Abstract
Objectives
Prospectively assess effects of select dietary fats on cognitive decline
Design
Prospective observational; 3-year follow-up
Setting
Subjects recruited at Northwestern University who participated in Women's Health Initiative Observational Study or control group of Diet Modification arm.
Participants
482 women ≥ 60 years
Measurements
We averaged dietary intake from a validated food frequency questionnaire (FFQ) administered twice (mean=2.7 years apart) before baseline cognitive assessment (mean=2.9 years after 2nd FFQ). Testing of memory, vision, executive function, language, and attention was performed at 2 time points, 3 years apart. We created a global Z-score for both time points by averaging all Z-scores for each participant and defined global cognitive change as the difference between follow-up and baseline Z-scores.
Results
Median intakes of saturated fats (SFA), trans-fats, (TFA), dietary cholesterol (DC) and monounsaturated fats (MUFA) were 18.53 g/d, 3.45 g/d, 0.201 g/d and 19.39 g/d, respectively. There were no associations between degree of cognitive decline and intakes of SFA (p=0.69), TFA (p=0.54) or DC (p=0.64) after adjusting for baseline cognition, total energy, age, education, reading ability, Apolipoprotein E (ε4) allele, BMI, estrogen and beta-blocker use, and intake of caffeine and other fatty acids. In contrast, compared with participants in the lowest quartile, MUFA intake was associated with lower cognitive decline in fully adjusted linear regression models, with decline of 0.21 + 0.05 SE in the lowest versus 0.05 + 0.05 SE in the highest quartiles (p=0.02). This effect of MUFA intake was primarily in the visual and memory domains (p=0.03 for both).
Conclusion
Higher intakes of SFA, TFA and DC in these women were not associated with cognitive decline, while MUFA intake was associated with less cognitive decline.
Keywords: Fatty acids, cognitive decline, monounsaturated fat intake and prospective
INTRODUCTION
Cognitive impairment is a common disorder among elderly persons. Age-related cognitive decline (ARCD) encompasses deterioration in several domains, including memory performance, executive functions, and speed of cognitive processing.1 The causes of cognitive decline are unknown, but previous studies have linked it to cardiovascular disease (CVD)2 and cardiovascular risk factors such as diabetes mellitus3 and abnormal blood pressure.4 Established modifiable risk factors for ARCD remain limited, but given its association with CVD, plausibly include dietary fatty acids, which have strong established roles in the etiology of CVD.5
Previous prospective studies of intake of dietary saturated fatty acids (SFA),6-11 trans-unsaturated fatty acids (TFA),6, 9 and mono-unsaturated fatty acids (MUFA)6-11 have had variable associations with cognitive decline with most suggesting deleterious effects of SFA and TFA. Of note, very few studies of fatty acids and cognitive decline have measured individual components of cognitive function beyond the standard Mini-Mental Status Exam6, 8, 9 and only one had multiple assessments of diet.9 Thus, it remains unclear if different fats have differential effects on specific elements of cognitive function.
We previously reported an association of higher dietary n-3, but not n-6, fatty acid intake with less cognitive decline in older women, but the corresponding associations with other fatty acids were not assessed.12 Given the established deleterious relationships of SFA and TFA with CVD and beneficial relation of MUFA with CVD, we hypothesized that they would have similar relationships with cognitive decline. In this study, we examined the prospective associations of these fatty acids with cognitive decline in a population of older women using comprehensive neuropsychiatric testing for multiple domains of cognitive decline.
METHODS
Study Sample
The Cognitive Change in Women (CCW) study is an ancillary study to the Observational study (OS) of the Women's Health Initiative (WHI) that was designed to examine associations of dietary and lifestyle factors with cognitive function in non-demented, community-dwelling women aged 60 years and older. Design and methods for CCW and WHI studies are described in detail elsewhere.13, 14 Briefly, women age 60 and over with visual and hearing acuity sufficient for valid neuropsychological testing and previously enrolled in the WHI Observational Study (OS) cohort or in the control (“usual diet”) group of the WHI Diet Modification arm at the Northwestern University and Evanston-Northwestern Healthcare WHI clinical centers were recruited, screened and excluded for history of dementia, stroke, and other neurologic illness, alcohol or substance abuse, or regular use of medications, known to affect cognitive function or that might preclude valid cognitive testing.
