternative splice variants of survivin as potential targets in cancer. Curr Drug Discov Technol. 2007;4:174–191. doi: 10.2174/157016307782109652. doi:10.2174/157016307782109652. [DOI] [PubMed] [Google Scholar]
- 23.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. doi:10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Chandele A, Prasad V, Jagtap JC, Shukla R, Shastry PR. Upregulation of survivin in G2/M cells and inhibition of caspase 9 activity enhances resistance in staurosporine-induced apoptosis. Neoplasia. 2004;6:29–40. doi: 10.1016/s1476-5586(04)80051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, et al. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry. 2001;40:1117–1123. doi: 10.1021/bi001603q. [DOI] [PubMed] [Google Scholar]
- 26.Simeone AM, Ekmekcioglu S, Broemeling LD, Grimm EA, Tari AM. A novel mechanism by which N-(4-hydroxyphenyl)retinamide inhibits breast cancer cell growth: the production of nitric oxide. Mol Cancer Ther. 2002;1:1009–1017. [PubMed] [Google Scholar]
- 27.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. doi:10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. doi:10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 29.Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ, et al. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20:1063–1068. doi: 10.1200/JCO.2002.20.4.1063. doi:10.1200/JCO.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 30.Islam A, Kageyama H, Takada N, Kawamoto T, Takayasu H, Isogai E, et al. High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19:617–623. doi: 10.1038/sj.onc.1203358. doi:10.1038/sj.onc.1203358. [DOI] [PubMed] [Google Scholar]
- 31.Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–5203. doi: 10.1200/JCO.2008.17.2064. doi:10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argyriou AA, Giannopoulou E, Kalofonos HP. Angiogenesis and anti-angiogenic molecularly targeted therapies in malignant gliomas. Oncology. 2009;77:1–11. doi: 10.1159/000218165. doi:10.1159/000218165. [DOI] [PubMed] [Google Scholar]
- 33.Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59:520–529. doi: 10.1002/ijc.2910590415. doi:10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 34.Altieri DC. New wirings in the survivin networks. Oncogene. 2008;27:6276–6284. doi: 10.1038/onc.2008.303. doi:10.1038/onc.2008.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. doi:10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 36.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. doi:10.1038/nrc968. [DOI] [PubMed] [Google Scholar]