Abstract
Conditionally replicating adenoviruses (CRAds) represent a promising modality for the treatment of neoplastic diseases, including Prostate Cancer. Selectively replicating viruses can be generated by placing a tissue or cancer-specific promoter upstream of one or more of the viral genes required for replication (for example, E1A, E1B). We have previously reported multiple cellular processes that can attenuate viral replication, which in turn compromises viral oncolysis and tumor kill. In this study, we investigated the importance of the cyclin-dependent kinase inhibitor p21/Waf-1, on viral replication and tumor growth. To our knowledge, this is the first report describing the importance of p21/Waf-1shRNA on the induction of an androgen responsive element (ARE) based promoter driving the E1A gene. As a proof of concept, the study emphasizes the use of RNA interference technology to overcome promoter weaknesses for tissue-specific oncolytic viruses, as well as the cellular inhibitor pathways on viral life cycle. Using RNA interference against p21/Waf-1, we were able to show an increase in viral replication and viral oncolysis of prostate cancer cells. Similarly, CRAd viruses that carry p21/Waf-1 shRNA (Ad5-RV004.21) were able to prevent tumor outgrowth that resulted in a marked increase in the mean survival time of tumor-bearing mice compared with CRAd without p21/Waf-1 shRNA (Ad5-RV004). In studies combining Ad5-RV004.21 with Adriamycin, a suprar-additive effect was observed only in CRAds that harbor shRNA against p21/Waf-1. Taken together, these findings of enhanced viral replication in prostate cancer cells by using RNA interference against the cdk inhibitor p21/Waf-1 have significant implications in the development of prostate-specific CRAd therapies.
Keywords: adenovirus, prostate cancer, p21/Waf-1, CRAd, shRNA
Introduction
Adenoviruses infect both quiescent and non quiescent cells1 and are known to replicate their genome inside the host cell nucleus.2,3 However, once inside the host cell the virus needs to overcome multiple challenges before it can efficiently replicate. Besides tumor suppressors like p53 and Rb, suboptimal cell cycle regulation is one of the hindrances that the virus encounters.4 Adenoviruses have evolved multiple methods that help overcome these barriers. It is now believed that the immediate adenovirus early gene, E1A, regulates the expression of host5–8 and viral genes9 and creates a cellular environment favorable for viral replication. Based on the importance of E1A in adenoviral biology, the majority of adenoviral gene therapy vectors rely on either replacing E1A gene from the virus backbone with the gene of interest or using tissue/cancer-specific promoters to limit viral replication to specific tissues and organs. We and other groups have previously shown the utility of creating conditionally replicating adenoviruses by placing prostate-specific promoters upstream of the E1A or E1B genes.10,11 Although these manipulations restrict viral replication to the tissue of interest, the use of such low activity promoters attenuates viral replication and therefore compromises viral cytotoxicity.
Previously, we have shown that p21/Waf-1 among other factors is one of the most clearly identified factors that impairs adenovirus replication.12 In cell lines where p21/Waf-1 was either absent or knocked down, we found that adenovirus replicates faster compared with cells in which p21/Waf-1 was intact.12 This finding of enhanced replication in the absence of p21/Waf-1 was recently validated by Shinna et al.13 In addition to suppression on viral replication, p21/Waf-1 has also been shown to have multiple inhibitory functions inside the cell. It is a direct downstream target of the tumor suppressor p53. Elevated levels of p21/Waf-1 is known to arrest cells in both G1 and G2 phases of the cell cycle by inhibiting cyclin-dependent kinase complexes.14–16 p21/Waf-1 interacts with procaspase-3 in mitochondria, which regulates caspase-3 activation and apoptotic cell death.17 Similarly, other investigators have reported the binding of p21/Waf-1 to the proliferation cell nuclear antigen to block DNA repair and replication.18
In this study, we describe a novel CRAd, which selectively replicates in prostate cells via prostate-specific rat probasin (PBN) promoter driving E1A, with enhanced therapeutic effect due to constitutive expression of an anti-p21/Waf-1 shRNA located in the fiber-gene region. To our knowledge, this is the first example where shRNA has been used to enhance the natural viral lifecycle for use in gene therapy. Knocking down p21/Waf-1 not only helped in virus replication but also enhances the induction of prostate-specific ARE-based promoter that controls E1A expression. Taken together, our data supports the feasibility of developing enhanced oncolytic viral gene therapies with shRNAs to modulate cellular pathways that attenuate viral replication.
Materials and methods
Reagents and antibodies
For western blot analysis the following antibodies were used: mAb–p21/Waf-1 (1:1000, Upstate, Charlottesville, VA), mAb–AR (1:1000, Santa Cruz Biotech, Santa Cruz, CA), mAb-β Actin (1:25 000) and Anti-mouse IgG HRP-conjugated (1:20 000 Sigma Aldrich, St Louis, MO). All restriction enzymes were purchased from New England Biolabs (Beverly, MA). Cell culture media were obtained from Cellgro (Herndon, VA). Trypan blue was purchased from Invitrogen (Carlsbad, CA). The majority of all other chemical reagents and compounds were ordered from Sigma, unless otherwise specified.
Cell culture and generation of stable cell lines
LNCaP and HEK293 cell lines were purchased from American Tissue Culture Collection (Manassas, VA). LAPC4 and C4-2 cell were obtained from Dr John Isaacs (Johns Hopkins University). DPL-S11 cells were generated in our laboratory by transfecting DPL adenoviral packaging cells with pDisplay-S11 plasmid (gift from Dr Victor W van Beusechem to Ronald Rodriguez) that encodes a bi-specific single-chain antibody (scFv) consisting of anti-epidermal growth factor receptor single-chain antibody 425 and anti-adenovirus fiber knob single-chain antibody.19 LNCaP, LAPC4, C4-2 and HEK293 cells were maintained in RPMI 1640 medium with L-Glutamine (Cellgro) and DMEM (Cellgro) and supplemented with heat-inactivated fetal bovine serum 10% (GIBCO, Carlsbad, CA), Ciprofloxacin Hydrochloride 5 μgml−1 (US Biological, Swampscott, MA), and Gentamicin 50 μgml−1 (Quality Biological Inc., Gaithersburg, MD). Cells were allowed to grow until 80–90% confluency and harvested with 0.05% trypsin/0.5mM EDTA (Cellgro) before each subsequent passage. Stable p21/Waf-1 knockdown C4-2 and LAPC4 cells or control cells were generated by transfection of plasmids. Briefly, cells were seeded into six-well plates at approximately 60–70% confluence 12–24 h before transfections. Plasmid DNA, pSuper-Puro-GFP or pSuper-Puro-GPF shRNA p21/Waf-1 using the sequence 5′-GATCCCCAGCGATGGAACTTCGACTTTTCAAGAGAAAGTCGAAGTTCCATCGCTTTTTTGGAAA-3′ (a kind gift from Professor Hinrich Gronemeyer, Institut de Génétique et de Biologie Moléculaire et Cellulaire)20 was used to transfect the C4-2 and LAPC-4 cells using Lipofectamine 2000 reagent (Life Technologies) according to the manufacturer’s instructions. Cell monolayers were trypsinized 24 h after transfection and transferred into T25 flasks or 100-mm diameter culture dishes. Cells were then selected by growth in complete medium containing 2 μgml−1 of puromycin for 4 weeks. Viable clones were pooled together and cultured for expansion in T75 Flasks and at the same time assayed by western blot to ensure p21/Waf-1 knockdowns.
Western blot analysis
Cells were washed with 1× PBS and re-suspended with five volumes of cold lysis buffer (50mM Tris-HCl, pH 7.5, 250mM NaCl, 5mM EDTA, 50mM NaF, 0.5% NP-40) supplemented with protease inhibitor cocktail (Roche, Indianapolis, IN). The cell lysate was incubated on ice for 30 min and then centrifuged for 10 min at 4 °C. Equal amounts of proteins were separated by SDS-PAGE, and the resolved proteins were then transferred to a nitro-cellulose membrane. After blocking with 5% nonfat milk in TBST overnight at 4 °C, the blot was incubated with primary antibody at 1 h at room temperature. The membrane was then probed with HRP-conjugated secondary antibody for 1 h and developed (ECL-Plus system, Amersham Pharmacia, Piscataway, NJ) using the manufacturer’s protocol.
