Abstract
For gamma knife planning, 2.4-mm-slice MRIs are taken under rigid frame fixation, so tiny tumors become visible. This study evaluated differences in the numbers of brain metastases between conventional contrast-enhanced MRI (6 ± 1 mm slice thickness) taken before patient referral and contrast-enhanced MRI for gamma knife planning. The numbers of metastases on the 2 images were counted by at least 2 oncologists. For gamma knife planning, spoiled gradient-recalled echo images were obtained after 0.1 mmol/kg gadolinium administration using a 1.5-T system. Images from 1045 patients with an interval between the 2 MRI studies of 6 weeks or less were analyzed. Increases in the number of metastases were found in 33.7% of the 1045 patients, whereas the number was identical in 62.3%. In 4.0%, the number decreased, indicating overdiagnosis at conventional MRI. These proportions did not differ significantly by the interval before gamma knife. An increase from single to multiple metastases was found in 16.0%. Meningeal dissemination was newly diagnosed in 2.3%. On planning images, the proportions of patients with 1, 2, 3, and 4 or more lesions were 37.6%, 19.3%, 9.3%, and 33.8%, respectively. In cases of colorectal cancer and hepatoma, the proportions of patients with a single metastasis (32 of 61 [52%] and 5 of 6 [83%], respectively) were higher than that of patients with other malignancies. In about one-third of the patients, an increased number of metastases were found on the thin-slice images. This should be kept in mind when deciding the treatment strategy for brain metastases.
Keywords: brain metastasis, frame-fixed, gamma knife, radiosurgery, thin-slice MRI
Metastatic brain tumors are the most common intracranial neoplasm in adults, occurring in approximately 10%–30% of adult cancer patients.1 The incidence of brain metastases is increasing because of an aging population, improvements in the systemic treatment of cancers, and advances in imaging modalities, such as MRI, to detect small metastases at follow-up screening examinations.1 MRI is a more sensitive diagnostic tool than CT for identifying brain metastases.2–5 One study showed that one-third of patients for whom a single brain metastasis was observed on contrast-enhanced CT had multiple brain metastases on gadolinium-enhanced MRI.4 At present, gadolinium-enhanced MRI is considered to be the imaging technique of choice for patients suspected of having brain metastases.6–8 However, recent guidelines and current opinions concerning the treatment of brain metastases are based on studies in which metastases were diagnosed through CT.9–11 It is debatable whether recommendations originating from these studies are applicable to all patients with brain metastases diagnosed through MRI. Recent developments in MRI techniques have unquestionably made an enormous contribution to further improving the detection of brain metastases. With the improvement in MRI techniques, more metastases may be detected, and the proportion of patients with a single brain metastasis may decrease compared with that estimated using conventional techniques.
Since the treatment strategy for brain metastases should change with the number of metastases, determining the exact number is of clinical importance. This study was undertaken to investigate the influence of changing the diagnostic technique from conventional MRI to MRI for gamma knife surgery (GKS) planning on the detected number of brain metastases. In particular, it was considered valuable to determine the proportion of patients who were initially suspected of having a single metastasis on conventional MRI but proved to have multiple metastases on thin-slice, frame-fixed MRI.
Materials and Methods
Patients
Patients included those treated at Nagoya Radiosurgery Center where GKS began in March 2004; since then, the MRI system has remained the same. Between March 2004 and November 2009, 1772 patients with various intracranial lesions were treated with GKS. Our criteria at the time of patient referral for accepting patients with brain metastases for GKS were: (i) maximum tumor diameter <3 cm; (ii) tumor number <10; (iii) no cerebrospinal fluid (CSF) dissemination; and (iv) Karnofsky performance status >50. During the period, 1086 patients with 4304 metastases fulfilling the following criteria were identified and they were included in the present study: (i) those with parenchymal brain metastases diagnosed with contrast-enhanced T1-weighted MRIs of 6 + 1 mm slice thickness, using a 1.5-T imager and a standard dose of a gadolinium agent (0.1 mmol/kg body weight) before referral to the radiosurgery center; (ii) those undergoing GKS within 90 days after the latest MRI; (iii) those receiving no chemotherapy since the latest MRI; (iv) those with no previous GKS, brain radiotherapy, or surgery; and (v) those who gave written informed consent.