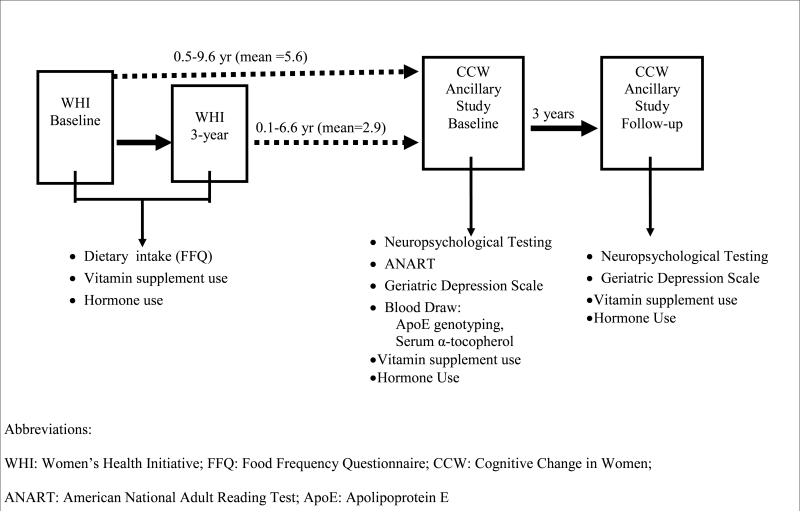
Of 1201 women who were sent mailings, 679 (57%) responded positively and 544 (80%) of these screened eligible and were enrolled. Of those, 482 completed both baseline and follow-up CCW exams, and 441 had complete data for the variables used in these analyses. Baseline CCW evaluations took place between Jan 1998 and Nov 2003 and follow-up assessments were between Jan 2001 and Nov 2006. Figure 1 shows the temporal relationship between WHI enrollment and CCW enrollment and follow-up, and key variables collected at each time-point. The WHI CCW study was reviewed and approved by the Institutional Review Boards at New England Research Institutes, Watertown, MA; Northwestern University Medical School, Chicago, IL; and Evanston-Northwestern Healthcare, Evanston, IL.
Figure 1.
Data Collection
Cognitive Function Assessment
At CCW enrollment and again after 3 years, CCW participants received an extensive cognitive test battery, including the CERAD word list learning, constructions, and word fluency tests,15, 16 Wechsler Memory Scale-Revised (WMS-R) Logical Memory, Visual Reproduction, and Digit Span tests,17 Boston Naming Test,18 F-A-S Word Fluency and Judgment of Line Orientation tests,19 Visual Target Cancellation Test,20 Trail Making Test parts A and B,21 and a modification of the Visual-Verbal Test.22 These 10 tests yielded 17 separate scores. The tests were grouped into four domains, including memory: CERAD Word List, WMS-R Logical Memory, WMS-R Visual Reproduction; executive function: Trail Making Part B, Visual Verbal Test; language: Boston Naming Test, CERAD word fluency, F-A-S Word Fluency Test; attention: Trail Making Part A, Digit Span; and visual: CERAD Constructions, Judgment of Line Orientation, and Visual Target Cancellation Test. In addition, the American National Adult Reading Test (ANART)23 was administered to measure reading ability and the 30-item Geriatric Depression Scale (GDS)24 to measure depressive symptoms.
Testing and scoring were performed by interviewer / technicians who had undergone standardized training at the Northwestern Alzheimer's Disease Center Clinical Core, and who underwent recalibration training approximately twice per year. Inter-rater reliability for scoring was assessed by randomly selecting 20 participant records which were re-scored by a different interviewer. Intra-class correlation (r) for continuous test scores exceeded 0.8 for all test scores and exceeded 0.9 for all but one, demonstrating excellent inter-rater reliability. For tests that were scored categorically or that had a limited range of possible scores, scorers showed 100% agreement (Cohen's kappa=1).
One hundred four participants (22%) exhibited at least one impaired score on memory tests at baseline, 68 (14%) on tests of visual spatial perception, 29 (6%) on tests of executive function, 22 (5%) on tests of language, and 10 (2%) on tests of attention. When the domain-specific impairments were considered together, 51 (11%) of participants exhibited mixed-domain impairment. A total of 65 women (13%) could not be classified by our mixed cognitive impairment variable because of missing values on one or more tests. Of the 51 women with impairment in two or more domains, 39 (76%) had memory impairment and 12 had impairment in two or more domains, not including memory.
