Abstract
α-Synuclein is a presynaptic protein that binds to phospholipid membranes and is involved in the pathogenesis of Parkinson's disease (PD). In this paper we describe the effects of adding wild-type α-synuclein (WT) and three familial PD mutants (A53T, A30P and E46K) to membranes containing 15–35 mol % anionic lipid. Tubules were observed to form in the membranes to an extent that depended on the α-synuclein variant, the anionic lipid content and the protein concentration. For all four variants tubule formation decreased with increasing anionic lipid content. Tubules were more readily observed with A30P and E46K than with WT or A53T. The results are consistent with a model wherein the helical content of α-synuclein increases with increasing anionic lipid content, and α-synuclein conformers with low helical content have a high propensity to induce tubule formation. This work, combined with previous work from our laboratory (Pandey et al., Biophys. J. 2009),1 shows that for WT adsorption of the protein has deleterious effects on the membrane when the anionic lipid concentration is less than 30 mol % (tubule formation) or greater than 40 mol % (re-organization of the bilayer, clustering of protein).
Keywords: Protein aggregation, supported lipid bilayers, epi-fluorescence microscopy, protein mis-folding
Introduction
α-Synuclein is a small (140 amino acids, 14 kDa) highly conserved neuronal protein that has been linked to neurodegeneration in Parkinson's disease (PD).2–4 Brain autopsies of patients suffering from PD reveal the presence of cytoplasmic inclusions (Lewy bodies) containing aggregated α-synuclein.5 While the majority of PD cases are sporadic in nature, three autosomal-dominant point mutations in the α-synuclein gene (A30P6, A53T7, and E46K8) have been linked to rare early-onset forms of PD. The role of α-synuclein in PD pathogenesis remains poorly understood.
Although the native function of α-synuclein has not been fully elucidated, the protein has been shown to regulate synaptic vesicle pools,9,10 dopamine release and reuptake11,12 and (in some studies) phospholipase D (PLD) activity.13,14 In solution the protein is unstructured.15 However, α-synuclein also avidly binds to membranes, and under these conditions the protein adopts an α-helical structure.1,16,17 The helical content of the membrane-bound protein increases with increasing anionic lipid content.18 Structural data indicate that α-synuclein binds to highly anionic membranes through the N-terminal region, which adopts an α-helical structure that sits on top of the membrane, while the C-terminal region remains unbound and unstructured.19–23 The N-terminal region (residues 1–95) consists of seven degenerate, 11-residue repeats, six of which contain a highly conserved motif `KTK(E/Q)GV'.15,18
The three α-synuclein mutants A53T, A30P, and E46K have different membrane-binding and aggregation propensities. Both A53T and wild-type α-synuclein (WT) adsorb to similar extents on rat brain vesicles24 and synthetic vesicles.25,26 A30P adsorbs less readily than WT on synthetic vesicles,25,26 lipid rafts27 and rat brain vesicles:24 it is assumed that the decreased adsorption is due to the presence of the helix breaking residue, proline. In contrast, E46K has been shown in some studies to adsorb more readily to negatively charged liposomes than WT.25,28 Relative to WT the mutants A53T29 and E46K25,30,31 form fibrils more rapidly in solution, while A30P29 fibrillizes more slowly. However, both A30P and A53T form potentially toxic prefibrillar oligomers (`protofibrils') more rapidly than WT.29,32
Biological membranes adopt various geometrically-distinct structures based on their function. For example, networks of tubular membranes with diameters ranging from 50–70 nm and lengths up to several micrometers in the trans Golgi network (TGN) participate in exporting protein and lipid cargo to different cellular destinations.33,34 In addition, tubule formation may be involved in the evolution of clathrin-coated pits in clathrin-mediated endocytosis.35 The role that proteins play in the formation of these structures is an area of active interest. A de novo-designed amphipathic α-helical peptide, Hel 13–5, induced tubule formation when added to egg phosphatidylcholine and Golgi-specific phospholipid liposomes.34 The proteins amphiphysin 1, endophilin 1, and epsin 1 have been shown to stimulate membrane tubulation in artificial bilayers, and this activity may relate to their role in modulating clathrin-mediated endocytosis. These proteins exhibit structural similarities with α-synuclein: all contain amphipathic α-helical domains. α-Synuclein has been observed to localize to the Golgi apparatus in bovine adrenal medullary chromaffin cells,36 and a recent study showed that the protein cooperates with polyunsaturated fatty acids to stimulate clathrin-mediated endocytosis.37 Given that α-synuclein localizes to the Golgi, modulates endocytosis, and shares structural similarity with proteins implicated in tubule formation, it is of interest to know whether α-synuclein is capable of forming tubules in membranes. In this paper we show conditions under which tubule formation follows α-synuclein adsorption to a phospholipid bilayer.
Tubule formation was monitored in supported lipid bilayers by epi-fluorescence microscopy. Supported lipid bilayers provide a convenient platform to examine soluble protein-lipid interactions; as two-dimensional structures they are ideal for imaging and unlike giant unilamellar vesicles (GUVs) they are stable under a wide range of solution conditions.38 Supported lipid bilayers can be formed on appropriately treated solid supports by vesicle fusion.39 A thin water layer is trapped between the bilayer and the support, allowing the lipids to retain their natural fluidity.40 In previous studies monitoring the adsorption of varying concentrations of α-synuclein on bilayers containing 30, 40 and 50 mol % PG, we observed that 40 mol % PG and 2.6 μM α-synuclein are required to observe the potentially deleterious effects of lipid de-mixing and protein clustering.1 Here we examined the adsorption of α-synuclein on bilayers containing 15, 25 and 35 mol % PG. Again potentially deleterious effects are observed, in this case tubule formation. It will be shown that tubule formation depends on: anionic lipid concentration, protein concentration, the identity of the α-synuclein variant, and protein incubation time. The biophysics of the process will be discussed along with physiological implications.