MRI for GKS Planning
The planning MRI for GKS was performed under Leksell stereotactic frame fixation with local anesthesia using a 1.5-T imager (Echo Speed 1.5T; GE Healthcare) equipped with a radiofrequency coil (Quadrature head coil; GE Healthcare), with a maximum amplitude of gradients of 33 mT/m and a maximum slew rate of 120 T/m/s. A standard dose of gadolinium diethylene triamine pentaacetic acid (0.1 mmol/kg body weight) was administered intravenously 10 minutes before acquisition of contiguous 2.4-mm spoiled gradient-recalled echo (SPGR) axial images (repetition time/echo time, 14.9/4.2 milliseconds; 30° flip angle; 256 × 256 matrix; 240 × 240 field of view; number of sections, 60; acquisition time, 360–420 seconds). Although these axial SPGR images were the key images for determining the tumor number and GKS planning in all cases, T2-weighted axial images and/or coronal/sagittal SPGR images were added on demand and were also used for diagnosis.
Image Analysis
The number of brain metastases on conventional MRIs (contrast-enhanced T1-weighted axial images) at referral was determined by the consensus of at least 2 radiation oncologists/neurosurgeons who were well trained in neuro-onocology imaging and had more than 10 years of neuroradiology experience. Reports from referring physicians were also taken into account, but we made the final decision on the number. Usually, other images such as T2-weighted and fluid-attenuated inversion recovery images were also available, with or without sagittal and/or coronal images, and the findings from these images were also taken into account. The number of brain metastases on GKS planning images was also determined by consensus of at least 2 of the radiation oncologists/neurosurgeons. These 2 evaluations were performed separately, and no feedback of the diagnosis was made to the initial diagnosis of brain metastases and tumor numbers.
Statistical Methods
The Mann–Whitney U-test was used for examining differences in time intervals between the conventional MRI and GKS imaging between the 2 groups with and without an increase in tumor number. The Spearman rank-correlation coefficient was used to examine the differences between the number of lesions identified on the conventional MRI and that identified on GKS imaging. To examine the differences in the proportion of patients with an increase in tumor number between pairs of groups, Fisher's exact test (for gender and primary site) or the Mann–Whitney U-test (for age) was used. Fisher's exact test was used to examine the differences in the proportion of multiple metastases according to the primary tumor site. A 2-sided P-value of .05 or less was considered to reflect statistical significance. All of these analyses were carried out using Dr SPSSII (SAS Institute Inc.).
Results
Table 1 shows characteristics of the patients at the time of the GKS. The mean age of the 1086 patients was 65 years (range: 30–91), and the male:female ratio was 1.7:1. The average of the interval between the conventional MRI before referral and the GKS imaging was 15.2 ± 11.3 days (range 1–85 days). To examine the influence of the interval before GKS, the patients were divided into 2 groups consisting of those with and without an increase in tumor number at planning MRI. The average intervals (±SD) between the 2 MRI studies were 16.1 ± 11.3 days for the group with an increase in tumor number and 14.8 ± 11.3 days for the group without an increase (P = .049), indicating that the number of brain metastases tended to increase with the interval. Therefore, patients with longer intervals were removed stepwise, and it was found that the intervals between the 2 groups were not different when only 1045 patients with an interval of 42 days or shorter were analyzed. For these 1045 patients, the average interval (±SD) between the 2 MRI procedures was 14.6 ± 9.2 days (range: 1–42 days) in the group with an increase in tumor number and 13.4 ± 8.5 days in the group with no increase (P = .063). For further analysis, mainly these 1045 patients with 4138 metastases were used; their median age was 65 years (range: 30–91) and the male: female ratio was 1.7:1.
