Abstract
Objective
To assess the impact of muscular strength on mortality in men with hypertension.
Background
Muscular strength is inversely associated with mortality in healthy men, but this association has not been examined in men with hypertension.
Methods
We followed 1506 hypertensive men aged ≥ 40 years enrolled in the Aerobics Center Longitudinal Study from 1980 to 2003. Participants received an extensive medical examination at baseline. Muscular strength was quantified by combining one repetition maximum (1-RM) measures for leg and bench press, and cardiorespiratory fitness (CRF) assessed by maximum exercise test on a treadmill.
Results
During an average follow-up of 18.3 years, 183 deaths occurred. Age adjusted death rates per 10 000 man-years across incremental thirds of muscular strength were 81.8, 65.5 and 52.0 (P<0.05 for linear trend). Multivariable Cox regression hazard ratios were 1.0 (reference), 0.81 (95% confidence interval, 0.57 to 1.14), and 0.59 (0.40 to 0.86) across incremental thirds of muscular strength. After further adjustment for CRF, those participants in the upper third of muscular strength still had a lower risk of death (0.66; 0.45 to 0.98). In the muscular strength and CRF combined analysis, men simultaneously in the upper third of muscular strength and high fitness group had the lowest mortality risk among all combination groups (0.49; 0.30 to 0.82), with men in the lower third of muscular strength and low fitness group as reference.
Conclusions
High levels of muscular strength appear to protect hypertensive men against all-cause mortality, and this is in addition to the benefit provided by CRF.
Keywords: Hypertension, muscular strength, cardiorespiratory fitness, mortality
INTRODUCTION
Hypertension is associated with an increased risk of all-cause and cardiovascular disease (CVD) mortality (1). Recent calculations show nearly 8 million deaths world-wide (about 13.5% of the global total) attributable to high blood pressure (2). It remains a major public health problem in the United States, with 58.4 million (28.7%) Americans aged 18 yr or older having hypertension (systolic blood pressure ≥ 140 and/or diastolic blood pressure ≥ 90 mm Hg) (1). Although awareness, treatment and control of hypertension appears to be improving during recent decades (1), this is still far from optimal; and with the ongoing obesity epidemic, there may be an increase in most CVD, including hypertension (3).
Cardiorespiratory fitness (CRF) provides strong and independent prognostic information about the overall risk of illness and death across a broad spectrum of medical conditions, including hypertension (4–6). It is generally accepted that regular exercise can help prevent the development of hypertension, lower blood pressure in normotensive and hypertensive adults, and reduce mortality risk in individuals with hypertension (7,8). The independent role of muscular strength in the prevention of chronic disease is now increasingly being recognized (9). Resistance-type physical activities are major determinants of muscular strength and are currently prescribed by prestigious health organizations for improving health and fitness (8,10). Findings from meta-analyses indicate that resistance training could produce a reduction of around 3 and 3.5 mm Hg for resting systolic and diastolic blood pressure (11,12), which represents approximate decreases of 2% and 4%, respectively (12). The blood pressure lowering effects of resistance training seem to be even more pronounced in individuals with existing hypertension (13,14). Taken together, these results suggest that moderate intensity resistance training is not contraindicated and could become part of the non-pharmacological intervention strategy to prevent and combat high blood pressure (11).
Several prospective studies have shown that muscular strength is inversely associated with all-cause (15–22) and cancer (22,23) mortality, but the association in persons with hypertension has yet to be examined. Any modifiable factor able to decrease the risk of mortality among hypertensive individuals would be of high interest from a public health perspective. We hypothesized that muscular strength would have an independent protective effect on all-cause mortality in hypertensive men.
METHODS
Data for the current report are from the Aerobics Center Longitudinal Study (ACLS), a prospective study on the association of clinical and lifestyle factors to health outcomes in patients examined at the Cooper Clinic in Dallas, Texas from 1970. Between 1980 and 1989, 10,265 men received muscular strength tests as part of the comprehensive medical examination. Participants came to the clinic for periodic preventive health examinations and for counseling on diet, exercise, and other lifestyle factors associated with an increased risk of chronic disease. Participants thus were volunteers, were not paid, and were not recruited to the study as would be the case for a clinical trial. Many were sent by their employers for the examination, some were referred by their doctors and others were self referred.