Materials and Methods
Materials
Stock solutions of L-α-phosphatidylcholine (egg PC), L-α-phosphatidylglycerol (egg PG), and 1-palmitoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-glycero-3-phosphocholine (NBD-PC) in chloroform were purchased from Avanti Polar Lipids, Inc. and used without further purification. Ethylenediaminetetra-acetic acid (EDTA) was purchased from Sigma Chemical Co. Sodium chloride (NaCl), sodium hydroxide (NaOH) and 4-(2-hydroxyethyl)-1-piperazineethanessulfonic acid (HEPES, free acid) were purchased from Mallinckrodt Chemicals. The Microcon YM-100 centrifugal filter units were obtained from Millipore.
Vesicle and supported lipid bilayer preparation
Lipid stock solutions in chloroform were mixed in the appropriate molar ratios, dried under a stream of nitrogen and placed under vacuum (on oil-free pump was used) for 1 hour. After drying the lipids were rehydrated in 100 mM NaCl solution. Large unilamellar vesicles were prepared by extruding 21 times through 100 nm polycarbonate membranes. The vesicle solution was then centrifuged for 5 minutes at 14,000 rpm (Eppendorf Minispin Plus). The vesicles were stored at room temperature and shielded from light. Supported lipid bilayers were formed by vesicle fusion inside a 60 μL perfusion chamber (Molecular Probes Inc.) on appropriately treated glass slides. The vesicles were mixed with 750 mM NaCl solution at a 1:1 ratio prior to fusion. After 15 minutes, excess vesicles were removed from the perfusion chamber using 750 mM NaCl solution, the pH was adjusted using 50 mM HEPES, 0.1 mM EDTA, and 750 mM NaCl, pH 7.4 buffer, and the slide was finally rinsed with 50 mM HEPES, 0.1 mM EDTA, pH 7.4 buffer to remove salt. Glass coverslips were washed in 7× detergent (MP Biomedicals), rinsed profusely in DI water, dried with a stream of nitrogen, and baked at 450 °C for 4 hours. The slides and vesicles were used within a day of preparation. At least 3 mL of buffer was passed through the perfusion chamber to ensure complete exchange. The bilayers were kept hydrated in 50 mM HEPES, 0.1 mM EDTA, pH 7.4 buffer prior to protein addition.
α-Synuclein expression and storage
Human α-synuclein (wild-type, A30P, E46K, or A53T) was expressed in E. coli and purified as described previously.18,41 Monomeric α-synuclein was isolated by gel filtration with a Superdex 200 column.1,17 The protein was then aliquoted and stored at −20 °C. Prior to each experiment; an aliquot was thawed and centrifuged through a Microcon YM-100 centrifugal filter unit with a molecular-weight cutoff of 100 kDa to remove any aggregates.
Imaging of supported lipid bilayers
A Nikon TE2000 fluorescence microscope equipped with a Cascade 512B CCD camera (Roper Scientific) was used (512 × 512 pixels; pixel size 16μm × 16μm). An X-Cite 120 arc lamp (EXFO) was used as a light source. For imaging, tail-labeled NBD-PC was present in the bilayer. The NBD fluorophore was imaged using a NBD filter set (Chroma Technology Corp.). Images were acquired using either a 40×, 1.30 NA objective or a 100×, 1.30 NA objective.
Results
Supported lipid bilayers, prepared by vesicle fusion, were used as a model membrane system.1,40 The bilayers were imaged using epi-fluorescence microscopy, and the fluidity of each bilayer was checked with fluorescence recovery after photobleaching (FRAP). For imaging, the bilayers contained 0.25 mol % tail-labeled NBD-PC. The bilayers were composed of varying amounts of egg phosphatidylglycerol (PG) and egg phosphatidylcholine (PC). The egg PG was prepared from egg PC by the action of phospholipase D; therefore, the tail compositions were identical for phospholipids used in this study. To determine if α-synuclein adsorption results in tubule formation, varying concentrations of α-synuclein (in 50 mM HEPES, 0.1 mM EDTA, pH 7.4) were added to the bulk solution. All experiments were conducted at pH 7.4 and room temperature.
Formation of lipid tubules
Figure 1 shows images acquired before and after a bilayer containing 15 mol % PG/ 84.75 mol % PC/0.25 mol % NBD-PC was exposed to 2.6 μM WT α-synuclein. In the absence of α-synuclein, the lipid bilayer was flat and uniform (Figure 1a). Upon addition of 2.6 μM WT α-synuclein, lipid tubules were clearly seen (Figure 1b, c, d). Control experiments where buffer-only solutions were introduced were conducted; in the absence of protein lipid tubules were never observed. As the bilayer was adjusted progressively out of focus (see progression from Figure 1b to Figure 1d), sections of the tubules came in and out of focus. One end of the tubules was attached to the surface of the membrane, while the other end was loose and underwent a fast wiggling motion. Due to this motion estimating the length and width of the tubules is difficult; we estimate that the majority have a length of 10 to 50 μm and place an upper bound on the width at 1 μm, which is the resolution limit of the system. A movie of tubule motion is available; to more clearly see the tubules gentle flow is applied, during which the tubules are observed to stretch out, when the flow stops they are observed to snap back. (Given that we know how much brighter the tubules are than the bilayer and assuming that they are labeled at the same percentage as the bilayer, a rough estimate of the tubule width could be determined when the tubules are stretched out, i.e. cylindrical in shape; unfortunately, application of flow causes the focus to drift, and the bilayers are significantly photobleached in the process of maintaining focus, rendering the information gathered insufficiently quantitative). If fluorescently labeled protein was used (see Pandey et. al.1 for details of labeling) it was observed that α-synuclein bound to both the tubules and the flat bilayer. The tubules were fragile and had a high tendency to shear off the membrane when excess labeled α-synuclein was exchanged out (an essential step for imaging the labeled protein). Therefore, to prevent the loss of tubules, unlabeled protein was used for all experiments herein. [A note on the presentation of the images in the paper: To make it easy to quickly focus the bilayer an aperture in the imaging system was brought in (this is the reason for the black edges in the figures). In those regions only background counts are acquired by the CCD camera; the bilayer is much brighter than the background, while the tubules are only a few percent brighter than the bilayer. Consequently, the tubules are not that easy to see (particularly when the images are printed). In Figures 2–4 we have adjusted the contrast to make the tubules more visible.]