Table 1.
Patient characteristics at the time of gamma knife surgery
| Patient characteristics | Interval between 2 MRI studies (d) |
||
|---|---|---|---|
| 1–85 | 1–42 | 1–14 | |
| Total number of patients | 1086 | 1045 | 640 |
| Total number of metastases | 4304 | 4138 | 2548 |
| Gender | |||
| Male | 683 (62.9%) | 651 (62.3%) | 403 (63.0%) |
| Female | 403 (37.1%) | 394 (37.7%) | 237 (37.0%) |
| Age (yrs; median [range]) | 65 [30–91] | 65 [30–91] | 66 [30–91] |
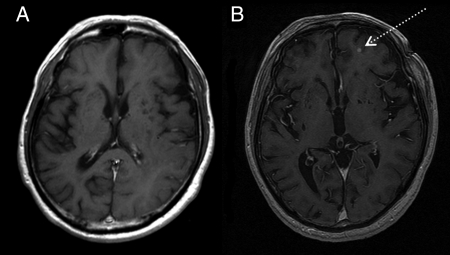
On GKS planning MRI, 1277 additional metastases were identified in 352 of the 1045 patients (33.7%). All newly detected lesions were identified as well-enhanced small masses (<5 mm) unaccompanied by edema or hemorrhage. Among the 352 patients with an increase in tumor number, CSF dissemination was also newly diagnosed in 8 patients (2.3%); newly diagnosed CSF dissemination was not counted as a new metastasis. For each patient, 1–39 additional metastases were identified with a mean of 4. A single additional lesion was identified in 37.8% of the 352 patients. Figure 1 shows conventional and planning MRIs for a patient in whom an additional metastasis is clearly depicted on MRI for GKS planning.
Fig. 1.
Comparison of a conventional contrast-enhanced MR image (A) and a contrast-enhanced SPGR image (B) for GKS planning. The interval between the 2 studies was 9 days. The latter clearly depicts a small lesion in the left frontal lobe (arrow).
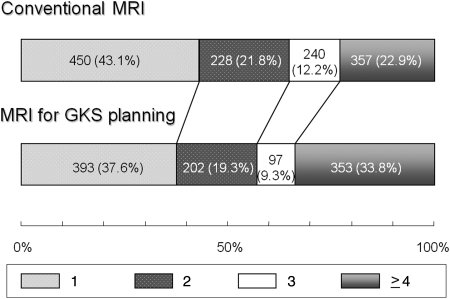
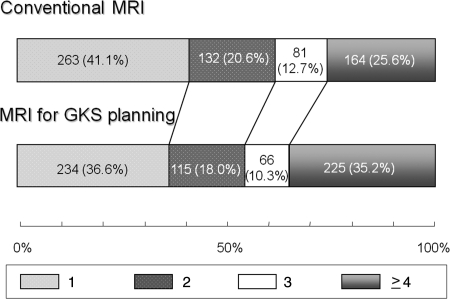
Figure 2 shows the proportions of patients with respective numbers of brain metastases at conventional and planning MRI among the 1045 patients. The proportion of patients detected as having a single metastasis decreased from 43.1% at conventional MRI to 37.6% at planning MRI (P = .001). In 450 patients found to have a single metastasis on conventional MRI, 72 (16.0%) proved to have multiple metastases. The proportion of patients found to have 4 or more metastases increased from 22.9% at conventional MRI to 33.8% at planning MRI (P = .001). In 33.7% of the patients, the identified number of tumors increased, whereas in 62.3% tumor numbers were identical. In the remaining 4.0%, the identified tumor number decreased at planning MRI. To further eliminate the influence of the length of the interval between the 2 MR studies, 640 patients with an interval of 14 days or less were similarly analyzed. Figure 3 shows the proportions of patients with respective numbers of brain metastases at conventional and planning MRI among the 640 patients. The results were similar to those obtained for the 1045 patients with an interval of 42 days or shorter.