Each baseline score was standardized to zero mean and unit standard deviation (Z-score). The follow-up scores were similarly standardized using the baseline means and standard deviations. A global Z-score was created for both CCW baseline and 3-year time points by averaging individual test Z-scores for each study participant, at each time point. Cognitive change was defined as the difference between follow-up and baseline global Z-score. This method of generating a composite score from multiple cognitive test scores has been employed previously by others.25
Dietary Assessment
Dietary intake data was assessed twice prior to baseline cognitive testing by a food frequency questionnaire (FFQ) that was developed for the WHI. This WHI FFQ has been found to have precision similar to other FFQs in terms of correlation of nutrient estimates, including fatty acids, with those obtained from four 24-hour dietary recalls and a 4-day food record.26 Briefly, the WHI FFQ includes 122 foods or food groups with questions on usual frequency of intake and portion size (compared to pictures of a medium portion size) over the “last 3 months”. Adjustment questions permitted more refined analyses of fat intake by asking about food preparation practices and types of added fats. For each dietary intake measure, we averaged the values from the 2 administrations of the WHI FFQ, collected 3 years apart (WHI baseline and 3-year visits) if they were both present. If the 3 year FFQ was missing (N=134), we carried the baseline measure forward. As seen in Figure 1, the first WHI FFQ was collected an average of 5.6 years prior to CCW baseline cognitive testing, and the second, an average of 2.9 years prior. Vitamin supplement use was assessed at both the baseline and 3-year follow-up WHI and CCW visits, neither of which included MUFA supplementation.
Individual dietary fats, including total SFA (gm), TFA (gm), dietary cholesterol (DC) (mg), n-3 polyunsaturated fatty acids (PUFAs, gm), n-6 PUFAs (gm), MUFAs (gm) and dietary carbohydrate (gm) intake data were computed from the WHI baseline and 3-year FFQs. We averaged these dietary macronutrients and calculated their percentage of total energy by dividing intake (gm/day) by energy intake (kcal) per day and multiplying by the energy content of that macronutrient. Individual saturated fatty acids, including palmitic (16:0), stearic (18:0) and myristic acid (14:0), were all strongly correlated (r=0.90-0.98), even when expressed as a proportion of total energy (r=0.73-0.93), which precluded their analysis separately.
Covariates
We treated age, American National Adult Reading Test (ANART) score at CCW baseline, dietary and supplemental vitamin D (mcg), dietary and supplemental beta-carotene (mcg), dietary and supplementary copper (mg), caffeine intake (mg), alcohol intake (gm) and dietary antioxidants, genistein (mg) and daidzein (mg), as continuous variables. We categorized educational attainment as high-school or less, some college and college degree or beyond. We assigned smoking status as never, past smoker (at least 100 cigarettes), and current smoker. We categorized race/ethnicity as non-Hispanic white, non-Hispanic black and all others. We assigned non-steroidal anti-inflammatory drug use as non-user, irregular user and regular user. We dichotomized physical activity into none/some activity of limited duration and moderate/strenuous physical activity (equivalent to 2-4 episodes/week of walking fast for ≥20 minutes or more). We calculated Body Mass Index (BMI) by dividing weight (kg) by height (m2) and categorized BMI into <25 kg/m2, 25-30 kg/m2 and >30 kg/m2. The following self-reported physician-diagnosed cardiovascular-related diseases were assessed either at the baseline WHI or CCW visit: diabetes at CCW, hyperlipidemia at WHI, cardiovascular disease at WHI, hypertension at CCW, and unipolar depression at CCW. None were included in final models due to not reaching statistical significance in bivariate analysis, which was determined a priori as <0.10. We ascertained use of cardiovascular-related medications, including cholesterol lowering medications, beta-blockers, diuretics, aspirin and estrogen hormone replacement therapy. We averaged dietary and supplemental vitamin E intake collected at WHI baseline and 3-year visits, as well as CCW baseline and 3-year visits (see Figure 1). We then categorized average intake based on the estimated requirement for Vitamin E for women 51 years of age and over (12 mg/day)27 as 1) consistently less than 12 mg/day; 2) fluctuating; 3) consistently greater than 12 mg/day. We dichotomized apolipoprotein E (ApoE) to reflect presence or absence of an ε4 allele.28
Statistical Analysis
To explore the associations of dietary fats and dietary cholesterol on change in cognitive function, we used least squares linear modeling methods. We computed two sequential linear regression models for 3-year change in global cognitive Z-score. We adjusted the first model for age, education, ApoE ε4 allele, reading ability in the American National Adult Reading Test (ANART), dietary total energy and baseline global cognitive Z-score. We then computed a second model controlling for factors that were associated with cognitive change in bivariate analysis with p< 0.10, including BMI, estrogen use, beta blocker use, dietary caffeine intake, and forced dietary intake in quartiles of MUFA, PUFA, SFA, TFA and DC into the model. Results are reported as the increase or decrease in cognitive function in standard deviation units per specified increment of dietary intake. We tested the interaction effects of dietary copper29 and ApoE ε428 with dietary fats on cognitive decline. All analyses were carried out using SAS statistical software (SAS Institute, Inc. Cary NC)
RESULTS
This cohort is comprised primarily of older, Caucasian (87%) women. Median intakes of SFA, TFA and DC were similar to levels found in the larger WHI cohort of older women (Table 1).30 Dietary MUFA intakes were slightly lower than levels found in this same comparison group, 11.5 (3.6) versus 14.4 (2.3).30 The proportions of calories from various dietary fats were moderately correlated (Table 2).