Figure 1.
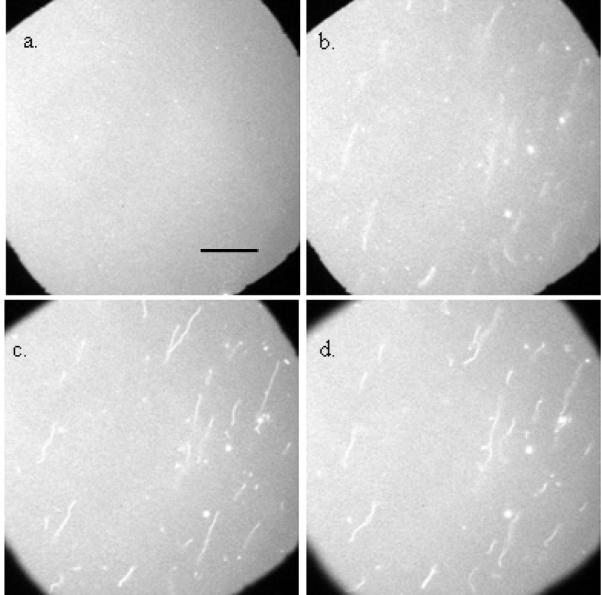
Tubule formation in PC/PG bilayers: Epi-fluorescence images of an 84.75 mol % PC/15 mol % PG/0.25 mol % NBD-PC bilayer. All the images are acquired at the same spot. Bilayer images were acquired (a) before the addition of protein, and (b, c, d) after the addition of 2.6 μM α-synuclein. In images obtained after the protein addition, the bilayer is adjusted progressively out of focus. The scale bar represents 40 μm.
Figure 2.
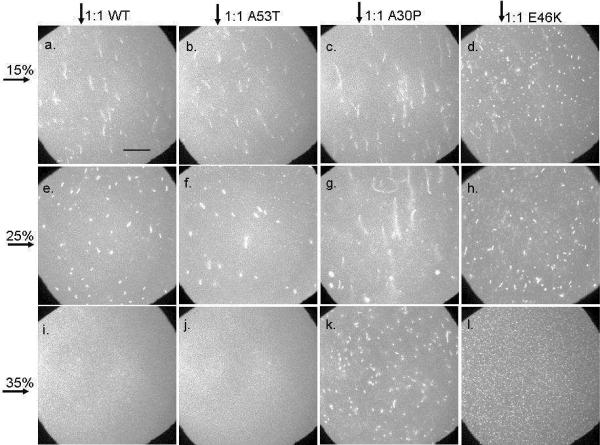
Effect of membrane charge density and protein variant identity on tubule formation: Epi-fluorescence images of bilayers with 15, 25, or 35 mol % PG (indicated by horizontal arrow) after the addition of 2.6 μM WT, A53T, A30P, or E46K (indicated by vertical arrow). Images were acquired 15 minutes after the addition of protein. Each bilayer contained 0.25 mol % NBD-PC. The scale bar represents 40 μm.
Figure 4.
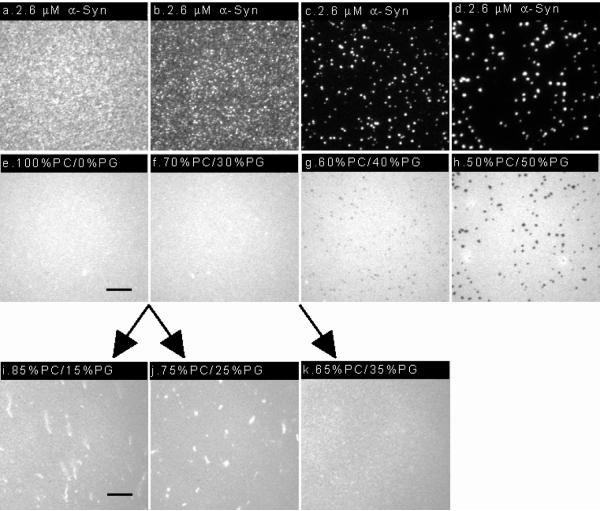
The variety of effects observed when 2.6 μM α-synuclein is added to bilayers of varying charge density: (a–h) Epi-fluorescence images acquired after the addition of labeled protein to bilayers containing 0, 30, 40 and 50 mol % PG. Protein images (a–d), lipid images (e–h), from our previous work.1 The bilayers contained 0.25 mol% NBD-PC and the protein was labeled with Alexa Fluor® 647. A 100×, 1.3 NA objective was used to acquire the images. The scale bar represents 10 μm. (i–k) Epi-fluorescence images acquired after the addition of unlabeled protein to bilayers containing 15, 25 and 35 mol % PG. The bilayers contained 0.25 mol% NBD-PC. A 40×, 1.3 NA objective was used to acquire the images. The scale bar represents 25 μm.
Effect of anionic lipid concentration and mutation
Supported lipid bilayers with 15, 25, and 35 mol % PG were used to examine the effect of membrane charge density on the formation of lipid tubules. Figure 2 shows representative images acquired after the bilayers were exposed to 2.6 μM (1:1 protein:lipid ratio, mol:mol) WT, A53T, A30P or E46K α-synuclein. The images were acquired 15 minutes after protein addition. The bilayers were uniformly fluorescent before the addition of protein (data not shown). Upon addition of 2.6 μM WT, lipid tubules were observed in the bilayers containing 15 and 25 mol % PG, respectively (Figures 2a, e). Similar results were obtained for 15 and 25 mol % PG bilayers when A53T was added (Figures 2b, f). For both WT and A53T, when the anionic PG content was increased from 15 to 25 mol %, the tubules shifted in appearance from long and thin to short and thick. When the PG content was further increased to 35 mol %, lipid tubules were no longer observed after adding WT (Figure 2i) or A53T (Figure 2j). In summary, with respect to tubule formation, WT and A53T behaved similarly.