Fig. 2.
Proportions of patients with 1, 2, 3, or 4 or more brain metastases at conventional and planning MRI among 1045 patients with an interval between the 2 MRI studies of 6 weeks or less. Figures in the bars indicate the actual numbers of patients.
Fig. 3.
Proportions of patients with 1, 2, 3, or 4 or more brain metastases at conventional and planning MRI among 640 patients with an interval between the 2 MRI procedures of 2 weeks or less. Figures in the bars indicate the actual numbers of patients.
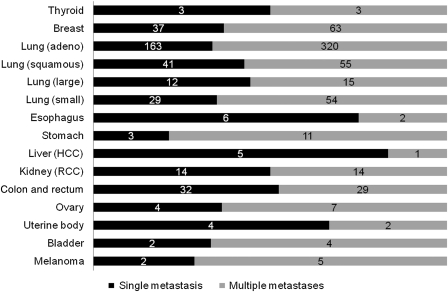
The proportion of patients with an increase in tumor number was analyzed with respect to the primary tumor site, patient age, and gender, but there were no differences in the proportions according to these factors. Figure 4 shows the proportion of single or multiple metastases at MRI for GKS planning according to the primary tumor site. In 61 patients with colorectal cancer, 32 (52%) had a single metastasis, and this proportion was significantly higher than that among 1025 patients with noncolorectal malignant tumor (376 of 1025 [36.7%], P = .020). In 6 patients with hepatoma, 5 (83%) had a single metastasis, and this proportion was significantly higher than that among 1080 patients with nonhepatoma malignancy (403 of 1080 [37.3%], P = .030).
Fig. 4.
Proportions of patients with single or multiple brain metastases at planning MRI for GKS according to the primary lesion. All analyzed patients were included, irrespective of the interval from conventional MRI. Figures in the bars indicate the actual numbers of patients. HCC, hepatocellular carcinoma; RCC, renal cell carcinoma.
In principle, newly detected lesions were also treated by GKS. At maximum, a total of 19 lesions were treated, but when the total tumor number was 20 or more, smaller lesions were not treated and whole-brain radiotherapy (WBRT) was recommended after GKS.
Discussion
Treatment strategy for brain metastases differs with the number of metastases. For a single brain metastasis, surgical resection may be indicated, but since the introduction of stereotactic irradiation (STI), the role of surgery has greatly diminished. In the United States, WBRT may even now be regarded as the gold standard of treatment for a single brain metastasis, but in Japan, such patients are most often treated by STI.12,13 Evidence regarding the choice of treatment is limited, but there may be a consensus that WBRT can decrease intracranial recurrences at distant sites compared with STI or surgery alone. In a randomized trial, Patchell et al.14 compared surgery alone and surgery followed by WBRT for a single brain metastasis; the recurrence rate at other sites in the brain was 37% vs 14%. Chougule et al.15 conducted a randomized study comparing GKS, WBRT, and GKS + WBRT in patients with 1–3 metastases, and the occurrence rate of new brain lesions was 43%, 23%, and 19%, respectively. Aoyama et al.16 compared single-fraction stereotactic radiosurgery (SRS) alone with WBRT + SRS in patients with 1–4 metastases; the 12-month actuarial rate of developing new brain metastases was 64% vs 42%. Therefore, all of these studies indicated that WBRT is useful in treating occult metastases. Results of the present study also suggest the usefulness of WBRT. WBRT has recently been shown to seldom produce dementia in patients with brain metastases.17 Recently, Kwon et al.18 concluded that WBRT plus hypofractionated stereotactic radiotherapy provides a high level of tumor control with minimal toxicity comparable to single-fraction SRS. In view of these recent trends, therefore, it is considered important to diagnose the number of metastases with the highest diagnostic accuracy and to determine the indication of SRS-alone treatment deliberately.