Table 1.
Baseline characteristics at CCW
| MUFA Quartiles | ||||
|---|---|---|---|---|
| Characteristic | 1 [0-9.75] | 2 [9.76-11.50] | 3 [11.51-13.26] | 4 [≥13.27] |
| SFA (SE) | 7.90 (2.15) | 10.99 (1.93) | 12.20 (2.06) | 13.26 (2.73) |
| TFA (SE) | 1.34 (0.54) | 1.98 (0.57) | 2.28 (0.72) | 2.92 (1.22) |
| DC (SE) | 152.0 (70.5) | 209.5 (86.8) | 249.9 (111.9) | 261.4 (129.0) |
| Baseline global z-score (SE) | 0.02 (0.61) | 0.04 (.49) | 0.05 (0.50) | -0.02 (0.58) |
| Follow-up global z-score (SE) | -0.13 (0.34) | -0.14 (0.39) | -0.06 (0.30) | 0.00 (0.28) |
| Age (years, SE) | 70.89 (6.70) | 71.36 (6.55) | 69.95 (6.67) | 70.17 (5.99) |
| PUFA (SE) | 5.12 (1.48) | 6.25 (1.78) | 6.99 (1.38) | 8.62 (2.16) |
| Caffeine Use (mg/day, SE) | 151.0 (107.3) | 160.5 (131.2) | 184.0 (120.7) | 160.5 (132.5) |
| ANART (reading ability, SE) | 9.04 (6.80) | 7.95 (6.06) | 8.48 (6.00) | 10.99 (8.29) |
| MMSE (SE) | 28.96 (1.44) | 28.87 (1.32) | 29.00 (1.16) | 28.87 (1.28) |
| BMI (kg/m2, SE) | 26.01 (5.42) | 26.64 (4.86) | 26.76 (5.36) | 27.18 (5.10) |
| <25 kg/m2 (N, %) | 59 (49%) | 46 (38%) | 49 (41%) | 45 (37%) |
| 25-30 kg/m2 (N, %) | 43 (36%) | 47 (39%) | 44 (37%) | 44 (36%) |
| >30 kg/m2 (N, %) | 18 (15%) | 27 (23%) | 27 (23%) | 32 (26%) |
| Education | ||||
| ≥High school (N, %) | 22 (18%) | 24 (20%) | 20 (17%) | 30 (25%) |
| Some college (N, %) | 27 (23%) | 26 (21%) | 32 (27%) | 30 (25%) |
| ≥College degree (N, %) | 71 (59%) | 71 (59%) | 68 (57%) | 61 (50%) |
| ApoE ε4 | ||||
| Yes (N, %) | 34 (31%) | 25 (23%) | 28 (24%) | 23 (20%) |
| No (N, %) | 76 (69%) | 86 (77%) | 89 (76%) | 93 (80%) |
| Estrogen Use | ||||
| Yes (N, %) | 34 (28%) | 31 (26%) | 42 (35%) | 37 (31%) |
| No (N, %) | 86 (72%) | 89 (74%) | 78 (65%) | 84 (69%) |
| Beta-Blocker Use | ||||
| Yes (N, %) | 10 (8%) | 22 (18%) | 14 (12%) | 14 (12%) |
| No (N, %) | 110 (92%) | 99 (82%) | 106 (88%) | 107 (88%) |
Abbreviations:
N: Number of subjects
SE: Standard error
MUFA: Monounsaturated fatty acid intake (% of Energy)
SFA: Saturated fatty acid intake (% of Energy)
TFA: Trans-fatty acid intake (% of Energy)
DC: Dietary Cholesterol intake (mg/day)
PUFA: Polyunsaturated fatty acid intake (% of Energy)
BMI: Body mass index
ApoEε4: Apolipoprotein E epsilon 4
ANART: American National Adult Reading Test
CCW: Cognitive Change in Women
MMSE: Mini Mental Status Exam
Table 2.