In contrast, tubules were observed at all PG concentrations after the addition of A30P (Figure 2c, g, k) or E46K (Figure 2d, h, l). Like WT the length of the tubules decreased as the anionic lipid content increased. The tubules formed after the addition of A30P were longer, for a given PG concentration, than those formed after the addition of WT. The tubules formed after the addition of E46K were shorter, for a given PG concentration, than those formed after the addition of WT. Additionally, the E46K-induced tubules were more numerous.
For all the variants, the lipid tubules shortened as the anionic concentration increased, and tubule formation was no longer observed for WT and A53T at 35 mol % PG.
Effect of protein concentration and mutation
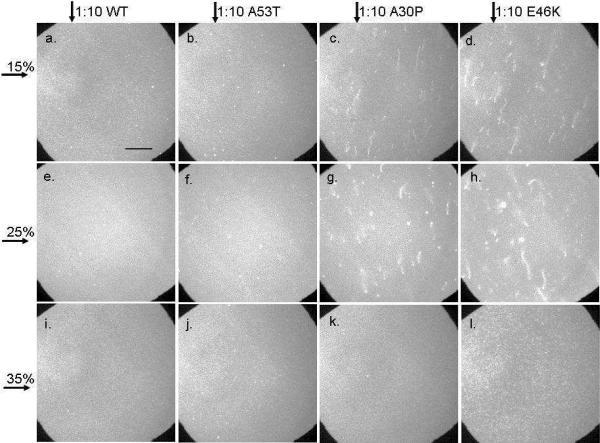
Three different concentrations of α-synuclein were examined: 2.6 μM (Figure 2), 0.26 μM (Figure 3) and 0.026 μM (not shown). From 2.6 to 0.026 μM the extent of tubule formation decreased. For WT and A53T, tubule formation was not observed, at all examined PG concentrations, after the addition of 0.26 μM protein (Figures 3a, b, e, f, i, j) or 0.026 μM protein (not shown). After the addition of 0.26 μM A30P tubule formation was observed at 15 (Figure 3c) and 25 (Figure 3g) mol % PG but not 35 mol % PG (Figure 3k). This was in contrast to the addition of 2.6 μM where tubules were observed in the presence of added A30P at all PG concentrations studied (Figure 2). The A30P-induced tubules appeared similar to those observed after the addition of 2.6 μM protein (Figure 2). After the addition of 0.26 μM E46K tubule formation was clearly observed at 15 (Figure 3d) and 25 (Figure 3h) mol % PG. In contrast, at 35 mol % PG (Figure 3l) very small tubules appeared to have formed, and these were right at the limit of detection. The E46K-induced tubules seen at 15 and 25 mol % PG were longer than those observed at the higher protein concentration (Figure 2). Tubules were not observed, at any of the examined PG concentrations, after the addition of 0.026 μM A30P or E46K (not shown).
Figure 3.
Effect of membrane charge density and protein variant identity on tubule formation: Epifluorescence images of bilayers with 15, 25, or 35 mol % PG (indicated by horizontal arrow) after the addition of 0.26 μM WT, A53T, A30P, or E46K (indicated by vertical arrow). Images were acquired 15 minutes after the addition of protein. Each bilayer contained 0.25 mol % NBD-PC. The scale bar represents 40 μm.
The effect of incubation time on the tubulation process was also examined, supporting information. For all four variants tubules were seen essentially immediately. The largest extent of growth occurred within the first five minutes, regardless of the identity of the α-synuclein variant.
Comparison with previous experiments
In previous work, we looked at the adsorption of 2.6 μM WT α-synuclein on bilayers containing 0, 30, 40 and 50 mol % PG.1 After α-synuclein addition the bilayers remained flat, and no tubules were observed. When 40 or 50 mol % PG was present the bilayers reorganized into PC-rich and PG-rich regions. In the presence of 30 mol % PG no de-mixing was observed in the membrane. This earlier work was done with protein that was labeled on the N-terminus (at ~20 mol %) with Alexa 647. If unlabeled WT α-synuclein was used the features observed at high anionic lipid content were reproduced: uniform, flat bilayer at 35 mol %; phase-separated, flat bilayer at 50 mol % PG, supporting information. In Figure 4 we show the combined results: Adsorption of 2.6 μM WT α-synuclein on bilayers containing 0, 30, 40 and 50 mol % PG (Figure 4a–h), previous work,1 and 15, 25 and 35 mol % PG (Figure 4i–k), this work. In the previous work labeled protein was used; images of both the protein (Figure 4a–d) and bilayer (Figure 4e–h) are shown.1 In this work unlabeled protein was used because, as previously mentioned, it is extremely difficult to rinse out the excess protein without shearing off some of the tubules, thereby altering the results; the images shown, therefore, are of the bilayer only (Figure 4i–k).
Discussion
Previously, we examined WT α-synuclein adsorption on PC/PG bilayers containing 30, 40 and 50 mol % PG.1 It was observed that: (i) At 30 mol % PG the bilayer remained uniformly mixed and the protein bound uniformly. (ii) At 40 and 50 mol % PG the addition of protein resulted in lipid de-mixing with PC-rich and PG-rich regions formed. Multilayers of protein bound on the PG-rich regions, but not the PC-rich regions. Additionally, it was observed that the amount of protein adsorbed on the surface was similar for bilayers containing 0 and 30 mol % PG; the amount of adsorbed protein on the bilayer increased only when multilayer formation was observed, 40 mol % PG and above. The results could be explained with a model wherein the helical content of α-synuclein increases with increasing anionic lipid content. As will be discussed below, this interdependence between helical content and anionic lipid content can also be used to account for the observation that tubule formation decreases with increasing anionic lipid content.