Only participants aged 40 yr or older and with resting blood pressure ≥ 140/90 mm Hg or previous physician diagnosed hypertension, were included in the present study (n=1668). The exclusion of younger participants was due to the low prevalence of hypertension at those ages (1). Among those finally selected, participants were excluded if they reported a history of myocardial infarction, stroke or cancer (n=81); they failed to achieve at least 85% of age-predicted maximum heart rate during the treadmill test (n=77); or they died during the first year of follow-up (n=4). These criteria resulted in 1506 men, all of them with a body mass index ≥ 18.5 kg/m2. Participants were predominantly white, well educated, and belonged to middle and upper socioeconomic strata. All participants gave informed consent to participate in the clinical examination and follow-up study. The Cooper Institute Institutional Review Board reviewed and approved the study protocol annually.
Clinical examination
The clinical examination, as well as measures of muscular strength and CRF, are described in detail elsewhere (24,25). Briefly, the baseline examination was completed subsequent to an overnight fast of at least 12 hours and included a physical examination and an array of clinical measurements. Body mass index was computed as weight in kilograms divided by height in meters squared, measured with a standard clinical scale and stadiometer. Resting systolic and diastolic blood pressure were measured in the seated position as the first and fifth Korotkof sounds, using standard auscultation methods after at least 5 min of sitting quietly (26). Two or more readings separated by 2 min were averaged. If the first two readings differed by more than 5 mm Hg, additional readings were obtained and averaged. Concentrations of total cholesterol and glucose were measured using automated techniques in accordance with the standards of the Centers for Disease Control and Prevention lipid standardization program. Hypercholesterolemia was defined as a total cholesterol concentration of ≥240 mg/dL. Diabetes mellitus was defined as a fasting plasma glucose concentration of ≥126 mg/dL, previous physician diagnosis of diabetes, or use of insulin. Participants completed a questionnaire on medical history, which included a personal history of myocardial infarction, stroke, hypertension, diabetes, or cancer; a family history of CVD; a family history of cancer; smoking status; alcohol intake; and physical activity (active or inactive). Physical inactivity was defined as reporting no physical activity during leisure time in the three months before the examination.
We assessed muscular strength in the upper and lower body using a standardized strength testing protocol and variable resistance weight machines (Universal Equipment, Cedar Rapids, IA, USA) (25). Upper body strength was assessed with a one repetition maximum (1-RM) supine bench press, and lower body strength was assessed with a 1-RM seated leg press. Initial loads were 70% of body weight for the bench press and 100% for the leg press. After a brief rest period, we added increments of 2.27 kg to 4.54 kg until maximum effort was achieved for each lift, usually after five or fewer trials. Participants were able to lift the initial load at least one time. They were instructed on proper breathing and lifting form for each movement. The intraclass correlation coefficient for 1-RM bench and leg press was 0.90 and 0.83, respectively, in a subgroup of 246 men who underwent two muscular strength assessments within one year (25).
A muscular strength index was computed by taking each individual’s 1-RM score for the bench press and leg press, expressed as weight lifted per kilogram body weight, and dividing the scores into three age groups (40–49, 50–59, and ≥60 years). Within each age group, the individual’s scores were standardized using the formula: standardized value = (value – mean)/standard deviation. These new scores for the bench press and leg press were averaged to express an age-specific standardized composite score for each individual, following our earlier studies (14,22,23). For the analyses we used thirds of this age-specific strength score.
We assessed CRF by a maximum treadmill test using a modified Balke protocol (24,27). Participants were encouraged to give maximum effort, and the test was stopped when participants reached volitional exhaustion or the doctor intervened for medical reasons. The mean percentage of age-predicted maximum heart rate (220–age) achieved during exercise was 102.3 (SD 7.1), indicating that a majority of participants achieved maximum effort. Exercise duration using this protocol is highly correlated with measured maximum oxygen uptake (r=0.92) (28). We estimated maximum metabolic equivalents (1 metabolic equivalent=3.5 ml oxygen uptake/kg/min) from the final treadmill speed and grade (29). We dichotomized CRF as low fitness and high fitness corresponding to the lower and the upper 50%, respectively, of the age-specific distribution of treadmill exercise duration from this particular population.
Mortality surveillance
All participants were followed from the date of their baseline examination until their date of death or until December 31, 2003. We computed man-years of exposure as the sum of follow-up time among decedents and survivors. The National Death Index was the primary data source for mortality surveillance, which has been shown to be an accurate method of ascertaining deaths in observational studies, with high sensitivity (96%) and specificity (100%) (30).
Statistical analysis
We described baseline characteristics of the study population by vital status and by thirds of muscular strength. Differences between survivors and decedents were tested using Student t test and χ2 test. Tests for linear trends across muscular strength categories were calculated using general linear models. We used Cox proportional hazards regression to estimate hazard ratios, 95% confidence intervals (CIs) and mortality rates (deaths per 10 000 man-years of follow-up), according to muscular strength categories. In multivariable analyses, we adjusted for age in model 1, and in model 2 for age, physical activity (active or inactive), smoking (current smoker or not), alcohol intake (≥ 5 drinks a week or not), body mass index, systolic and diastolic blood pressure, total cholesterol, presence or not of diabetes, abnormal electrocardiogram, and family history of CVD. Additional analyses were done after further adjustment for CRF in model 3, entered as a continuous variable (treadmill test duration in minutes). A total of 666 (44.2%) participants reported a family history of CVD.