The number of identified brain metastases is known to change with the imaging modality and method and the use and dose of contrast media. Shalen et al.19 found that a high-dose infusion of contrast media followed by delayed CT imaging increased sensitivity for detecting metastases by as much as 67% compared with immediate CT scanning. If diagnosis had been based solely on the findings of CT performed immediately after contrast administration, 11.5% of the studies would have produced false-negative results.20 Then, the value of gadolinium-enhanced MRI vs single- or double-dose (delayed) CT for the diagnosis of brain metastases from solid tumors was investigated.21–23 From these studies, it was concluded that gadolinium-enhanced MRI is superior to contrast-enhanced CT.24 Thereafter, Sze et al.6 recommended contrast enhancement for the detection of brain metastasis, and Yuh et al.25 suggested high-dose (0.3 mmol/kg) gadolinium for the detection of early or small metastases. Higher-contrast doses were judged to be better than delayed imaging with standard-contrast doses. Sze et al.26 suggested the beneficial effects of a triple dose in cases of equivocal findings or solitary metastasis. However, the use of the more expensive triple-dose regimen for routine screening is not warranted. Moreover, enhancement itself is not necessarily specific to metastases. Reviewers identified false-positive cases more frequently in triple-dose studies. This was attributed to an increase in detected artifacts, better vascular demonstration, and nontumoral enhancement, such as vascular malformations. Ginsberg and Lang27 argued for the usefulness of postcontrast magnetization transfer saturation imaging rather than triple-dose gadolinium. In addition, Haba et al.28 concluded that a half-dose MRI with magnetization transfer saturation can replace a single-dose MRI. Elster and Chen29 concluded that nonenhancing white matter abnormalities have a low probability of representing metastatic disease.
MRI devices continue to be improved. The 3.0-T units are currently available and may be superior to the more commonly used 1.5-T imagers. Eighty-four vs 81 brain metastases were detected when comparing 3.0- with 1.5-T triple-dose MRI, respectively. The signal contrast to noise ratio has also been improved.30 The present study showed that even with a 1.5-T imager, the identified tumor number can increase with thin-slice imaging under rigid frame fixation that prevents even subtle movement of the head. The decrease in tumor number seen in 4.0% of the patients also suggests that the imaging procedure for GKS planning is more accurate in the identification of nontumorous contrast enhancement including artifacts, vessels, and initial small cerebral infarctions.
According to the previous autopsy and CT imaging studies, the rate of multiple brain metastases ranges from 58% to 86%, with a mean of 66%; however, in an MRI-based study, only 19% of 336 patients had a single lesion. The percentages of patients with 2, 3, 4, and 5 or more lesions were 16%, 13%, 10%, and 40%, respectively.31 In the present study, the subjects were patients who were considered to be appropriate for GKS, so the proportion of patients with a single metastasis (37.6%) appears to be higher than the true incidence. The proportion of such patients decreased by 5.5% (from 43.1% to 37.6%) using the 2.4-mm-slice MRI under rigid frame fixation. Sixteen percent of 450 patients who were diagnosed as having a single metastasis on conventional MRI proved to have multiple metastases. If a 3.0-T imager and triple-dose gadolinium were used, this proportion might increase further.32 Therefore, oncologists must be aware of our findings when determining the optimal treatment strategy.
Conclusions
In about one-third of the patients in this study, increased numbers of metastases were found on the thin-slice SPGR images for GKS planning. An increase from single to multiple metastases was found in 16.0%. Physicians should keep these findings in mind when deciding treatment strategy for brain metastases.
Acknowledgments
The authors are grateful to Dr Naoki Hayashi, Mr Hideto Nakazawa, Mr Takashi Kimura, and Ms Yukari Miwa for their valuable help in this research.
Conflict of interest statement. None declared.