Recent experiments have shown that the helical content of membrane-bound α-synuclein increases with the anionic lipid content; within error, the same helical contents were observed for WT and E46K, whereas significantly lower helical content was observed for A30P.18 In the case of α-synuclein bound to highly anionic membranes the structure of the protein has been determined.19–23,42 The N-terminal region (residues 1–60) and highly hydrophobic central NAC domain (residues 61–95) form an 11/3 α-helix that resides on top of the membrane.19,43 The highly charged C-terminus (residues 96–140) remains unstructured and unbound. For the charged residues this is, from a free energy perspective, a highly favorable configuration: the cationic residues (all lysines) run down the two sides of the helix, allowing for close interaction with the anionic lipids; while the anionic residues (aspartate and glutamate) are all solvent exposed.19,21,44 From a free energy perspective the situation is less favorable for the hydrophobic residues: the majority of these are on the face that inserts into the membrane, but seven hydrophobic residues (Met 5, Val 16, Leu 38, Val 49, Val 71, Val 82, and Phe 94)19,43 are solvent exposed. Considering the free energy landscape, experiments showing that helical content increases as the anionic lipid content increases can be rationalized: A trade-off occurs as the anionic lipid content increases, with the expense of solvating some of the hydrophobic residues paid by the increasing electrostatic interaction.
The deformation of a membrane requires energy. For the process to be spontaneous it must result in an overall reduction in the free energy. One proposed mechanism involves the adsorption of amphipathic helices on membrane surfaces – the insertion of hydrophobic residues into the upper monolayer of the membrane leads to a local spontaneous curvature change (or area asymmetry) between the two monolayers.45,46 Membranes have a limited ability to withstand such asymmetric alterations (the free energy cost is high); consequently, there is a strong tendency to resolve the asymmetry via a shape change, which reduces the free energy. With regards to the results in this paper there are two questions to consider: (1) A variety of shape transformations may occur; are tubules an expected shape? (2) Given the energetic cost of tubule formation is it reasonable to observe them?
Recent calculations suggest that, in general, the adsorption of amphipathic helices on lipid bilayers is a very effective means of inducing membrane shape transformations.47,48 In general, the answer to our second question is that shape transformations upon amphipathic helix adsorption is not unexpected. The two studies looked at a variety of parameters and for the purposes of this paper two relevant ones are inclusion depth and inclusion area fraction. Both studies found that the positive spontaneous curvature increased as the helix penetrated more deeply. At a given depth, half-insertion into the hydrocarbon core48 or ~40 % of the monolayer thickness,47 the sign of the spontaneous curvature was observed to switch from positive to negative. In our system a positive spontaneous curvature would push the bilayer away from the support, while a negative spontaneous curvature would push it towards the solid support.
In the case of α-synuclein bound to 30 mol % phosphatidylserine (PS) vesicles it has been determined that the center of the helix penetrates ~1 – 4 Å into the membrane.21 This is a small insertion into the membrane; depending on the composition the thickness of a lipid monolayer is ~20 – 25 Å. The helical content of α-synuclein is dependent on the charge density of the membrane, and the charge density of the membrane is in turn dependent on the specific lipid composition, i.e. the charge density of a bilayer with 30 mol % anionic lipid varies depending on the area of the charged lipid. Given that the areas of PS and PG lipids are similar we expect that for α-synuclein bound to membranes containing 35 mol % PG the penetration depth is small relative to the thickness of the monolayer. Pushing the protein further into the membrane would increase the positive spontaneous curvature, increasing the drive to bend away from the solid support. The consequence for our two questions is: (1) Tubules are indeed a possible shape (they would form when the bilayer bends away from the solid support). (2) Not only are shape transformations expected, in general, but more specifically the energetic cost is expected to decrease as the protein inserts more deeply.
The primary observation of this paper is that tubule formation increases with decreasing anionic lipid content. In our previous work with WT the amount of bound protein varied with anionic lipid content only when multilayer formation occurred, 40 mol % PG and higher.1 Increasing area fraction of bound protein with decreasing anionic lipid content can therefore be ruled out as an explanation for the observations herein. From other work it is known that α-synuclein helicity decreases with decreasing anionic lipid content.18 We suggest (as one possible model) that the inverse relation between tubule formation and anionic lipid content may reflect the deeper penetration of α-synuclein into the membrane as the protein becomes less structured. Conceivably, unstructured α-synuclein may penetrate more deeply because (i) more of the hydrophobic residues are able to insert into the membrane, and/or (ii) the lysine residues are not aligned as they are on the surface of helical α-synuclein, and consequently they lose the ability to anchor the protein to the membrane and hinder insertion. In such a model, the NAC region of unstructured α-synuclein is expected to have the greatest propensity to penetrate the membrane because it has only three charged residues.
