Pleuropulmonary blastoma (PPB) is a rare pediatric tumor of the lung that arises during fetal and infant lung development and is part of an inherited cancer syndrome (OMIM entry 601200). PPBs are composed of both epithelial and mesenchymal components. The early stage of disease features cystic expansion of the airspaces lined by benign-appearing epithelium. Mesenchymal cells susceptible to malignant transformation reside within the cyst walls and form a dense layer beneath the lining epithelium known as the cambium layer. Sarcomatous overgrowth by the mesenchymal cells growing as solid masses dictates clinical outcome for children with this tumor, however, not all cystic PPBs are destined to progress to sarcomas (1). The natural history of the tumor suggests a multi-step genetic pathogenesis (2).
Approximately 20% of children with PPB have a family history of pediatric neoplasia, most notably cystic nephroma of the kidney and rhabdomyosarcoma (3). We mapped the PPB locus to chromosome 14q with a family-based linkage study on four families with inherited predisposition to PPB (4) (Fig. S1, S2). Of 72 genes within the 7 Mb candidate region, DICER1, was an attractive candidate because of its known role in lung development. DICER1 encodes a ~218 kDa RNase III endonuclease that is a key component of a highly conserved cellular pathway responsible for generation of small RNAs (miRNAs and siRNAs) (5). miRNAs negatively regulate gene expression and appear to play critical roles in stem cell maintenance, organogenesis, cell cycle progression, and oncogenesis. Conditional loss of Dicer1 in the developing mouse lung results in cystic airway dilation, disruption of branching morphogenesis and mesenchymal expansion that resembles the early stage of PPB (6).
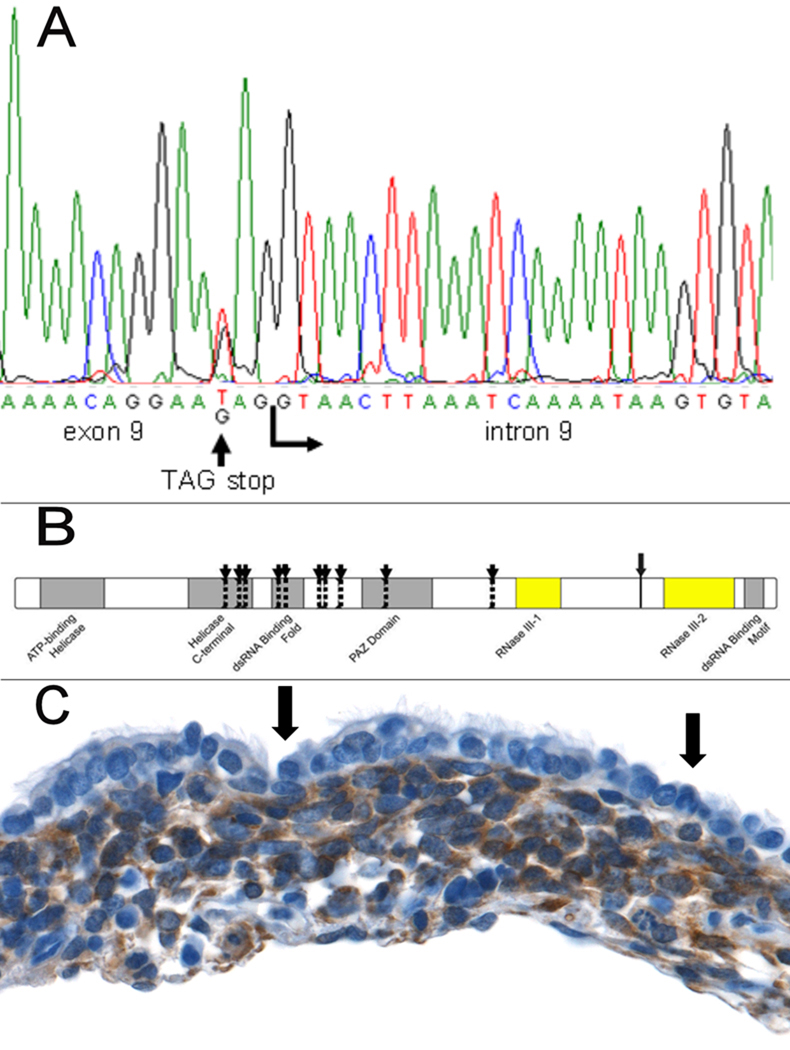
Heterozygous germline mutations in DICER1 were identified by direct sequencing in affected members from each of 11 families (four included in the linkage study and seven additional families) (Fig. 1A, Fig. S3, Table S1). For ten of the 11 PPB families, the observed mutations result in proteins truncated proximal to DICER1’s two carboxy-terminal RNase III functional domains (Fig. 1B) and therefore are likely loss of function defects. The missense mutation (L1583R) detected in the 11th family (family C) involves an evolutionary conserved amino acid and the non-polar to polar change was neither a previously reported sequence variant (NCBI SNP database Build 128) nor was it detected in 360 cancer-free controls tested by Pyrosequencing.™
Figure 1. DICER1 mutations and expression in PPB.