We also examined the combined effects of muscular strength and CRF on all-cause mortality. For this analysis we created six categories for combinations of strength and CRF on the basis of thirds of muscular strength and we dichotomized these in to low fitness and high fitness groups for CRF. We compared the effect of each combination of strength and fitness status with the reference group (low fitness, low strength). Cumulative hazard plots grouped by exposure suggested no appreciable violations of the proportional hazards assumption. We calculated two sided P values and we considered those <0.05 as significant. Analyses were done using SAS statistical software, version 9.2.
RESULTS
During an average follow-up of 18.3 years and 27 560 man-years of observation, 183 (12.2%) deaths occurred. Table 1 shows the baseline characteristics of the participants on the basis of vital status and thirds of muscular strength. Muscular strength, as well as exercise test duration and maximum metabolic equivalents, were significantly higher in survivors than in decedents. A direct gradient of treadmill test duration across thirds of muscular strength was observed. Age, fasting blood glucose, systolic blood pressure, and prevalence of current smokers, alcohol intake ≥ 5 drinks weekly, diabetes mellitus and family history of premature CVD were higher in decedents. Body mass index, levels of glucose, diastolic blood pressure and prevalence of physical inactivity were higher in those with lower levels of muscular strength.
Table 1.
Baseline characteristics of hypertensive men in Aerobics Centre Longitudinal Study, 1980–2003, according to vital status and by thirds of muscular strength.
| Characteristic | Vital Status
|
Muscular strength (thirds)
|
||||||
|---|---|---|---|---|---|---|---|---|
| All (n=1506) | Survivors (n=1323) | Decedents (n=183) | P value* | Lower (n=499) | Middle (n=508) | Upper (n=499) | P for linear trend | |
| Age (years) | 50.2 (7.4) | 49.7 (7.2) | 54.0 (7.9) | <0.001 | 50.5 (7.4) | 50.2 (74.2) | 49.8 (7.7) | 0.33 |
| Body mass index (kg/m2) | 26.9 (3.7) | 26.9 (3.7) | 27.1 (3.3) | 0.35 | 28.2 (4.4) | 26.7 (3.1) | 25.8 (3.0) | <0.001 |
| Treadmill time (minutes) | 17.5 (4.8) | 17.7 (4.8) | 15.8 (4.7) | <0.001 | 15.3 (4.3) | 17.6 (4.4) | 19.6 (4.8) | <0.001 |
| Maximum metabolic equivalents † | 11.4 (2.4) | 11.5 (2.4) | 10.6 (2.2) | <0.001 | 10.4 (2.0) | 11.4 (2.1) | 12.4 (2.5) | <0.001 |
| Upper body strength: | ||||||||
| kg | 66.3 (15.0) | 66.7 (14.9) | 63.0 (15.6) | 0.002 | 57.3 (11.7) | 65.1 (10.8) | 76.2 (15.8) | <0.001 |
| kg/kg of body weight | 0.8 (0.2) | 0.8 (0.2) | 0.7 (0.2) | <0.001 | 0.6 (0.1) | 0.8 (0.1) | 0.9 (0.2) | <0.001 |
| Lower body strength: | ||||||||
| kg | 132.3 (26.4) | 132.7 (26.0) | 129.6 (29.0) | 0.15 | 121.4 (26.1) | 131.9 (22.5) | 143.6 (25.7) | <0.001 |
| kg/kg of body weight | 1.5 (0.3) | 1.6 (0.3) | 1.5 (0.2) | 0.008 | 1.3 (0.2) | 1.5 (0.1) | 1.8 (0.2) | <0.001 |
| Composite strength score | 0.00 | 0.03 (0.88) | −0.23 (0.82) | <0.001 | −0.87 (0.45) | −0.05 (0.33) | 0.91 (0.66) | <0.001 |
| Total cholesterol (mg/dL) | 221.9 (43.2) | 221.1 (41.4) | 227.7 (54.5) | 0.053 | 222.6 (39.1) | 221.6 (49.1) | 221.5 (40.8) | 0.91 |
| Fasting blood glucose (mg/dL) | 104.4 (20.0) | 103.6 (16.9) | 110.5 (34.7) | <0.001 | 106.8 (26.4) | 103.0 (15.4) | 103.5 (16.2) | 0.005 |
| Blood pressure (mmHg) | ||||||||
| Systolic | 131.7 (13.9) | 131.1 (13.7) | 136.1 (14.6) | <0.001 | 131.2 (13.1) | 130.9 (13.2) | 133.0 (15.3) | 0.03 |
| Diastolic | 89.9 4 (8.5) | 89.3 (8.6) | 89.9 (7.6) | 0.36 | 90.1 (8.5) | 88.7 (8.0) | 89.4 (8.9) | 0.03 |
| No (%) physically inactive | 339 (22.5) | 301 (22.8) | 38 (20.8) | 0.55 | 144 (28.9) | 123 (24.2) | 72 (14.4) | <0.001 |