Several lines of evidence suggest that α-synuclein may bind phospholipid membranes in a relatively unstructured conformation (with lower α-helical content) under specific conditions. First, CD data indicate that α-synuclein adopts a structure with reduced α-helical content and thermal stability in the presence of vesicles with decreasing amounts of anionic lipid.18 Second, data from our recent studies addressing mechanisms by which α-synuclein modulates lipid clustering, and the structure of the clusters formed, are fully consistent with a model wherein the protein adopts a less helical conformation upon binding to membranes with reduced anionic lipid content.1,17 Third, NMR data suggest that α-synuclein binds to vesicles containing a mixture of anionic and zwitterionic lipids in at least two `modes' (or conformations) with different α-helical contents (in one conformation the protein consists of a short α-helical segment spanning residues 1–24 followed by a disordered stretch spanning residues 25–140).28
There are three other possible explanations for the inverse relation between tubule formation and anionic lipid content: (1) Other models that posit a role for helical conformers are also plausible. For example, α-synuclein has recently been shown to bind to liposomes or micelles as either a broken or extended helix, and the interconversion between these two forms is modulated by familial PD mutations.49,50 Accordingly, it is possible that one of these two helical forms promotes tubule formation and is preferentially populated at the surface of membranes with low anionic lipid content. (2) The spontaneous curvature is affected by the degree of structure of the protein. The existing theoretical work47,48 models the protein as a cylindrical rod; a priori it is difficult to predict how giving the protein structure, or lack thereof, would affect the spontaneous curvature. A significant effect, at first glance, seems unlikely, but without theory or simulations to give more depth to our understanding we leave it open as a possibility. (3) That changing the lipid mixture raises the free energy cost of tubule formation; this would occur if the spontaneous curvature of the membrane became less positive, or switched sign to negative, as the lipid mixture changed. In this case it is unlikely, the more PG present the greater the positive spontaneous curvature of the PG lipids;51 thus one would expect more tubule formation with increasing PG, not less. Additionally, the amount of PG required to abolish tubule formation depends on the identity of the α-synuclein variant. In particular A30P, as will be discussed below, results in tubule formation at higher PG content than WT.
While the overall pattern, decreasing amounts of tubule formation with increasing anionic lipid content, occurs with each of the four variants, there are differences observed among the variants. From other studies we know the following: (i) In circular dichroism experiments significantly less helical content was observed for A30P than WT; as the PG content increased from 10 to 25 mol % the helical content increased as follows: 4 ± 2 to 18 ± 7 % for WT and 0 to 4 ± 3 % for A30P. The helical content of WT and E46K was observed, within the error, to be the same.18 (ii) E46K adsorbs more readily to membranes than WT,25 A30P adsorbs less readily24–27 and A53T adsorbs roughly to the same extent.24–26 The observed WT results are compared with those obtained for each of the three variants below:
WT versus A30P
Tubules are observed after A30P adsorption at higher anionic lipid content and lower protein concentration (relative to WT). The differences which arise can be attributed to structural differences in the adsorbed protein: A30P is less helical, adsorption results in a larger perturbation of the membrane, and tubules form at a higher anionic lipid content, despite the fact that A30P adsorbs less avidly than WT.
WT versus E46K
Tubules are observed after E46K adsorption at higher anionic lipid content and lower protein concentration (relative to WT). Additionally, there are significantly more nucleation sites for tubule formation, consistent with the observation that E46K adsorbs more readily (E46K adsorbs both more quickly and to a greater extent than WT.25,28,52,53). Because WT and E46K show the same extent of helical content18 the observed differences can, at least in part, be attributed to the increased amount of adsorption and therefore increased area fraction of the protein inclusions; recent calculations show that with increasing area fraction of protein inclusions the membrane spontaneous curvature increases.47 The fact that E46K adsorbs more rapidly may also contribute to the observed differences: In solution α-synuclein is unstructured; upon adsorbing to a membrane it adopts structure. If protein adsorbs rapidly interactions between neighboring protein molecules could hinder the adoption of helical structure. Between this work and the circular dichroism work18 we cannot compare the rate of protein adsorption, leaving open the possibility that it occurred more quickly in the experiments reported herein.
WT versus A53T
The addition of WT or A53T results in essentially the same extent of tubule formation. When 2.6 μM WT1 or A53T54 is added to membranes containing 40 and 50 mol % PG similar results are again seen: The addition of protein results in the formation of PG-rich and PC-rich regions, and multilayers of protein adsorb to the PG-rich regions. At present we do not know how the helical content of A53T varies with anionic lipid content. The degree of adsorption of both variants to membranes is roughly equivalent; from that perspective the lack of difference in tubule-forming propensity is unsurprising.
Protein-induced alteration of membrane geometry leading to the formation of vesicles and tubules is involved in membrane trafficking processes.35,55–57 Various studies indicate that α-synuclein-lipid interactions could play a role in the regulation of vesicle trafficking.36,37,58,59 That α-synuclein adsorption can alter the structure of lipid bilayers has been previously suggested by Eliezer and colleagues,60 who monitored the adsorption of α-synuclein to large unilamellar vesicles (LUVs). Due to technique limitations no comment could be made as to the change in structure. Herein, we have shown that under certain conditions the adsorption of α-synuclein on membranes results in tubule formation. During the preparation of this manuscript, Langen and colleagues published evidence suggesting that α-synuclein induces tubulation and increased curvature in large vesicles, and these effects correlate with membrane disruption.61 In the experiments herein the bilayer was on a solid support. The presence of the solid support changes some bilayers properties, e.g. it damps out membrane undulations, while leaving others relatively unchanged, e.g. lipid diffusion. That the bilayer-support interaction raises the energetic cost of tubule formation seems likely, but the degree is unknown, and one factor that might mitigate the cost is that supported bilayers have little ability to expand, and thus to accommodate inclusions (where there may be a large system gain in free energy from their adsorption) the bilayers buckle, moving away from the support. Tubules have previously been observed in supported bilayers, upon heating gel phase bilayers above their phase transition temperature62 and upon addition of lyso phosphatidylcholine to phosphatidic acid/phosphatidylcholine mixtures.38
Between the studies outlined here and our previous work1 we have examined WT α-synuclein binding to membranes ranging from 0 to 50 mol % PG. Three kinds of behavior are observed: (i) Between 0 and 30 mol %: Tubules form in the membrane. (ii) 30 mol % to 40 mol %: The bilayer remains flat, the lipids remain mixed and protein adsorbs uniformly at ~monolayer coverage. (iii) 40 mol % and above: The bilayer remains flat, the lipids de-mix forming PC-rich and PG-rich phases, and multilayers of protein adsorb on the PG-rich regions. All of these observations are consistent with a model wherein the protein helical content increases with increasing anionic lipid content. Cell membranes contain up to ~30 mol % anionic lipid;63 it is therefore interesting to note that 30 mol % PG is the point where no obvious deleterious effects of α-synuclein adsorption are observed. Parkinson's is an age-related disease; as a part of aging the parameters that the cell ordinarily holds under tight control are no longer so carefully regulated. In turn, this loss of regulation may account for the observed increase in anionic lipid content with aging.64,65 Our work shows that `slipping' in either direction – more anionic lipid or less anionic lipid – may be deleterious to the cell: either tubules may be formed more readily than usual in the membrane (with potentially negative consequences to membrane integrity or to cellular processes such as membrane trafficking and endocytosis66,67), or the protein may form potentially neurotoxic, membrane-bound aggregates.1,17
Conclusions
The results of this study show that: (i) Tubules form upon α-synuclein adsorption on supported membranes. (ii) The tubule formation process is dependent on protein concentration, anionic lipid concentration, and α-synuclein variant identity. The observed dependence of tubule formation on these parameters was related to the protein conformation (unstructured versus helical) and the membrane binding affinity of the different variants. The results described here further support the possible role of α-synuclein in cellular transport processes.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01 NS049221 (J.-C.R. and J.S.H.) and awards from the Showalter Trust (J.S.H. and J.-C.R.) and the Department of Energy (DE-FG02-08ER64579) (J.-C. R.). The authors thank Jagadish Hindupur and John Hulleman for assistance with α-synuclein expression and purification.