References
- 1.Wen PY, Black PM, Loeffler JS. Cancer: principles and practice of oncology. In: DeVita V, Hellman S, Rosenberg SA, editors. Metastatic Brain Cancer. 6th ed. Philadelphia: Lippincott, Williams, & Wilkins; 2001. pp. 2655–2670. [Google Scholar]
- 2.Davis PC, Hudgins PA, Peterman SB, Hoffman JC., Jr Diagnosis of cerebral metastases: double-dose delayed CT vs contrast-enhanced MR imaging. Am J Neuroradiol. 1991;12:293–300. [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki K, Yamamoto M, Hasegawa Y, et al. Magnetic resonance imaging and computed tomography in the diagnoses of brain metastases of lung cancer. Lung Cancer. 2004;46:357–360. doi: 10.1016/j.lungcan.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol. 1999;44:275–281. doi: 10.1023/a:1006308808769. [DOI] [PubMed] [Google Scholar]
- 5.Akeson P, Larsson EM, Kristoffersen DT, Jonsson E, Holtas S. Brain metastases—comparison of gadodiamide injection-enhanced MR imaging at standard and high dose, contrast-enhanced CT and non-contrast-enhanced MR imaging. Acta Radiol. 1995;36:300–306. [PubMed] [Google Scholar]
- 6.Sze G, Milano E, Johnson C, Heier L. Detection of brain metastases: comparison of contrast-enhanced MR with unenhanced MR and enhanced CT. Am J Neuroradiol. 1990;11:785–791. [PMC free article] [PubMed] [Google Scholar]
- 7.Davey P. Brain metastases: treatment options to improve outcomes. CNS Drugs. 2002;16:325–338. doi: 10.2165/00023210-200216050-00005. [DOI] [PubMed] [Google Scholar]
- 8.Kaal EC, Taphoorn MJ, Vecht CJ. Symptomatic management and imaging of brain metastases. J Neurooncol. 2005;75:15–20. doi: 10.1007/s11060-004-8094-5. [DOI] [PubMed] [Google Scholar]
- 9.Soffietti R, Cornu P, Delattre JY, et al. EFNS Guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. Eur J Neurol. 2006;13:674–681. doi: 10.1111/j.1468-1331.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- 10.Soffietti R, Costanza A, Laguzzi E, Nobile M, Ruda R. Radiotherapy and chemotherapy of brain metastases. J Neurooncol. 2005;75:31–42. doi: 10.1007/s11060-004-8096-3. [DOI] [PubMed] [Google Scholar]
- 11.Kaal EC, Niel CG, Vecht CJ. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4:289–298. doi: 10.1016/S1474-4422(05)70072-7. [DOI] [PubMed] [Google Scholar]
- 12.McPherson CM, Suki D, Feiz-Erfan I, et al. Adjuvant whole-brain radiation therapy after surgical resection of single brain metastases [published online ahead of print February 14, 2010] Neuro-Oncology. doi: 10.1093/neuonc/noq005. doi:10.1093/neuonc/noq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibamoto Y, Sugie C, Iwata H. Radiotherapy for metastatic brain tumors. Int J Clin Oncol. 2009;14:281–288. doi: 10.1007/s10147-009-0915-2. [DOI] [PubMed] [Google Scholar]
- 14.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 15.Chougule PB, Burton-Williams M, Saris S, et al. Randomized treatment of brain metastasis with gamma knife radiosurgery, whole brain radiotherapy or both. Int J Radiat Oncol Biol Phys. 2000;48(suppl):114. [Google Scholar]
- 16.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 17.Shibamoto Y, Baba F, Oda K, et al. Incidence of brain atrophy and decline in mini-mental state examination score after whole-brain radiotherapy in patients with brain metastases: a prospective study. Int J Radiat Oncol Biol Phys. 2008;72:1168–1173. doi: 10.1016/j.ijrobp.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 18.Kwon AK, Dibiase SJ, Wang B, Hughers SL, Milcarek B, Zhu Y. Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer. 2009;115:890–898. doi: 10.1002/cncr.24082. [DOI] [PubMed] [Google Scholar]