Pearson correlations of dietary exposures
| Variable | Median (IQR) | MUFA | SFA | TFA | DC |
|---|---|---|---|---|---|
| MUFA | 11.5 (3.6) | 1.00 | |||
| SFA | 11.1 (4.0) | 0.67 | 1.00 | ||
| TFA | 1.9 (1.2) | 0.65 | 0.45 | 1.00 | |
| DC | 200.8 (149.7) | 0.37 | 0.49 | 0.23 | 1.00 |
Abbreviations:
IQR: Inter-quartile range
MUFA: Monounsaturated fatty acid intake (% of Energy)
SFA: Saturated fatty acid intake (% of Energy)
TFA: Trans-fatty acid intake (% of Energy)
DC: Dietary Cholesterol intake (mg/day)
We found that higher intake of dietary MUFA was associated with less cognitive decline in both partially and fully adjusted models. Compared with participants in the lowest quartile, MUFA intake was associated with lower cognitive decline in fully adjusted linear regression models, with decline of 0.21 ± 0.05 SD in the lowest versus 0.05 ± 0.05 SD in the highest quartiles (p=0.02) after adjustment for total energy, age, education, ANART (reading ability) score, cognitive z-score at baseline, ApoE status, SFA, TFA and DC. (Table 3). In partially adjusted models, SFA intake was associated with less cognitive decline (p=0.01), with decline of 0.18 ± 0.03 in the lowest quartile versus 0.03 ± 0.03 in the highest. However, this association with cognitive decline was null in fully adjusted models (p=0.69), primarily due to confounding by MUFA intake. There was no association between TFA and cognitive decline in partial (p=0.07) or fully adjusted models (p=0.54).
Table 3.
Regression coefficients (and SEs) for multivariable linear regression analysis of fatty acid intake in quartiles on prospective cognitive function z-score; N=482
| Quartile of Fatty Acid Intake | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | p-value | |
| MUFA | [0-9.75] | [9.76-11.50] | [11.51-13.26] | [≥13.27] | |
| Partial* | -0.16 (0.03) | -0.16 (0.03) | -0.10 (0.03) | 0.00 (0.03) | <0.01 |
| Full** | -0.21 (0.05) | -0.23 (0.04) | -0.16 (0.04) | -0.05 (0.05) | 0.02 |
| SFA | [0-9.12] | [9.13-11.11] | [11.12-13.00] | [≥13.01] | |
| Partial* | -0.18 (0.03) | -0.11 (0.03) | -0.10 (0.03) | -0.03 (0.03) | 0.01 |
| Full** | -0.20 (0.05) | -0.16 (0.04) | -0.16 (0.04) | -0.13 (0.04) | 0.69 |
| TFA | [0-1.45] | [1.46-1.92] | [1.93-2.64] | [≥2.65] | |
| Partial* | -0.13 (0.03) | -0.16 (0.03) | -0.07 (0.03) | -0.06 (0.03) | 0.07 |
| Full** | -0.17 (0.04) | -0.19 (0.04) | -0.13 (0.04) | -0.17 (0.04) | 0.54 |
| DC | [0-136.17] | [136.18-203.53] | [203.54-281.58] | [≥281.59] | |
| Partial* | -0.11 (0.04) | -0.10 (0.03) | -0.11 (0.03) | -0.10 (0.04) | 0.97 |
| Full** | -0.14 (0.04) | -0.14 (0.04) | -0.18 (0.04) | -0.19 (0.04) | 0.64 |
Partial: Adjusted for global z-score at baseline, dietary total energy (kcal), age, education, American National Adult Reading Test score and apolipoprotein E (ε4) allele
Full: Further adjusted for BMI, estrogen use, caffeine use, beta-blocker use and other fatty acid (MUFA, PUFA, SFA, TFA, % energy; DC, mg/day) intake
Abbreviations:
SE: Standard error
MUFA: Monounsaturated fatty acid intake (% energy)
SFA: Saturated fatty acid intake (% energy)
TFA: Trans-unsaturated fatty acid intake (% energy)
DC: Dietary cholesterol (mg/day)
In sensitivity analyses, all of the above associations were minimally changed in models without baseline cognitive z-score included or in an energy substitution model for dietary protein intake. The energy substitution model included carbohydrates, alcohol and other variables in the fully adjusted models and can be interpreted as follows, compared with participants in the lowest quartiles, dietary MUFA intake in exchange for an equivalent amount of energy from dietary protein, was minimally changed from the associations with cognitive decline in our primary analysis. There was no change in the associations when an indicator variable for the number of FFQs completed was added to fully adjusted models.
Given the association of MUFA intake with less global cognitive decline, we tested its associations with individual cognitive domains, including memory, executive function, language and visual (Table 4). MUFA intake was associated with less decline in the visual domain and memory domains (p=0.03 for both). There was no evidence of effect modification by ApoE e4 (p>0.55) or dietary copper (p>0.29) with intakes of SFA, TFA, DC and MUFA. Additionally, there was no evidence of effect modification by SFA (p=0.90), TFA (p=0.98) or PUFA (p=0.37) with MUFA intake.
Table 4.