Reporter-based quantification of viral replication
As described previously,12,21 we have applied a reporter system to quantify adenoviral replication in real time by linking green fluorescent protein (GFP) expression to the viral major late gene fiber through an Internal Ribosome Entry Site (IRES). In brief, this replication-deficient reporter virus, FFIG, (a kind gift from Dr Gary Ketner, Johns Hopkins Bloomberg School of Public Health) expresses GFP in a replication-dependent manner when co-infected with a replicating adenovirus. For reporter experiments, cells were plated into 96-well plates at 1 × 104 per well overnight, and co-infected the next day with various replication competent adenoviruses at different MOI of 5–10 p.f.u. per cell and the reporter virus FFIG at an MOI of 10–20 p.f.u. per cell. The GFP fluorescence signals were measured at the indicated time points using a multi-plate fluorometer (FLUOstar Optima Microplate Reader), using an excitation wavelength of 485±20nm and an emission wavelength of 530±25 nm. Background fluorescence was measured in cells, which were infected with FFIG virus alone. GFP data are plotted as fold GFP induction relative to the mock-infected cells. All samples were performed in either triplicate or quadruplicate with error bars representing the s.e.m.
3-(4,5)-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay
The metabolic viability of the cells was monitored using an MTS assay kit (CellTiter 96) from Promega (Madison, WI) as described by Hoti et al.21 Briefly cells were seeded onto 96-well plates and cultured in the presence of test agents for indicated time intervals. A mixture of MTS and phenazine methosulfate (an electron-coupling reagent; final concentrations, 333 μgml−1 and 25 μM) was added, and cells were incubated for 30 min at 37 °C Formazan formed from the reduction of MTS was quantified by measurement of absorbance of the medium at 490nm using a microplate reader (All data have been normalized to the background signals).
Northern blot analysis
Total RNAs were isolated from cells infected with adenovirus Ad5-RV004.21 carrying shRNAp21 at different time points using Trizol. Fifteen micrograms of total RNAs were resolved on 15% acrylamide/8M urea gel, transferred onto nylon membranes and UV cross-linked. Membranes were hybridized in the hybridization solution at 42 °C with gamma P32-labeled oligo designed against the processed sense strand of p21. The blots were autoradiographed with an intensifying screen at −80 °C for 1 day and scanned with Molecular Imager FX (Bio Rad, Hercules, CA).
Generation of recombinant adenoviruses
Ad5wtΔE3 (CN702) is an adenovirus type 5 with a deletion in E3 region. It was generated as described earlier.22,23 Prostate-specific CRAd, Ad5-PSE/PBN-E1A (Ad5-RV004) was constructed as previously reported.21 Briefly shuttle plasmid RpsToad-PSE/PBN-E1A that carries prostate-specific enhancer and rat probasin promoter driving E1A was linearized with EcoRI restriction endonucleases. After gel purification the linearized RpsToad-PSE/PBN-E1A vector was transformed into the electro-competent AdEasier-1 (BJ5183-AD-1) cells for homologous recombination. The desired clones (pAd5-PSE/PBN-E1A) after screening were transformed into non-recombinant strain DH10B cells for large-scale DNA amplification. For viral propagation the recombinant plasmid pAd5-PSE/PBN-E1A was linearized with PacI and transfected into adenovirus packaging cell line DPL-S11 to generate recombinant adenoviruses. Virus amplification was done in the same DPL-S11 cell line. Similarly for generating a prostate-specific conditionally replicating adenovirus (Ad5-RV004.21) that carries p21/Waf-1 shRNA after the fiber region, an adenoviral pFEX system was used to recombine a shuttle plasmid RpsToad-PSE/PBN-E1A using homologous recombination between the two LTRs of the adenovirus in BJ5183 bacterial competent cells.24 After getting the desired recombinant, the fiber-less viral DNA that carried prostate-specific enhancer and rat probasin promoter (pAd5-FexPSE/PBN-E1A) was linearized with Pac1 and transfected into the fiber-expressing FBJ cell line (a generous gift of Dr David Johns, Johns Hopkins University) to generate prostate-specific pseudo-typed Ad5-FexPSE/PBN-E1A virus. The Ad5-RV004.21 that carries p21/Waf-1 shRNA and a wild-type fiber was produced by the transfection and infection experiment using a transient vector RpUC-WT-FIB shRNAp21 and a pseudo-typed fiber-less adenovirus Ad5-FexPSE/PBN-E1A in 293Cre57 cells. The schematic diagrams of both viruses are shown as supplementary figure 1A. Once Ad5-RV004.21 was made and purified, RCA (replication competent adenovirus) was ruled out using PCR amplification with a set of primers E1A 5′-CGTTCCGGGTCAAAGTTGG-3′ E1A 5′-CCTCCGTGGCAGATAATATGTC-3′ spanning the wild type E1A promoter and E1A gene. PCR amplifications were performed to confirm the presence of p21/Waf-1shRNA in Ad5-RV004.21 preps using a set of primers P21 5′–GAACGCTGACGTCATCAA-3′, P21 5′-AAGT TCCATCGCTGGG-3′ specific to the H1 promoter and p21/Waf-1 region of shRNA (Supplemental Figure 1B and C).
For the comparison of a single shRNA construct against luciferase in two different regions of adenovirus, that is, E1 region and fiber region, two different replication-deficient adenoviruses, Ad5-Track-U6-shRNALuc or Ad5-Fex-U6-shRNALuc were made using either AdEasy-125 or pFEX systems.24 Briefly shRNA against luciferase in E1 region of the adenovirus was cloned in BglII/Not1 site of the shuttle pAdTrack plasmid. Shuttle plasmid carrying shRNA against the luciferase (pAd-TrackshRNA-Luc) was then recombined with AdEasy-1 backbone in bacterial strain BJ5183 using homologous recombination. Viruses were made and amplified after transfection of the linearized recombinant vector pAd5-Track-U6-shRNALuc into the DPL cells. Unlike Ad5-Track-U6-shRNALuc virus that carries Luc shRNA in the E1 region the Ad5-Fex-U6shRNA-Luc has shRNA against luciferase after the fiber region by taking advantage of the pFEX system. The shRNA against luciferase was cloned into the Not1 site of a shuttle plasmid Rpuc-WTFib.24 Ad5-Fex-U6shRNALuc virus was made by transfection and infection of a pseudotyped Ad5-FexTrack virus (0.5 MOI) together with shuttle plasmid Rpuc-WTFib, shRNA-Luc (4 ug ml−1) and pUC-Cre (2 ug ml−1) in DPL-S11 cells.
Output to input assay
Viral output to input assays were performed using Adeno-X Rapid Titer Kit (Clontech Laboratories Inc., Mountain View, CA). Briefly, cells were infected with adenovirus (1 MOI) in six-well plates. Seventy-two hours post-infection, cells were harvested in the same medium and subjected to three rounds of freeze–thaw cycles. Total infectious viruses were measured by tittering them on HEK293 cells according to the Adeno-X Rapid Titer Kit protocol. The ‘amplification ratio’ of a virus produced from an infected cell (output) to the amount originally used to infect the cells (input) were determined and reported as the output to input ratio.
Luciferase assay
Here, 1 × 104 cells per well were plated in a 96-well plate 1 day before infection. Cells were infected with adenovirus carrying luciferase (1 MOI) together with either adenovirus carrying shRNA against luciferase in the E1A region or after the fiber region (10 MOI). The Luciferase assays (Dual-Glo Luciferase Assay System, Promega, WI) were performed at 24–48 h post infection. All of the shRNA knock-down experiments were performed in quadruplets and normalized to the total GFP measured in the cells. Luciferase activity is reported as percentage relative light-forming units normalized to GFP.