- 19.Shalen PR, Hayman LA, Wallace S, Handel SF. Protocol for delayed contrast enhancement in computed tomography. Radiology. 1981;139:397–401. doi: 10.1148/radiology.139.2.7220885. [DOI] [PubMed] [Google Scholar]
- 20.Sze G, Stimac GK, Bartlett C, et al. Multicenter study of gadopentetate dimeglumine as an MR contrast agent: evaluation in patients with spinal tumors. Am J Neuroradiol. 1990;11:967–974. [PMC free article] [PubMed] [Google Scholar]
- 21.Mastronardi L, Lunardi P, Puzzilli F, Schettini G, Lo Bianco F, Ruggeri A. The role of MRI in the surgical selection of cerebral metastases. Zentralbl Neurochir. 1999;60:141–145. [PubMed] [Google Scholar]
- 22.Yuh WT, Engelken JD, Muhonen MG, Mayr NA, Fisher DJ, Ehrhardt JC. Experience with high-dose gadolinium MR imaging in the evaluation of brain metastases. Am J Neuroradiol. 1992;13:335–345. [PMC free article] [PubMed] [Google Scholar]
- 23.Yuh WT, Fisher DJ, Runge VM, et al. Phase III multicenter trial of high-dose gadoteridol in MR evaluation of brain metastases. Am J Neuroradiol. 1994;15:1037–1051. [PMC free article] [PubMed] [Google Scholar]
- 24.Seute T, Leffers P, ten Velde GP, Twijinstra A. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI) Cancer. 2008;112:1827–1834. doi: 10.1002/cncr.23361. [DOI] [PubMed] [Google Scholar]
- 25.Yuh WT, Tali ET, Nguyen HD, Simonson TM, Mayr NA, Fisher DJ. The effect of contrast dose, imaging time, and lesion size in the MR detection of intracerebral metastasis. Am J Neuroradiol. 1995;16:373–380. [PMC free article] [PubMed] [Google Scholar]
- 26.Sze G, Johnson C, Kawamura Y, et al. Comparison of single- and triple-dose contrast material in the MR screening of brain metastases. Am J Neuroradiol. 1998;19:821–828. [PMC free article] [PubMed] [Google Scholar]
- 27.Ginsberg LE, Lang FF. Neuroradiologic screening for brain metastases—can quadruple dose gadolinium be far behind? Am J Neuroradiol. 1998;19:829–830. [PMC free article] [PubMed] [Google Scholar]
- 28.Haba D, Pasco Papon A, Tanguy JY, Burtin P, Aube C, Caron-Poitreau C. Use of half-dose gadolinium-enhanced MRI and magnetization transfer saturation in brain tumors. Eur Radiol. 2001;11:117–122. doi: 10.1007/s003300000493. [DOI] [PubMed] [Google Scholar]
- 29.Elster AD, Chen MY. Can nonenhancing white matter lesions in cancer patients be disregarded? Am J Neuroradiol. 1992;13:1309–1315. [PMC free article] [PubMed] [Google Scholar]
- 30.Ba-Ssalamah A, Nobauer-Huhmann IM, Pinker K, et al. Effect of contrast dose and field strength in the magnetic resonance detection of brain metastases. Invest Radiol. 2003;38:415–422. doi: 10.1097/01.RLI.0000067488.57101.bd. [DOI] [PubMed] [Google Scholar]
- 31.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastases. J Clin Oncol. 2006;24:1295–1303. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 32.Donahue BR, Goldberg JD, Golfinos JG, et al. Importance of MR technique for stereotactic radiosurgery. Neurooncology. 2003;5:268–274. doi: 10.1215/S1152851703000048. [DOI] [PMC free article] [PubMed] [Google Scholar]