Multivariable linear regression analysis of MUFA intake (% of energy) in quartiles on prospective change in mean (SE) cognitive function domains z-score; N=482
| Cognitive Function Domain | MUFA Quartiles | p-value | |||
|---|---|---|---|---|---|
| 1 [0-9.75] | 2 [9.76-11.50] | 3 [11.51-13.26] | 4 [≥13.27] | ||
| Memory | -0.23 (0.08) | -0.30 (0.06) | -0.17 (0.06) | -0.05 (0.07) | 0.03 |
| Executive Function | -0.14 (0.10) | -0.18 (0.08) | -0.22 (0.08) | -0.04 (0.10) | 0.25 |
| Language | -0.08 (0.07) | -0.20 (0.05) | -0.13 (0.05) | -0.06 (0.06) | 0.16 |
| Attention | -0.19 (0.10) | -0.16 (0.07) | -0.16 (0.07) | -0.18 (0.09) | 0.99 |
| Visual | -0.26 (0.08) | -0.20 (0.06) | -0.18 (0.06) | 0.02 (0.08) | 0.03 |
Adjusted for baseline cognitive test z-score, dietary total energy (kcal), age, education, American National Adult Reading Test score and apolipoprotein E (ε4) allele, BMI, estrogen use, caffeine use and beta-blocker use, PUFA (% energy), SFA (% energy), TFA (% energy) and DC (mg/day)
Abbreviations:
SE: Standard Error
MUFA: Monounsaturated fatty acid intake (% of Energy)
SFA: Saturated fatty acid intake (% energy)
TFA: Trans-unsaturated fatty acid intake (% energy)
DC: Dietary cholesterol (mg/day)
DISCUSSION
In this cohort of older women, we found that MUFA intake was associated with less cognitive decline over a three year period. Previous studies generally but not invariably support this association. One previous prospective study found increased dietary MUFA intake to be associated with less cognitive decline,10 a second found a trend in the same direction,9 a third found a trend in the same direction in restricted analyses,6 and three others were null.7, 8, 11 Among the null studies, none of them had multiple measures of diet, although one assessed diet through a measure of fatty acid composition of erythrocyte membranes.7 However, that study assessed cognitive decline exclusively with the Mini-Mental State Examination, which is likely not as sensitive as the neuropsychological test battery used in this study.
MUFAs are thought to be one of the major protective components of the traditional Mediterranean diet, where they are consumed primarily from olive oil (46 g/d or 4 tbs/d).10 Two recent prospective studies of Mediterranean diet have found greater adherence to be associated with less cognitive decline or Alzheimer Disease (AD) incidence.31, 32 One of these studies found an effect of Mediterranean diet on an individual cognitive domain, namely memory.31 This finding is consistent with the observed protective effect of MUFAs on memory in the WHI CCW. In addition, we found an association of MUFAs with less decline in visual-spatial abilities (copying and matching), a finding not previously made to our knowledge. Decline in visuospatial function has been associated with driving errors in older adults 33 and has also been suggested as a potential predictor (along with amnestic impairment) of transition from mild cognitive impairment to Alzheimer's disease.34
Several pathways may explain the apparent relationship of MUFA intake with cognitive function. MUFAs and MUFA derivatives have anti-inflammatory effects in vivo.35, 36 This may be important since chronic inflammation appears as a precursor of symptomatic AD.37-39 Oxidative stress has also been demonstrated in patients with mild cognitive impairment and AD,40 and derivatives from MUFAs, including low molecular weight phenols, have been found to have anti-oxidant effects.41 MUFAs may also exert their potentially beneficial effects on cognition indirectly by decreasing cardiovascular risk through reducing macrophage uptake of plasma oxidized low-density lipoprotein, reduction of apolipoprotein B and reduction of triglycerides.42-44
Intakes of SFA, TFA and DC were not associated with cognitive decline in this cohort of relatively healthy elderly women. SFA had a protective effect on cognitive decline in the partial model, which is likely due to confounding by MUFA, which was moderately correlated with SFA. However, there was no association between SFA and cognitive decline in the fully adjusted model. Previous prospective studies found dietary SFA intake to be either deleterious(5-8) or null10, 11 in associations with cognitive decline. Similarly, previous prospective studies found TFA intake to be either deleterious9 or trending towards deleterious effects6. Of note, these studies had substantially higher absolute and relative SFA and TFA intakes than did the women in this study, and hence the deleterious effects of these fatty acids may be limited to individuals with higher levels of intake.
Strengths of this study include the use of a battery of sensitive cognitive tests, rather than a global cognitive instrument in order to better detect the fairly small changes that were anticipated over 3 years in this group of generally healthy, well-educated women who were cognitively intact at baseline. Assessment of multiple domains of cognition allowed for a more detailed characterization of cognitive effects. Another strength of this study is the administration of a validated FFQ at two time-points rather than a single measurement, as was done in most prospective studies of MUFAs and cognitive decline.6-11 Finally, the WHI and CCW measured a wide variety of characteristics of subjects, allowing for the ability to control for various potential confounders and interactions in a systematic fashion.