BALB/C-nude mice and tumor implantations
Four to six-week-old athymic BALB/c nude male mice, weighing approximately 20–24 g were obtained from Harlan (Indianapolis, IN). Mice were quarantined for a minimum of 5 days in the SPF Grade Animal House under 12 h light/dark cycles at 24–25 °C with a relative humidity of 50–55%. Institutional guidelines were followed in handling the animals. Tumors were established by subcutaneous (s.c.) injection with C4-2 cells (1 × 106) resuspended in 100 ul phosphate-buffered saline (Ph 7.4; BioSource, Rockville, MD) mixed 1:1 with Matrigel (BD Biosciences, Palo Alto, CA), in both dorsal flanks of the animals. Once palpable tumors were established, animals were randomized into control and treatment arms.
Statistical analysis
All experiments were done in triplicate or quadruplicate and plotted with s.e.m. All statistical analysis was performed using Prism 4.0 (GraphPad Inc.) or Excel running on IBM-PC compatible computer on the Windows XP-operating system. Statistical comparisons for paired data were analyzed by the Student’s t-test for the in vitro assays, whereas ANOVA was used to analyze the statistical significance for in vivo xenograft models. Statistical significance was defined as P<0.05.
Results
RNA interference against p21/Waf-1 enhances adenovirus replication
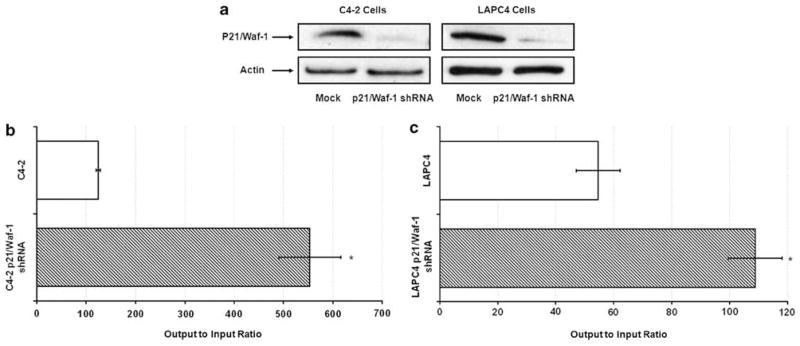
We have previously used histone deacetylase inhibitors (HDACI) to enhance the expression of adenovirus receptors for cancer gene therapy.12,26 Although adenovirus infectivity was increased, we found that HDACI-induced p21/Waf-1 expression, resulted in reduced adenovirus replication and cytotoxicity. Moreover, total adenovirus yields were increased over three-fold in cells, which lacked p21/Waf-1. We hypothesized that the potentcy of our oncolytic viruses could be markedly enhanced by expressing short hairpin RNAs (shRNAs) targeting p21/Waf1. To test this hypothesis we first used p21/Waf1 shRNA and control vectors to establish two prostate cancer cell line models, C4-2 and LAPC-4, with native or reduced p21/Waf-1 expression (Figure 1a). These cell lines were infected with equal multiplicity of infection (MOI) of a prostate-specific CRAd, Ad5-RV004, and total viral output was quantified. Cells with reduced p21/Waf-1 expression produced two- to five-times more infectious adenovirus compared with cells containing vector alone (Figures 1b and c). Similarly, the yield of wild-type adenovirus (CN702) was nearly doubled in cell lines with reduced p21/Waf-1 expression (supplementary figure 2A). These results suggest that oncolytic adenoviruses armed with p21/Waf1 shRNA expression cassettes would have enhanced replication kinetics and therapeutic efficacy.
Figure 1.
Knocking down p21/Waf-1 increases adenovirus titers. Western blot analysis showing stable knockdown of p21/Waf-1 in C4-2 and LAPC-4 cells using shRNA against p21/Waf-1 (a), Prostate specific conditionally replicating adenovirus (Ad5-RV004) replicates better in p21/Waf-1 knockdown C4-2 (b) and LAPC-4 cells (c) compared with the control cell lines. Data plots represent output to input ratio from three individual experiments at 96 h post infection and is given as the mean±s.e of triplicate samples. *P<0.05.
Knocking down p21/Waf-1 induces prostate-specific promoter by upregulating AR expression
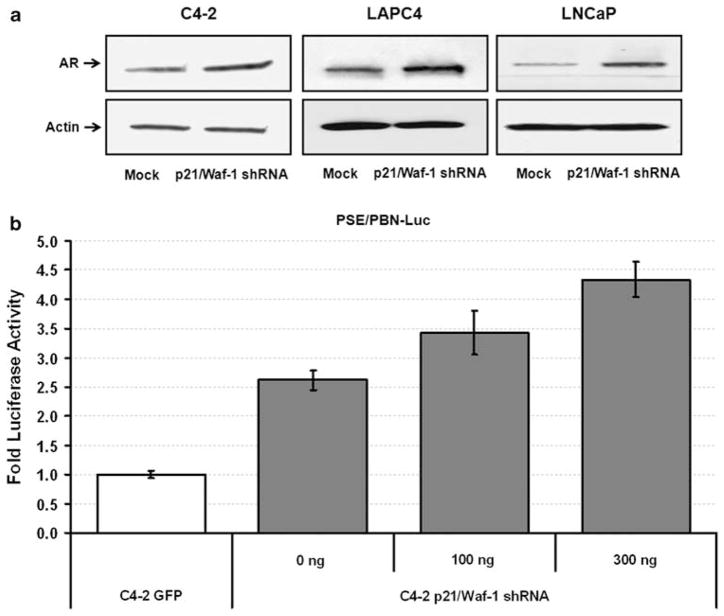
One of the interesting findings that we noticed was that knocking down p21/Waf-1 with shRNA resulted in an increased expression of the androgen receptor. As shown in Figure 2a C4-2, LAPC4 and LNCaP cells with stable knockdown for p21/Waf-1 expression had higher AR expression compared with the mock cells. To further verify that this increase in the total AR expression was indeed a functional increase; a reporter assay was performed using rat probasin promoter and prostate-specific enhancer (PSE/PBN) driving luciferase expression. Briefly, stable p21/Waf-1 knockdown C4-2 cells or control cells were transfected with equal amounts of reporter plasmids (pRL–PSE/PBN–FLuc together with a renella luciferase-expressing plasmid pCMV-RL for normalization). C4-2 cells with p21/Waf-1 knockdown had twofold-induced expression of luciferase compared with the mock C4-2 cells (Figure 2b). This induced expression of the PSE/PBN promoter in p21/Waf-1 knockdown cells was found to be dose dependent as further overexpression of the same p21/Waf-1 shRNA construct (pSuper-puro–EGFP–shRNA p21/Waf-1) in these stable C4-2 p21/Waf-1 knockdown cells showed an increase in the total amount of Luciferase expression. We repeated the same experiment in C4-2 cells to examine the luciferase expression driven by PSE/PBN and included a control GFP shRNA27 instead of plasmid alone and observed similar results of PSE/PBN induction only in cells lacking p21/Waf-1 (supplementary data 2B). This data shows that shRNA against p21/Waf-1 is responsible for the induced expression of the ARE-based PSE/PBN promoter and can be translated in the construction of our gene therapy vector.
Figure 2.
Induced expression of androgen receptor in p21/Waf-1 knockdown cell lines. Western blot analysis showing overexpression of AR in stable p21/Waf-1 knockdown C4-2, LAPC-4 and LNCaP cells, β-actin was included to serve equal amount of loading across the wells (a). Firefly luciferase assay was performed to study the induction of AREs-based prostate-specific enhancer and rat probasin promoter (PSE/PBN) in p21/Waf-1 knockdown C4-2 or control cells. Overexpression of the same shRNA construct against p21/Waf-1 in stably selected p21/Waf-1 knockdown C4-2 cells showed further increase in the promoter inducibility suggesting the dose-dependent response of the p21/Waf-1 knockdown in AR induction. Firefly luciferase activity was normalized to Renilla luciferase expression and plotted as fold luciferase expression. Data set represent mean±s.e. of the quadruplicate experiment (b).