Footnotes
Supporting Information Epi-fluorescence images of the time evolution of tubule formation are available. Epi-fluorescence images of 35 and 50 mol % PG bilayers after the addition of μM α-synuclein is added are available. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Pandey AP, Haque F, Rochet J-C, Hovis JS. Biophys J. 2009;96:540. doi: 10.1016/j.bpj.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Maroteaux L, Campanelli JT, Scheller RH. J. Neurosci. 1988;8:2804. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, Kittel A, Saitoh T. Neuron. 1995;14:467. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- (4).Cookson MR. Annu Rev Biochem. 2005;74:29. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]
- (5).Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. Nature. 1997;388:839. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- (6).Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Nat. Genet. 1998;18:106. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- (7).Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandradekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Science. 1997;276:2045. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- (8).Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Tortosa EG, del Ser T, Munoz DG, de Yebenes JG. Annals of Neurology. 2004;55:164. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- (9).Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. J Neurosci. 2002;22:8797. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Murphy DD, Rueter SM, Trojanowski JQ, Lee VMY. J Neurosci. 2000;20:3214. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Sidhu A, Wersinger C, Vernier P. FEBS Lett. 2004;565:1. doi: 10.1016/j.febslet.2004.03.063. [DOI] [PubMed] [Google Scholar]
- (12).Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. J Neurosci. 2006;26:11915. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ahn BH, Rhim H, Kim SY, Sung YM, Lee MY, Choi JY, Wolozin B, Chang JS, Lee YH, Kwon TK, Chung KC, Yoon SH, Hahn SJ, Kim MS, Jo YH, Min DS. J Biol Chem. 2002;277:12334. doi: 10.1074/jbc.M110414200. [DOI] [PubMed] [Google Scholar]
- (14).Jenco JM, Rawlingson A, Daniels B, Morris AJ. Biochemistry. 1998;37:4901. doi: 10.1021/bi972776r. [DOI] [PubMed] [Google Scholar]
- (15).Weinreb PH, Zhen WG, Poon AW, Conway KA, Lansbury PT. Biochemistry. 1996;35:13709. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- (16).Davidson WS, Jonas A, Clayton DF, George JM. J Biol Chem. 1998;273:9443. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- (17).Haque F, Pandey AP, Cambrea LR, Rochet J-C, Hovis JS. J Phys Chem B. 2010;114:4070. doi: 10.1021/jp1006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zakharov SD, Hulleman JD, Dutseva EA, Antonenko YN, Rochet JC, Cramer WA. Biochemistry. 2007;46:14369. doi: 10.1021/bi701275p. [DOI] [PubMed] [Google Scholar]
- (19).Bussell R, Jr., Eliezer D. J Mol Biol. 2003;329:763. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- (20).Chandra S, Chen XC, Rizo J, Jahn R, Sudhof TC. J Bio Chem. 2003;278:15313. doi: 10.1074/jbc.M213128200. [DOI] [PubMed] [Google Scholar]
- (21).Jao CC, Der-Sarkissian A, Chen J, Langen R. Proc Natl Acad Sci. 2004;101:8331. doi: 10.1073/pnas.0400553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Bisaglia M, Tessari I, Pinato L, Bellanda M, Giraudo S, Fasano M, Bergantino E, Bubacco L, Mammi S. Biochemistry. 2005;44:329. doi: 10.1021/bi048448q. [DOI] [PubMed] [Google Scholar]
- (23).Ulmer TS, Bax A, Cole NB, Nussbaum RL. J. Biol. Chem. 2005;280:9595. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- (24).Jensen PH, Nielsen MS, Jakes R, Dotti CG, Goedert M. J Biol Chem. 1998;273:26292. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- (25).Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. FEBS Lett. 2004;576:363. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- (26).Perrin RJ, Woods WS, Clayton DF, George JM. J Biol Chem. 2000;275:34393. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- (27).Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. J Neurosci. 2004;24:6715. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bodner CR, Maltsev AS, Dobson CM, Bax A. Biochemistry. 2010;49:862. doi: 10.1021/bi901723p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Conway KA, Lee S-J, Rochet J-C, Ding TT, Williamson RE, Lansbury PT., Jr. Proc Natl Acad Sci. 2000;97:571. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. J Bio Chem. 2005;280:7800. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- (31).Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, Lansbury PT., Jr. Biochemistry. 2007;46:7107. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- (32).Li J, Uversky VN, Fink AL. Biochemistry. 2001;40:11604. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- (33).De Matteis MA, Luini A. Nat Rev Mol Cell Biol. 2008;9:273. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- (34).Lee S, Furuya T, Kiyota T, Takami N, Murata K, Niidome Y, Bredesen DE, Ellerby HM, Sugihara G. J Biol Chem. 2001;276:41224. doi: 10.1074/jbc.M104705200. [DOI] [PubMed] [Google Scholar]