Limitations of this study include the modest sample size and follow-up time of 3 years, which may preclude detection of modest long-term effects on cognition. This limits the ability to adjust for potential confounding among dietary fatty acids and to detect non-linear associations. This may contribute to why associations between SFA and TFA intake and cognitive decline were null. Limitations of using FFQs for dietary assessment include restrictions imposed by a fixed list of foods, reliance on memory, perception of portion sizes, and interpretation of questions. Also, blood fatty acid measurements, which are objective but can also have limitations of measurement error, were not available for our analyses. However, the FFQ used in this study was validated against a 4-day food record, which does not share these limitations. As with any observational study, we can not rule out the possibility of residual confounding by unknown risk factors, such as a generally healthier lifestyle of individuals with a higher MUFA intake. Finally, our study population consisted of primarily healthy, highly educated, older, Caucasian women, which limits the generalizability of these findings to other populations.
In conclusion, we found dietary MUFA intake to be associated with less cognitive decline in older women. Dietary intakes of SFA, TFA and DC were not associated with cognitive decline. Further larger, prospective studies with longer follow-up time are needed to confirm the possible protective effects of MUFAs for cognitive decline
ACKNOWLEDGMENTS
This work was exclusively funded by the National Institutes of Health (T32 AT000051 and R01 AG018695-01A1).
Dr. Mukamal is the principal investigator on an ongoing study funded by Harvard Medical School for which BIDMC received a donation of docosahexaenoic acid (DHA) and placebo capsules from Martek Corporation. Dr Naqvi is a co-investigator on that trial. Martek provided no other resources or funds and has no role in the conduct or analysis of that study. Martek had no role whatsoever in the current manuscript. There are no other financial or personal interests to disclose.
Sponsor's Role:
This work was exclusively funded by the National Institutes of Health (T32 AT000051 and R01 AG018695-01A1).
APPENDIX
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.
Short List of WHI Investigators Program Office
(National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers
(Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women's Health Initiative Memory Study
(Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit: http://www.whiscience.org/publications/WHI_investigators_longlist.pdf
Footnotes
Asghar Naqvi: no other potential conflicts of interest
Brian Harty: no conflicts of interests to disclose
Kenneth J. Mukamal: no other potential conflicts of interest
Anne Stoddard: no conflicts of interests to disclose
Mara Vitolins: no conflicts of interests to disclose
Julie E. Dunn: no conflicts of interests to disclose
REFERENCES
- 1.Von Dras DD, Blumenthal HT. Dementia of the aged: disease or atypical-accelerated aging? Biopathological and psychological perspectives. J Am Geriatr Soc. 1992;40:285–294. doi: 10.1111/j.1532-5415.1992.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 2.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 3.Naor M, Steingruber HJ, Westhoff K, Schottenfeld-Naor Y, Gries AF. Cognitive function in elderly non-insulin-dependent diabetic patients before and after inpatient treatment for metabolic control. J Diabetes Complications. 1997;11:40–46. doi: 10.1016/1056-8727(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 4.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The Honolulu-Asia Aging Study. JAMA. 1995;274:1846–1851. [PubMed] [Google Scholar]
- 5.Hunter JE, Zhang J, Kris-Etherton PM. Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review1,3. Am J Clin Nutr. 2009 doi: 10.3945/ajcn.2009.27661. [DOI] [PubMed] [Google Scholar]
- 6.Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–1579. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 7.Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes--The EVA Study. Am J Clin Nutr. 2003;77:803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 8.Eskelinen MH, Ngandu T, Helkala EL, et al. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry. 2008;23:741–747. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 9.Devore EE, Stampfer MJ, Breteler MM, et al. Dietary fat intake and cognitive decline in women with type 2 diabetes. Diabetes Care. 2009;32:635–640. doi: 10.2337/dc08-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solfrizzi V, Colacicco AM, D'Introno A, et al. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol Aging. 2006;27:1694–1704. doi: 10.1016/j.neurobiolaging.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Vercambre MN, Boutron-Ruault MC, Ritchie K, Clavel-Chapelon F, Berr C. Long-term association of food and nutrient intakes with cognitive and functional decline: a 13-year follow-up study of elderly French women. Br J Nutr. 2009;102:419–427. doi: 10.1017/S0007114508201959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn J, et al. Past dietary intake of N-3 fatty acids and cognitive change: The Cognitive Change in Women (CCW) WHI ancillary study. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2008;4:T174–T175. [Abstract] [Google Scholar]