Construction and replication kinetics of the prostate-specific CRAd virus carrying shRNA against p21/Waf-1
The p21/Waf-1 protein has been shown to interact directly with adenoviral E1A protein, resulting in an inactivation of p21 cyclin-dependent kinase activity.28 We therefore hypothesized that replicating adenovirus, which expresses a p21 shRNA will have enhanced replication potential. However, due to the organization of most conditionally replicating viruses we explored alternative regions for expression of the shRNA cassette. For example, most Conditionally replicating adenoviruses (CRAds) use a tissue or cancer-selective promoter upstream of the viral immediate early or early gene that is E1A or E1B in the E1 region for tissue specificity.22,29 Alternatively, the natural viral early gene promoters are retained and cancer specificity is achieved by modifications to the viral E1A or E1B genes.30–32 To find out what other region of the adenovirus will be permissive for functional knockdown, we performed a head to head comparison of the same shRNA construct against luciferase in both the E1 vs the fiber region of adenovirus. HEK293 cells infected with overexpressing luciferase adenovirus (1MOI) were co-transduced with non replicative viruses that carry shRNA against luciferase either in E1 (AdTrack shRNA–Luc) or fiber region (AdTrack–Fex–shRNA–Luc) at an MOI of 10. After 48 h, plates were read for Luciferase and normalized to the viral GFP expression. As shown in supplementary Figure 3, both viruses were able to knockdown luciferase expression to similar levels. We conclude that the fiber gene region is a viable region for housing shRNA expression cassettes in CRAds. Based on this finding, we made a prostate-specific CRAd Ad5-RV004.21 that expresses E1A gene under the control of human prostate-specific enhancer and rat probasin promoter (PSE/PBN) whereas the shRNA against p21/Waf-1 driven by H1 promoter was placed after the fiber region as described in the Materials and methods section.
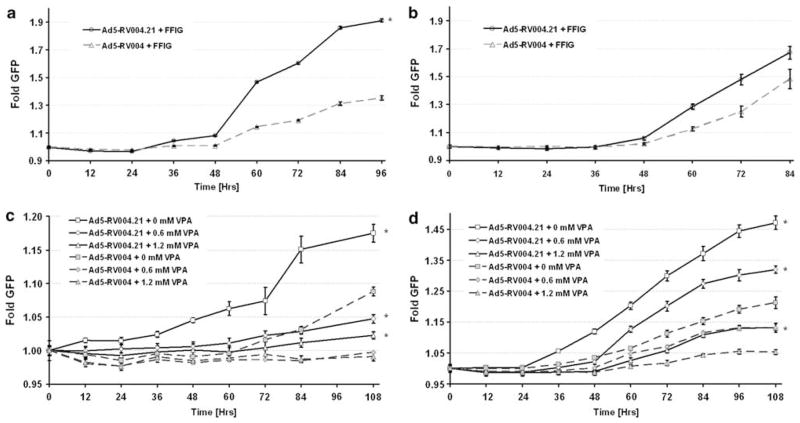
To show whether or not Ad5-RV004.21 (which contains a shRNA against p21/Waf-1) was able to replicate better than Ad5-RV004 (lacking p21/Waf-1 shRNA) we used the FFIG reporter system, which places a GFP gene under the control of the major late promoter (MLP), allowing us the ability to measure viral replication in real time in a non-invasive high-throughput assay. LNCaP cells plated at a density of 5 × 104 cells per well were infected with MOI of five with test viruses (Ad5-RV004.21 or Ad5-RV004) in the presence of a reporter virus FFIG (MOI 10) in complete medium supplemented with 5 nM of R1881. As shown in Figure 3, there was a significant difference in the replication kinetics between the two viruses (P<0.05), which started as early as 48 h post infection (Figure 3a). This shows that the CRAd virus that carries a shRNA against p21/Waf-1 (Ad5-RV004.21) replicates faster than a virus without the p21/Waf-1 shRNA (Ad5-RV004) in LNCaP cells. Similar results of enhanced Ad5-RV004.21 viral replication over Ad5-RV004 were also obtained in the androgen-independent C4-2 cell line (Figure 3b). We also compared the replication potency of the non-armed Ad5-RV004 and the p21/Waf-1 shRNA armed Ad5-RV004.21 in C4-2-proficient and p21/Waf-1 knockdown prostate cancer cells. As shown in 72 h post infection output to input assay (supplementary Figure 4), Ad5-RV004.21 was far better in producing infectious particles compared with Ad5-RV004 in wild type C4-2 cells. There was no difference in replication potential between the two viruses in C4-2 p21/Waf-1 Knock down cells, further emphasizing the importance of p21/Waf-1 in the biology of viral replication. We have previously shown that HDACI suppresses viral oncolysis in a process, which involves but is not totally dependent on HDACI induction of p21/Waf-1.12 To show if shRNA-based p21/Waf-1 knockdown can overcome HDACI suppression of viral replication, we infected LNCaP and C4-2 cells with either Ad5-RV004.21 or Ad5-RV004 in the presence of different concentrations of VPA (0, 0.6, 1.2mM). As shown in the Figures 3c and d, Ad5-RV004.21 replicate better than Ad5-RV004 (Figures 3c and d).
Figure 3.
Replication kinetics of Ad5-RV004.21 vs Ad5-RV004 in prostate cancer cells. Ad5-RV004.21 or Ad5-RV004 were assessed for kinetics and degree of viral replication by measuring green fluorescent protein (GFP) expression from the major late promoter in the reporter virus FFIG. As the MLP is only active in late viral replication, this activity is surrogate for viral replication. Ad5-RV004.21 or Ad5-RV004 were used at an equal multiplicity of infection (MOI) of 5 that were accessed for kinetics and degree of viral replication by co-infecting with reporter FFIG virus (10 MOI). In both LNCaP and C4-2 cells Ad5-RV004.21 showed higher fold replication compared with the Ad5-RV004 (a and b). LNCaP or C4-2 cells treated with VPA at different concentrations (0, 0.6, 1.2mM) were infected with ether Ad5-RV004.21 or Ad5-RV004 (5 MOI) in the presence of a reporter FFIG virus (10 MOI). GFP expression was measured and plotted as fold induction of GFP over the FFIG background. In both cells lines, Ad5-RV004.21, which carries shRNA against p21/Waf-1 was able to replicate faster than Ad5-RV004, which lacked p21/Waf-1 shRNA (c and d). *P<0.05.