(A) DICER1 sequence alteration identified in one of the families in the linkage study. (B) Protein locations of DICER1 mutations in 11 PPB families. Vertical dotted lines with arrows indicate the location of truncating or insertions/deletions in 10 families. Each of these occurs proximal to the RNase III domains. The larger arrow with the solid line marks the location of the missense mutation between the RNase III domains. (C) DICER1 protein is absent in benign-appearing tumor-associated epithelium (arrows) and is present in the mesenchymal tumor cells forming the cambium layer underneath (anti-DICER1 with brown chromagen and hematoxylin counterstain; original magnification x400).
Apart from PPB-associated tumors in a subset of family members, the majority of obligate carriers with DICER1 mutations are phenotypically normal indicating that loss of one DICER1 allele is compatible with normal development and insufficient for tumor formation. Consistent with this observation, mice haploinsufficient for Dicer1 show no overt phenotypic abnormalities (7). DICER1 immunohistochemistry of PPB tumors suggests expression from the wild-type allele is lost in tumor-associated epithelium in six of the seven families harboring PPBs with a residual epithelial cystic component, but is retained in the mesenchymal tumor cells (Fig. 1C, Fig. S5). The presence of inactivating missense mutations in mesenchymal tumor cells from all 10 families assayed is considered unlikely. The areas of absent staining in the tumoral epithelium were segmental in most cases but were clearly evident in areas overlying cambium layers. The genetic basis behind this altered expression in epithelium is currently unknown but the phenotype recapitulates that seen in the Dicer1−/− conditional mouse model. Interestingly, the tumoral epithelial cells lacking DICER1 protein expression were morphologically normally differentiated.
Based on our preliminary studies, we hypothesize that PPB is initiated through a novel mechanism of non-cell autonomous cancer initiation and speculate that loss of DICER1 in the epithelium of the developing lung adversely impacts production of diffusible factors that regulate mesenchymal growth and predisposes these cells to additional genetic aberrations. If, or how, DICER1 haploinsufficiency in the mesenchymal cells contributes to pathogenesis remains an open question. Given that hereditary PPB is associated with an increased risk for development of other more common malignancies, DICER1-dependent tumor suppressive mechanisms uncovered in PPB may also apply to other more common cancers.
Supplementary Material
Footnotes
Supporting Online Material
Materials and Methods
Supplemental Text
Figures S1 to S5
Tables S1 to S5
Supplemental References
References
- 1.Hill DA, et al. Am. J. Surg. Pathol. 2008;32:282. doi: 10.1097/PAS.0b013e3181484165. [DOI] [PubMed] [Google Scholar]
- 2.Priest JR, et al. Cancer. 1997;80:147. [PubMed] [Google Scholar]
- 3.Priest JR, et al. J. Pediatr. 1996;128:220. doi: 10.1016/s0022-3476(96)70393-1. [DOI] [PubMed] [Google Scholar]
- 4.Materials and methods are available as supporting online material on Science Online
- 5.Bartel DP. Cell. 2004;116:281. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X. Proc. Natl. Acad. Sci. U. S. A. 2006;103:2208. doi: 10.1073/pnas.0510839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morita S, et al. PLOS One. 2009;4:e4212. doi: 10.1371/journal.pone.0004212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.