- 13.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 14.Dunn JE, Weintraub S, Stoddard AM, Banks S. Serum alpha-tocopherol, concurrent and past vitamin E intake, and mild cognitive impairment. Neurology. 2007;68:670–676. doi: 10.1212/01.wnl.0000255940.13116.86. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 16.Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology. 1994;44:609–614. doi: 10.1212/wnl.44.4.609. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Wechsler Memory Scale-Revised Manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 18.Goodglass HK, Edith . The assessment of aphasia and related disorders / Harold Goodglass with the collaboration of Edith Kaplan. 2nd ed. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 19.Benton AL. Contributions to neuropsychological assessment : a clinical manual. 2nd ed. Oxford University Press; New York: 1994. [Google Scholar]
- 20.Weintraub S. Neuropsychological assessment of mental state. In: Mesulam M-M, editor. Principles of behavioral and cognitive neurology. Oxford University Press; New York: 2000. p. 540. [Google Scholar]
- 21.Reitan R. Validity of the trail-making test as an indication of organic brain damage. Perceptual Motor Skills. 1958;8:271–276. [Google Scholar]
- 22.Feldman MD, J . The Visual-Verbal Test. Western Psychological Services; Los Angeles: 1959. [Google Scholar]
- 23.Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 25.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67:1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Medicine . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 28.Notkola IL, Sulkava R, Pekkanen J, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer's disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 29.Morris MC, Evans DA, Tangney CC, et al. Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch Neurol. 2006;63:1085–1088. doi: 10.1001/archneur.63.8.1085. [DOI] [PubMed] [Google Scholar]
- 30.Howard BV, Van Horn L, Hsia J, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 31.Feart C, Samieri C, Rondeau V, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302:638–648. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dawson JD, Uc EY, Anderson SW, Johnson AM, Rizzo M. Neuropsychological Predictors of Driving Errors in Older Adults. J Am Geriatr Soc. doi: 10.1111/j.1532-5415.2010.02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iachini I, Iavarone A, Senese VP, Ruotolo F, Ruggiero G. Visuospatial memory in healthy elderly, AD and MCI: a review. Curr Aging Sci. 2009;2:43–59. doi: 10.2174/1874609810902010043. [DOI] [PubMed] [Google Scholar]
- 35.Vassiliou EK, Gonzalez A, Garcia C, Tadros JH, Chakraborty G, Toney JH. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-alpha both in vitro and in vivo systems. Lipids Health Dis. 2009;8:25. doi: 10.1186/1476-511X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borniquel S, Jansson EA, Cole MP, Freeman BA, Lundberg JO. Nitrated oleic acid up-regulates PPAR-gamma and attenuates experimental inflammatory bowel disease. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin WS, Stanley LC, Ling C, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker DG, Kim SU, McGeer PL. Complement and cytokine gene expression in cultured microglial derived from postmortem human brains. J Neurosci Res. 1995;40:478–493. doi: 10.1002/jnr.490400407. [DOI] [PubMed] [Google Scholar]
- 39.Wood JA, Wood PL, Ryan R, et al. Cytokine indices in Alzheimer's temporal cortex: no changes in mature IL-1 beta or IL-1RA but increases in the associated acute phase proteins IL-6, alpha 2-macroglobulin and C-reactive protein. Brain Res. 1993;629:245–252. doi: 10.1016/0006-8993(93)91327-o. [DOI] [PubMed] [Google Scholar]
- 40.Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2009 doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 41.Briante R, Febbraio F, Nucci R. Antioxidant properties of low molecular weight phenols present in the mediterranean diet. J Agric Food Chem. 2003;51:6975–6981. doi: 10.1021/jf034471r. [DOI] [PubMed] [Google Scholar]
- 42.Moreno JA, Lopez-Miranda J, Perez-Martinez P, et al. A monounsaturated fatty acid-rich diet reduces macrophage uptake of plasma oxidised low-density lipoprotein in healthy young men. Br J Nutr. 2008;100:569–575. doi: 10.1017/S0007114508911508. [DOI] [PubMed] [Google Scholar]
- 43.Gebauer SK, West SG, Kay CD, Alaupovic P, Bagshaw D, Kris-Etherton PM. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose-response study. Am J Clin Nutr. 2008;88:651–659. doi: 10.1093/ajcn/88.3.651. [DOI] [PubMed] [Google Scholar]
- 44.Gumbiner B, Low CC, Reaven PD. Effects of a monounsaturated fatty acid-enriched hypocaloric diet on cardiovascular risk factors in obese patients with type 2 diabetes. Diabetes Care. 1998;21:9–15. doi: 10.2337/diacare.21.1.9. [DOI] [PubMed] [Google Scholar]