Dose-dependent downregulation of p21/Waf-1 by Ad5-RV004.21
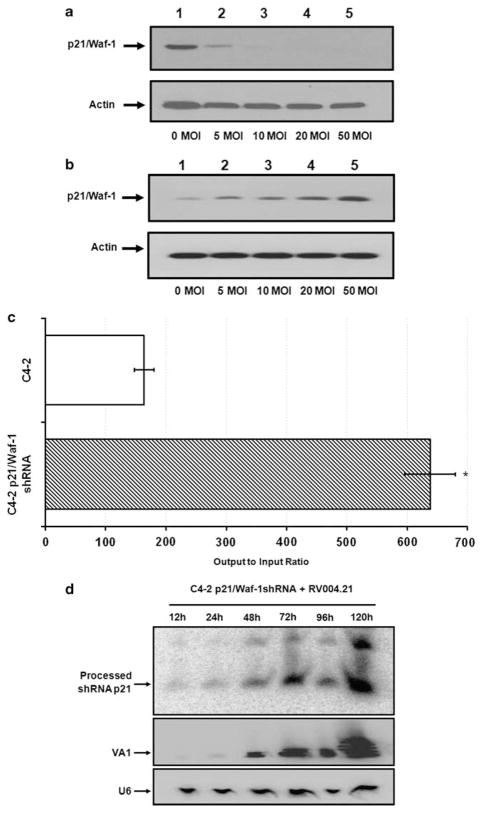
Gene silencing using RNA interference is known to work in a dose-dependent manner.33 To find out if that was still the case with an adenovirus carrying shRNA against p21/Waf-1, C4-2 cells were infected with Ad5-RV004.21 or Ad5-RV004 at different MOI’s (0–50). Cells were harvested in RIPA buffer approximately 24 h post-infection for western blot analysis. As shown in Figure 4a, levels of p21/Waf-1 protein were completely decreased to undetectable levels with high MOIs of the Ad5-RV004.21 in C4-2 cells. Interestingly, there was a dose-dependent increase of p21/Waf-1 after Ad5-RV004 infection (Figure 4b). To further elucidate these findings we made use of C4-2-p21/Waf-1shRNA knockdown or control cell lines and infected them with Ad5-RV004.21. Virus titer assays were performed 96 h post infection on HEK293 cells. As shown in Figure 4c, viral titers recovered from the stable p21/Waf-1 knockdown cells were significantly higher than that of control C4-2 cells. To explain these higher titers of Ad5-RV004.21 in cells which were selected to express a p21 shRNA, we looked at the levels of the processed p21/Waf-1 shRNA in the adenoviral-infected cells. Total RNA from the C4-2-p21/Waf-1shRNA cells infected with Ad5-RV004.21 at 0.5 MOI were subjected to northern blot analysis and probed with an oligo designed against the processed sense strand of the p21/Waf-1 shRNA. The same blot was used for an endogenous adenoviral Pol III gene (VA1), which results in a non-coding RNA as a viral control as well as endogenous U6 RNA, as a cellular control. As shown in the Figure 4d, there was a tremendous increase in the amount of the processed p21 shRNA over time compared with the initial 12 h infection. Similarly, higher levels of VA1 were also detected over time in these C4-2-p21/Waf-1–shRNA cells. The high levels of processed p21–shRNAs in the cell lines that already expressed shRNA against p21/Waf-1 might explain higher titers in our output to input viral titer assays. In other words, the stable p21/Waf-1 shRNA C4-2 cells were not completely devoid of basal p21/Waf-1 expression and even minor basal expression of p21/Waf-1 can have profound effects on viral replication.
Figure 4.
Downregulation of p21/Waf-1 by Ad5-RV004.21 in a dose-dependent manner: Western blot showing p21/Waf-1 expression in C4-2 cells with increasing MOIs (0, 5, 10, 20, 50) of Ad5-RV004.21 virus (a) or Ad5-RV004 virus (b) after 24 h of infection. Output to input assay of the Ad5-RV004.21 virus from cells stably expressing shRNA against p21/Waf-1 96 h post infection (c). Northern blot analysis performed on the total RNA extracted from the stably expressing p21/Waf-1-shRNA C4-2 cells infected with Ad5-RV004.21 (MOI 0.5). A non-coding VA1 adenoviral RNA was probed with VA1 probe that accounts for the presence of replicating virus and a probe complementary to U6 was used to ensure equal amount of loading across different lanes (d). *P<0.05.
Combinatory studies of Adriamycin with Ad5-RV004.21 or Ad5-RV004 virus in C4-2 cells
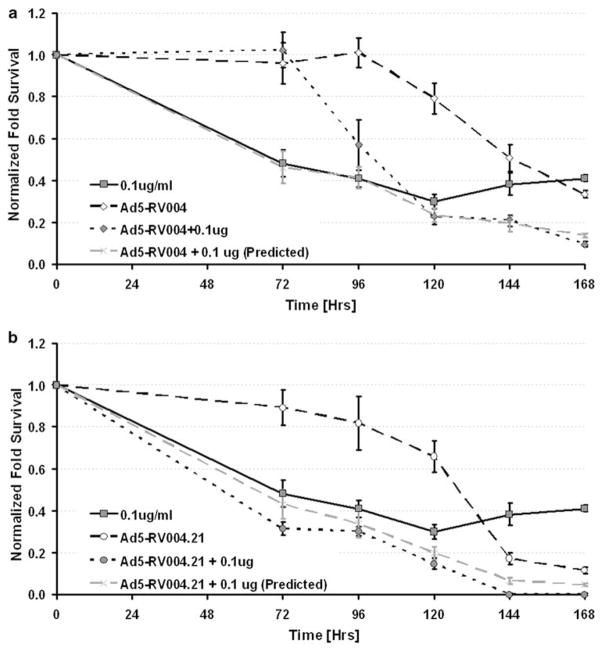
Combination treatment of viruses with radiation or chemotherapy hold promise as a new strategy for cancer treatment. However, there are reports of attenuated cytotoxicity of CRAds when combined with chemotherapies (for example, doxorubicin34). To investigate the oncolytic activity of Ad5-RV004 or Ad5-RV004.21 in the presence of adriamycin, C4-2 cells were infected with either Ad5-RV004 or Ad5-RV004.21 (MOI=2) in the presence or absence of adriamycin (0.1 ug ml−1) in 96-well plates. Cell cytotoxicity was measured by MTS assay after 72 h of treatment and followed every 24 h for a total of 7 days. All treatments were normalized to background MTS-treated media and plotted as a fold decrease in cell survival to the untreated control C4-2 cells. Data for the Ad5-RV004 or Ad5-RV004.21 was plotted separately in the presence or absence of 0.1 ug ml−1 adriamycin. The predicted value for the combinatory effect was calculated and is given by the dotted gray line. As shown in Figure 5a, the treatment of adriamycin alone was able to kill almost 50% of the C4-2 cells by 72 h of treatment. In Ad5-RV004-infected cells, the oncolysis of the infected cells was not seen till 120 h post infection and when both treatments were combined their additive effect became obvious by 168 h in C4-2 cells (Figure 5a). On the other hand, C4-2 cells infected with Ad5-RV004.21 alone was able to kill about 20% of the infected C4-2 cells by 96 h (Figure 5b), and when both treatments were combined together a supra-additive effect was observed from the beginning of the treatment as shown by the closed circles (Figure 5b). This supra-additive effect of the Ad5-RV004.21 in the combinatory studies appears to be the direct result of the p21/Waf-1shRNA, which enhanced drug-induced cell death.
Figure 5.
Combinatory studies of Ad5-RV004.21 or Ad5-RV004 with adriamycin. Growth inhibition and cytotoxicity of C4-2 cells treated with Ad5-RV004 (MOI=2) (a) or Ad5-004.21 (MOI=2) viruses (b) alone, or in combination with Adriamycin (0.1 ug ml−1) was accessed by MTS assay at different time points (24–168 h) and plotted as fold survival over background. Dotted gray line represents the predicted combined killing of the virus and drug. Data shown represent mean±s.e. of the quadruplicate experiment.