- (35).Farsad K, Ringstad N, Takei K, Floyd SR, Rose K, De Camilli P. J Cell Biol. 2001;155:193. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Tompkins MM, Gai WP, Douglas S, Bunn SJ. Brain Res. 2003;984:233. doi: 10.1016/s0006-8993(03)03040-3. [DOI] [PubMed] [Google Scholar]
- (37).Gedalya T, Loeb V, Israeli E, Altschuler Y, Selkoe D, Sharon R. Traffic. 2009;10:218. doi: 10.1111/j.1600-0854.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Gieger K, Lamberson ER, Hovis JS. Langmuir. 2009;25:71. doi: 10.1021/la8033269. [DOI] [PubMed] [Google Scholar]
- (39).Sackmann E. Science. 1996;271:43. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- (40).Seu KJ, Pandey AP, Haque F, Proctor EA, Ribbe AE, Hovis JS. Biophys J. 2007;92:2445. doi: 10.1529/biophysj.106.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Rochet JC, Conway KA, Lansbury PT., Jr. Biochemistry. 2000;39:10619. doi: 10.1021/bi001315u. [DOI] [PubMed] [Google Scholar]
- (42).Drescher M, Veldhuis G, van Rooijen BD, Milikisyants S, Subramaniam V, Huber M. J Am Chem Soc. 2008;130:7796. doi: 10.1021/ja801594s. [DOI] [PubMed] [Google Scholar]
- (43).Mihajlovic M, Lazaridis T. Proteins. 2008;70:761. doi: 10.1002/prot.21558. [DOI] [PubMed] [Google Scholar]
- (44).Bussell R, Ramlall TF, Eliezer D. Protein Science. 2005;14:862. doi: 10.1110/ps.041255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Huttner WB, Zimmerberg J. Curr Opin Cell Biol. 2001;13:478. doi: 10.1016/s0955-0674(00)00239-8. [DOI] [PubMed] [Google Scholar]
- (46).Zimmerberg J, Kozlov MM. Nature Rev Mol Cell Biol. 2006;7:9. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- (47).Campelo F, McMahon H, Kozlov M. Biophys J. 2008;95:2325. doi: 10.1529/biophysj.108.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Zemel A, Ben-Shaul A, May S. J Phys Chem B. 2008;112:6988. doi: 10.1021/jp711107y. [DOI] [PubMed] [Google Scholar]
- (49).Ferreon A, Moran C, Ferreron J, Deniz A. Angew Chem Int Ed Engl. 2010;49:3469. doi: 10.1002/anie.201000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. J Biol Chem. 2010;285:28261. doi: 10.1074/jbc.M110.157214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Logisz CC, Hovis JS. Biochem. et Biophys. Acta. 2005;1717:104. doi: 10.1016/j.bbamem.2005.10.002. [DOI] [PubMed] [Google Scholar]
- (52).Haque F. Conformational Dynamics of Wild Type and Mutant α-Synuclein on Membrane Surfaces. Purdue University; 2008. [Google Scholar]
- (53).Stöckl M, Fischer P, Wanker E, Herrmann A. J Mol Biol. 2008;375:1394. doi: 10.1016/j.jmb.2007.11.051. [DOI] [PubMed] [Google Scholar]
- (54).Pandey AP. Clustering of α-Synuclein and Tubule Formation on Supported Lipid Bilayers. Purdue University; 2008. [Google Scholar]
- (55).Sens P, Johannes L, Bassereau P. Curr Opin Cell Biol. 2008;20:476. doi: 10.1016/j.ceb.2008.04.004. [DOI] [PubMed] [Google Scholar]
- (56).Takei K, Slepnev VI, Haucke V, De Camilli P. Nature Cell Biology. 1999;1:33. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- (57).Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Nature. 2002;419:361. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- (58).Lotharius J, Brundin P. Hum Mol Genet. 2002;11:2395. doi: 10.1093/hmg/11.20.2395. [DOI] [PubMed] [Google Scholar]
- (59).Narayanan V, Guo Y, Scarlata S. Biochemistry. 2005;44:462. doi: 10.1021/bi0487140. [DOI] [PubMed] [Google Scholar]
- (60).Rhoades E, Ramlall TF, Webb WW, Eliezer D. Biophys J. 2006;90:4692. doi: 10.1529/biophysj.105.079251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Varkey J, Isas JM, Mizuno N, Jensen MB, Bhatia VK, Jao CC, Petrlova J, Voss JC, Stamou DG, Steven AC, Langen R. Journal of Biological Chemistry. 2010;285:32486. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Tamm L, McConnell H. Biophys J. 1985;47:105. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Zimmerberg J, McLaughlin S. Curr Biol. 2004;14:R250. doi: 10.1016/j.cub.2004.02.060. [DOI] [PubMed] [Google Scholar]
- (64).Giusto NM, Salvador GA, Castagnet PI, Pasquare SJ, Ilincheta de Boschero MG. Neurochem Res. 2002;27:1513. doi: 10.1023/a:1021604623208. [DOI] [PubMed] [Google Scholar]
- (65).Riekkinen P, Rinne UK, Pelliniemi TT, Sonninen V. Arch Neurol. 1975;32:25. doi: 10.1001/archneur.1975.00490430047006. [DOI] [PubMed] [Google Scholar]
- (66).Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, LaBaer J, Rochet J-C, Bonini NM, Lindquist S. Science. 2006;313:324. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet J-C, McCaffery JM, Barlowe C, Lindquist S. Proc Natl Acad Sci. 2008;105:145. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.