In vivo oncolytic activity of the prostate specific Ad5-RV004.21 virus
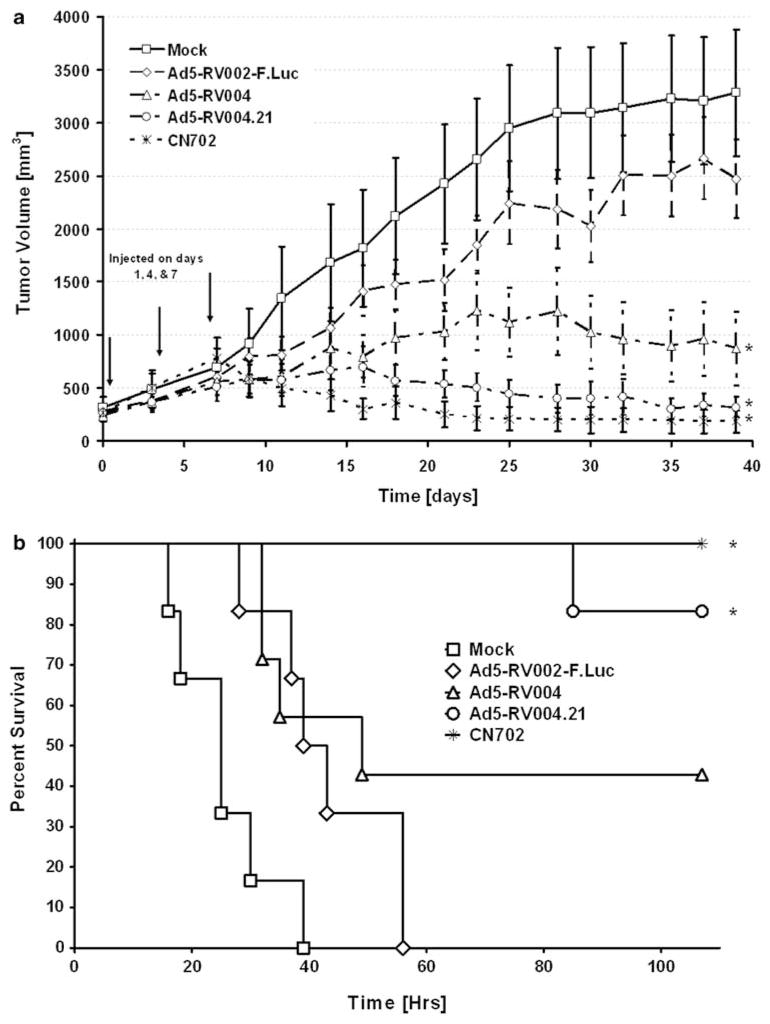
We next evaluated the benefit of adenoviral p21/Waf-1 knock-down by comparing the oncolytic activity of Ad5-RV004.21 vs the non-shRNA containing Ad5-RV004 and the E3-deleted wild-type adenovirus CN702 along with controls (buffer only and the replication-defective luciferase expressing adenovirus Ad5-RV002-F-Luc virus). C4-2 cells were subcutaneously inoculated into the dorsal rear flank region of athymic nude mice as described in the Materials and methods section. When tumor volume reached approximately 0.2 cm3, animals were randomized into four groups. Equal number of infectious viruses (that is, 1 × 108 plaque forming units/tumor of CN702, Ad5-RV004.21, Ad5-RV004, Ad5-RV002-F-Luc virus or mock infection phosphate-buffered saline) was administered intra-tumorally on day 1, 4 and 7. Tumor volume was measured every third day for 35 days and plotted as shown in Figure 6a. Profound tumor growth was observed in the PBS control and in replication incompetent Ad5-RV002-F-Luc virus, with tumor volume approaching the maximum allowable size of 2.0 cm3 by end of 24th day. Although, Ad5-RV004 suppressed tumor growth in a statistically significant manner compared with the control groups, this activity was not as dramatic as the Ad5-RV004.21-treated animals. Ad5-RV004.21 virus was able to suppress xenograft tumor growth similarly to that of wild-type (CN702) virus and there was no significant difference between the CN702 and Ad5-RV004.21 treated tumors. We also compared the survival benefit of these animals treated with either Ad5-RV004 or Ad5-RV004.21 viruses. Animals were monitored daily for 100 days for signs and symptoms of any weakness or ulcerative tumors and were removed either after they died naturally or showed a heavy tumor burden (when tumor sizes were ≥2.0 cm3). Survival was evaluated by Kaplan–Meier analysis and groups compared by the log-rank test using a P<0.05 as a cutoff for statistical significance. As shown in Figure 5b, all negative control groups (Ad5-RV002-F-Luc or PBS) died within 60 days of treatment. However, 40% of the Ad5-RV004 (n=5) treated mice survived within duration of the experiment (>100 days) compared with 85 and 100% survival of Ad5-RV004.21- and CN702-treated animals. A significant difference was found between the PBS-treated mocks vs all three Ad5-RV004, Ad5-RV004.21 and wild-type CN702 treatments. However, when all the viral-treated groups were compared with the non-replicative Ad5-RV002-F.Luc-treated animals, only Ad5-RV004.21 and wild-type CN702-treated groups were found significant and no statistical significance were observed between the Ad5-RV002-F.Luc controls vs Ad5-RV004-treated animals (Figure 5b). This phenomena of enhanced tumor suppression associated with improved survival by Ad5-RV004.21 compared with Ad5-RV004 appears to be a direct result of p21-Waf-1 knockdown.
Figure 6.
In vivo oncolytic activity of Ad5-RV004.21 virus. Tumor xenograft model using C4-2 prostate cancer cells were injected at a density of 1 × 106 cells into the dorsal rear flank region of the athymic nude mice to examine the efficacy of Ad5-RV004 or Ad5-RV004.21 viruses. Equal number of infectious viruses, that is, 1 × 108 plaque forming units/tumor of either CN702 (wild type), Ad5-RV004.21, Ad5-RV004, Ad5-RV002-F-Luc virus, or mock infection (phosphate-buffered saline) were administered on day 1, 4 and 7 and measured every other day for 38 days. There was a significant difference between the tumor volumes in animals that were treated with wild type CN702 virus and Ad-RV004 virus (P<0.05); however, no significant difference was observed in CN702 vs Ad5-RV004.21-treated animals (P>0.05) (a). Kaplan–Meier plot showing survival of mice bearing s.c. C4-2 tumor xenografts treated intra-tumorally with different viruses (CN702, Ad5-RV004, Ad5-RV004.21 or Ad5-002-F-Luc) or PBS. Animals were killed when tumor area was ≥2.0cm2. A median survival vs time was evaluated using a log-rank test (P<0.05). All mock-treated groups (Ad5–RV002-F–Luc or PBS) did not survive beyond 60 days of treatment. Animals treated with CN702 (wild-type virus) had 100% survival, whereas 40% of the Ad5-RV004 (n=5) and 85% of Ad5-RV004.21-treated animals survived beyond 100 days of treatment. There was a significant difference in the survival of the PBS-treated mocks vs the three Ad5-RV004, Ad5-RV004.21 and CN702 viral treatment groups, or Ad5-RV002-F.Luc Control virus vs CN702 or Ad5-RV004.21-treated animals. However, no statistical difference was observed between the Ad5-RV002-F.Luc controls-treated group vs Ad5-RV004-treated animals (b). *P<0.05.
Discussion
Despite escalating research efforts for the treatment of prostate cancer, advanced prostate cancer (PCa) remains incurable. Traditional chemotherapeutic strategies do provide some benefits by nominally extending life expectancy (less than 2.5 months) for hormone-resistant disease,35 yet resistance to such therapies remains a serious clinical problem. One strategy for approaching this recalcitrant disease has been the development of prostate-specific CRAds. Prostate-specific CRAds are generated by placing the adenoviral genes responsible for controlling replication (E1A, E1B or E4) under the control of a prostate-specific promoter. Early prostate-specific CRAds used the PSA promoter and enhancer to control E1A expression10 or the rat probasin promoter to control E1A plus the PSA promoter and enhancer to control E1B.22 Although this strategy has shown clinical efficacy in early clinical trials36,37 the potency of these viruses has been inadequate for a single modality therapy. We have previously reported different cellular mechanisms that might be responsible for attenuating adenovirus replication,12,21 including upregulation of p21/Waf-1 by HDACI. The cell-cycle-dependent kinase inhibitor, p21/waf-1, has been linked to many other functions in addition to its activity to arrest the cells at G1 or G2. It is also known to interact with proliferating cell nuclear antigen, which can inversely affect DNA repair and replication.38 Similarly, p21/Waf-1 was found to interact with procaspase-3, inhibiting Fas-mediated cell death.17 Other investigators have also reported the protective effect of p21/Waf-1 against drug-induced apoptosis. Looking at these inhibitory functions of cyclin-dependent kinase inhibitor-p21/Waf-1, we became interested in exploring the role of p21/Waf-1 in adenoviral oncolysis. It has been recently reported that overexpression of Androgen receptor in cell lines downregulate the expression of p21/Waf-1;39 however, to our knowledge the converse effect of p21/Waf-1 on androgen receptors has never been studied. We report for the first time that knockdown of p21/Waf-1 by shRNA in prostate cancer results in increased AR expression and AR-dependent promoter activity compared with controls. Using a shRNA against p21/Waf-1 in the viral backbone, we were able to show accelerated viral replication and enhanced viral cytotoxicity against prostate cancer cells.
During the past several years, numerous studies have shown efficacious silencing of genes by shRNAs in an adenoviral backbone.40,41 Both exogenous and endogenous genes have been silenced, and promising results have been obtained across multiple organs and tissues. However, there are reports describing the inhibition of RNA interference by adenoviruses, which are thought to be the function of one of adenoviral endogenous genes, the noncoding VA1 RNA. The VA1 RNA is a 160-nt long RNA that is expressed at very high levels during the adenoviral life cycle42 and has been thought to have a role in suppressing adenovirus mRNA-specific RNAi response inside infected cells. VA1 RNA has also been shown to inhibit the biogenesis of both micro-RNAs and siRNAs by inhibiting nuclear export of the pre-micro-RNA and shRNA precursors and by inhibiting Dicer function by binding to Dicer directly.43 Keeping these effects of VA1 in mind we looked at the processed p21/Waf-1 shRNA levels in parallel with non-coding VA1 RNA of the adenovirus (Figure 4d) but did not find any significant effect of the VA1 on the processing of p21/Waf-1 shRNA in the viral backbone.
In this study, we developed prostate-specific CRAds using the Cre-recombinase-based pFex system24 where the shRNA against p21/Waf-1 was placed downstream of the fiber gene. The CRAd was rendered prostate-specific by placing the E1A gene under the control of the PSA enhancer fused to the rat probasin promoter. Using the FFIG reporter assay we were able to show real-time enhanced replication of the Ad5-RV004.21 in different prostate cancer cell lines. This phenomenon was attributed to the p21/Waf-1 knockdown because the same virus lacking the shRNA against p21/Waf-1 was unable to show as efficient replication or as high viral titers in prostate cancer cells (Figure 3). We further investigated these findings in an in vivo model. Animal xenografts were injected with the same dose (3 × 10 8 PFU) of Ad5-RV004.21 or CN702 (E3 deleted wild-type virus) and showed no significant difference in tumor regression at any given time point throughout the course of study. However, Ad5-RV004 (no shRNA) treatment was less effective in suppressing tumor growth when compared with either wild-type CN702 or Ad5-RV004.21 treatments (Figure 5). Other contributing factors to the difference in oncolytic activity between Ad5-RV004 and Ad5-RV004.21 might be the tumor microenvironment. Tumors have a disorganized vasculature, which results in an unbalanced blood supply and significant perfusion heterogeneities. As a consequence, many regions within tumors are transiently or chronically hypoxic. It has been previously reported that hypoxic conditions lead to cell cycle arrest through p21/Waf-1 overexpression and induction of apoptosis that is independent of p53.44,45 Similarly there is data that show adenovirus replication compromised in hypoxic tissues.46 Our findings using an adenovirus that can knockdown p21/Waf-1 expression might help in resolving these observations and suggest that any processes that result in overexpression of p21/Waf-1 might be counter-productive in current oncolytic adenoviral therapy. An additional benefit in employing p21/Waf-1 knockdown in prostate cancer cells was a desire to enhance bio-sensitization of our CRAd vectors to conventional chemotherapeutic agents. In our preliminary studies with p21/Waf-1 knockdown C4-2 cells, both adriamycin and HDACI showed an increase in cell death compared with the wild-type cell lines (data not shown). This finding was further confirmed when CRAd viruses were engineered to express shRNA against the p21/Waf-1. A supra-additive effect was observed at any given time point during the course of the experiment only in combinatory studies using adriamycin and Ad5-RV004.21, (Figure 5) further strengthening the importance of p21/Waf-1 shRNA in adenoviral-based bio-sensitization of drug targeted therapies. Other utilities of this approach could be in combination with radiation therapies as it has been reported that overexpression of p21/Waf-1 leads to the protective cellular mechanism during radiation therapies.47,48
In summary, our findings show the first use of a p21/Waf-1 shRNA incorporated into the backbone of a prostate-specific CRAd to augment its natural life cycle and a novel approach to induce promoters containing AREs, which drive the viral replication genes. To our knowledge this is the first report using RNAi to induce promoter activity enhancing the therapeutic efficacy of a CRAd.
Supplementary Material
Acknowledgments
This study was supported in part by grants from; FAMRI-the Flight Attendant Medical Research Institute YCSA award to Naseruddin Höti and The National Institutes of Health 1R01CA121153-01A2 to Dr Ronald Rodriguez.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on Cancer Gene Therapy website (http://www.nature.com/cgt)
References
- 1.Imai E, Isaka Y. Strategies of gene transfer to the kidney. Kidney Int. 1998;53:264–272. doi: 10.1046/j.1523-1755.1998.00768.x. [DOI] [PubMed] [Google Scholar]
- 2.Greber UF, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 3.Pombo A, Ferreira J, Bridge E, Carmo-Fonseca M. Adenovirus replication and transcription sites are spatially separated in the nucleus of infected cells. EMBO J. 1994;13:5075–5085. doi: 10.1002/j.1460-2075.1994.tb06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Israel H, Kleinberger T. Adenovirus and cell cycle control. Front Biosci. 2002;7:d1369–d1395. doi: 10.2741/ben. [DOI] [PubMed] [Google Scholar]
- 5.Morimoto K, Gosselink J, Kartono A, Hogg JC, Hayashi S, Ogawa E. Adenovirus E1a regulates lung epithelial Icam-1 expression by interacting with transcriptional regulators at its promoter. Am J Physiol Lung Cell Mol Physiol. 2009;296:L361–L371. doi: 10.1152/ajplung.90331.2008. [DOI] [PubMed] [Google Scholar]
- 6.Horwitz GA, Zhang K, McBrian MA, Grunstein M, Kurdistani SK, Berk AJ. Adenovirus small e1a alters global patterns of histone modification. Science. 2008;321:1084–1085. doi: 10.1126/science.1155544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari R, Pellegrini M, Horwitz GA, Xie W, Berk AJ, Kurdistani SK. Epigenetic reprogramming by adenovirus e1a. Science. 2008;321:1086–1088. doi: 10.1126/science.1155546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller DL, Myers CL, Rickards B, Coller HA, Flint SJ. Adenovirus type 5 exerts genome-wide control over cellular programs governing proliferation, quiescence, and survival. Genome Biol. 2007;8:R58. doi: 10.1186/gb-2007-8-4-r58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones N, Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci USA. 1979;76:3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. [PubMed] [Google Scholar]
- 11.Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P, et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–722. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- 12.Hoti N, Chowdhury W, Hsieh JT, Sachs MD, Lupold SE, Rodriguez R. Valproic acid, a histone deacetylase inhibitor, is an antagonist for oncolytic adenoviral gene therapy. Mol Ther. 2006;14:768–778. doi: 10.1016/j.ymthe.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Shiina M, Lacher MD, Christian C, Korn WM. RNA interference-mediated knockdown of p21(WAF1) enhances anti-tumor cell activity of oncolytic adenoviruses. Cancer Gene Ther. 2009;16:810–819. doi: 10.1038/cgt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niculescu AB, III, Chen X, Smeets M, Hengst L, Prives C, Reed SI. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol. 1998;18:629–643. doi: 10.1128/mcb.18.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuse T, Yamada K, Asai K, Kato T, Nakanishi M. Heat shock-mediated cell cycle arrest is accompanied by induction of p21 CKI. Biochem Biophys Res Commun. 1996;225:759–763. doi: 10.1006/bbrc.1996.1247. [DOI] [PubMed] [Google Scholar]
- 16.Gehen SC, Vitiello PF, Bambara RA, Keng PC, O’Reilly MA. Downregulation of PCNA potentiates p21-mediated growth inhibition in response to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L716–L724. doi: 10.1152/ajplung.00135.2006. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki A, Kawano H, Hayashida M, Hayasaki Y, Tsutomi Y, Akahane K. Procaspase 3/p21 complex formation to resist fas-mediated cell death is initiated as a result of the phosphorylation of p21 by protein kinase A. Cell Death Differ. 2000;7:721–728. doi: 10.1038/sj.cdd.4400706. [DOI] [PubMed] [Google Scholar]
- 18.Cayrol C, Knibiehler M, Ducommun B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene. 1998;16:311–320. doi: 10.1038/sj.onc.1201543. [DOI] [PubMed] [Google Scholar]
- 19.van Beusechem VW, Mastenbroek DC, van den Doel PB, Lamfers ML, Grill J, Wurdinger T, et al. Conditionally replicative adenovirus expressing a targeting adapter molecule exhibits enhanced oncolytic potency on CAR-deficient tumors. Gene Therapy. 2003;10:1982–1991. doi: 10.1038/sj.gt.3302103. [DOI] [PubMed] [Google Scholar]
- 20.Nebbioso A, Clarke N, Voltz E, Germain E, Ambrosino C, Bontempo P, et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat Med. 2005;11:77–84. doi: 10.1038/nm1161. [DOI] [PubMed] [Google Scholar]
- 21.Hoti N, Li Y, Chen CL, Chowdhury WH, Johns DC, Xia Q, et al. Androgen receptor attenuation of Ad5 replication: implications for the development of conditionally replication competent adenoviruses. Mol Ther. 2007;15:1495–1503. doi: 10.1038/sj.mt.6300223. [DOI] [PubMed] [Google Scholar]
- 22.Yu DC, Chen Y, Seng M, Dilley J, Henderson DR. The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res. 1999;59:4200–4203. [PubMed] [Google Scholar]
- 23.Li Y, McCadden J, Ferrer F, Kruszewski M, Carducci M, Simons J, et al. Prostate-specific expression of the diphtheria toxin A chain (DT-A): studies of inducibility and specificity of expression of prostate-specific antigen promoter-driven DT-A adenoviral-mediated gene transfer. Cancer Res. 2002;62:2576–2582. [PubMed] [Google Scholar]
- 24.Lupold SE, Kudrolli TA, Chowdhury WH, Wu P, Rodriguez R. A novel method for generating and screening peptides and libraries displayed on adenovirus fiber. Nucleic Acids Res. 2007;35:e138. doi: 10.1093/nar/gkm914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachs MD, Ramamurthy M, Poel H, Wickham TJ, Lamfers M, Gerritsen W, et al. Histone deacetylase inhibitors upregulate expression of the coxsackie adenovirus receptor (CAR) preferentially in bladder cancer cells. Cancer Gene Ther. 2004;11:477–486. doi: 10.1038/sj.cgt.7700726. [DOI] [PubMed] [Google Scholar]
- 27.Huang B, Kochanek S. Adenovirus-mediated silencing of huntingtin expression by shRNA. Hum Gene Ther. 2005;16:618–626. doi: 10.1089/hum.2005.16.618. [DOI] [PubMed] [Google Scholar]
- 28.Chattopadhyay D, Ghosh MK, Mal A, Harter ML. Inactivation of p21 by E1A leads to the induction of apoptosis in DNA-damaged cells. J Virol. 2001;75:9844–9856. doi: 10.1128/JVI.75.20.9844-9856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nettelbeck DM, Rivera AA, Balague C, Alemany R, Curiel DT. Novel oncolytic adenoviruses targeted to melanoma: specific viral replication and cytolysis by expression of E1A mutants from the tyrosinase enhancer/promoter. Cancer Res. 2002;62:4663–4670. [PubMed] [Google Scholar]
- 30.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 31.Leitner S, Sweeney K, Oberg D, Davies D, Miranda E, Lemoine NR, et al. Oncolytic adenoviral mutants with E1B19K gene deletions enhance gemcitabine-induced apoptosis in pancreatic carcinoma cells and anti-tumor efficacy in vivo. Clin Cancer Res. 2009;15:1730–1740. doi: 10.1158/1078-0432.CCR-08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh M, Wang H, Ishidoya S, Abe H, Moriya T, Hamada H, et al. Oncolytic virotherapy for prostate cancer by E1A, E1B mutant adenovirus. Urology. 2007;70:1243–1248. doi: 10.1016/j.urology.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 33.Raponi M, Arndt GM. Double-stranded RNA-mediated gene silencing in fission yeast. Nucleic Acids Res. 2003;31:4481–4489. doi: 10.1093/nar/gkg484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graat HC, Witlox MA, Schagen FH, Kaspers GJ, Helder MN, Bras J, et al. Different susceptibility of osteosarcoma cell lines and primary cells to treatment with oncolytic adenovirus and doxorubicin or cisplatin. Br J Cancer. 2006;94:1837–1844. doi: 10.1038/sj.bjc.6603189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mike S, Harrison C, Coles B, Staffurth J, Wilt TJ, Mason MD. Chemotherapy for hormone-refractory prostate cancer. Cochrane Database Syst Rev. 2006;4:CD005247. doi: 10.1002/14651858.CD005247.pub2. [DOI] [PubMed] [Google Scholar]
- 36.DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- 37.Small EJ, Carducci MA, Burke JM, Rodriguez R, Fong L, van Ummersen L, et al. A phase I trial of intravenous CG7870, a replication-selective, prostate-specific antigen-targeted oncolytic adenovirus, for the treatment of hormone-refractory, metastatic prostate cancer. Mol Ther. 2006;14:107–117. doi: 10.1016/j.ymthe.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Cooper MP, Balajee AS, Bohr VA. The C-terminal domain of p21 inhibits nucleotide excision repair In vitro and In vivo. Mol Biol Cell. 1999;10:2119–2129. doi: 10.1091/mbc.10.7.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang LG, Ossowski L, Ferrari AC. Overexpressed androgen receptor linked to p21WAF1 silencing may be responsible for androgen independence and resistance to apoptosis of a prostate cancer cell line. Cancer Res. 2001;61:7544–7551. [PubMed] [Google Scholar]
- 40.Yoo JY, Kim JH, Kim J, Huang JH, Zhang SN, Kang YA, et al. Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition. Gene Therapy. 2008;15:635–651. doi: 10.1038/gt.2008.3. [DOI] [PubMed] [Google Scholar]
- 41.Chu L, Gu J, Sun L, Qian Q, Qian C, Liu X. Oncolytic adenovirus-mediated shRNA against Apollon inhibits tumor cell growth and enhances antitumor effect of 5-fluorouracil. Gene Therapy. 2008;15:484–494. doi: 10.1038/gt.2008.6. [DOI] [PubMed] [Google Scholar]
- 42.Mathews MB, Shenk T. Adenovirus virus-associated RNA and translation control. J Virol. 1991;65:5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu S, Cullen BR. Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and MicroRNA biogenesis. J Virol. 2004;78:12868–12876. doi: 10.1128/JVI.78.23.12868-12876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmaltz C, Hardenbergh PH, Wells A, Fisher DE. Regulation of proliferation-survival decisions during tumor cell hypoxia. Mol Cell Biol. 1998;18:2845–2854. doi: 10.1128/mcb.18.5.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen BH, Hermiston TW. Effect of hypoxia on Ad5 infection, transgene expression and replication. Gene Therapy. 2005;12:902–910. doi: 10.1038/sj.gt.3302448. [DOI] [PubMed] [Google Scholar]
- 47.Wang YA, Elson A, Leder P. Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci USA. 1997;94:14590–14595. doi: 10.1073/pnas.94.26.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park SH, Park JY, Weiss RH. Antisense attenuation of p21 sensitizes kidney cancer to apoptosis in response to conventional DNA damaging chemotherapy associated with enhancement of phospho-p53. J Urol. 2008;180:352–360. doi: 10.1016/j.juro.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.