| No (%) of current smokers | 220 (14.6) | 182 (13.8) | 38 (20.8) | 0.012 | 86 (17.2) | 65 (12.8) | 69 (13.8) | 0.11 |
| No (%) with alcohol intake ≥ 5 drinks weekly | 812 (53.9) | 700 (52.9) | 112 (61.2) | 0.035 | 271 (54.3) | 261 (51.4) | 280 (56.1) | 0.31 |
| Baseline medical conditions No (%): | ||||||||
| Hypercholesterolemia | 450 (29.9) | 389 (29.4) | 61 (33.3) | 0.28 | 157 (35.1) | 145 (28.5) | 148 (29.7) | 0.59 |
| Diabetes mellitus | 93 (6.2) | 69 (5.2) | 24 (13.1) | <0.001 | 38 (7.6) | 28 (5.5) | 27 (5.4) | 0.26 |
| No (%) with family history of cardiovascular disease | 666 (44.2) | 562 (42.3) | 104 (56.8) | <0.001 | 215 (43.1) | 218 (42.9) | 233 (46.7) | 0.40 |
Values are means (standard deviations) unless stated otherwise.
Comparison between survivors and decedents.
Estimated from final treadmill speed and elevation.
Table 2 shows age adjusted death rates and hazard ratios (model 1) for death from all causes; hazard ratios after further adjustment for physical activity, smoking, alcohol intake, body mass index, systolic and diastolic blood pressure, total cholesterol, diabetes, abnormal electrocardiogram and family history of CVD (model 2); and after additionally adjusting for CRF (model 3). All-cause mortality rates were 1.25 (81.8/65.5) and 1.57 (81.8/52) times greater for those in the lowest third of muscular strength compared to those in the middle and upper thirds of muscular strength, respectively. The age adjusted results of model 1 showed an inverse risk of death from all causes across incremental thirds of muscular strength (P<0.05 for linear trend). Those participants in the upper muscular strength group had a 36% significantly lower risk of all-cause mortality compared with those in the lower group. After the additional adjustments (model 2), risks of death remained progressively lower with higher levels of muscular strength (P<0.05 for linear trend). Hypertensive men in the upper muscular strength group had a 41% significantly lower risk of all-cause mortality. Finally, after further adjustment for CRF (model 3), the association between muscular strength and risks of death was attenuated (P=0.11 for linear trend), yet those participants in the upper third of muscular strength still had a 34% lower risk of death. Excluding deaths that occurred during the first three years of follow-up did not materially change these results. Similarly, the results were virtually the same when including those participants who did not achieve 85% of age-predicted maximum heart rate during the treadmill test.
Table 2.
Rates and hazards ratios for all-cause mortality in hypertensive men by thirds of muscular strength.
| Muscular strength | No of deaths (No of participants) | Age adjusted rate per 10 000 man-years | Hazard ratio (95% CI)
|
||
|---|---|---|---|---|---|
| Model 1* | Model 2† | Model 3‡ | |||
| Lower | 74 (499) | 81.8 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Middle | 61 (508) | 65.5 | 0.80 (0.57 to 1.12) | 0.81 (0.57 to 1.14) | 0.86 (0.60 to 1.21) |
| Upper | 48 (499) | 52.0 | 0.64 (0.44 to 0.91) | 0.59 (0.40 to 0.86) | 0.66 (0.45 to 0.98) |
| P for linear trend | 0.048 | 0.02 | 0.11 | ||
Adjusted for age.
Adjusted for age, physical activity (active or inactive), current smoking, alcohol intake (≥ 5 drinks weekly), body mass index, systolic and diastolic blood pressure, total cholesterol, diabetes, abnormal electrocardiogram and family history of cardiovascular disease.
Adjusted for age, physical activity (active or inactive), current smoking, alcohol intake (≥ 5 drinks weekly), body mass index, systolic and diastolic blood pressure, total cholesterol, diabetes, abnormal electrocardiogram, family history of cardiovascular disease and CRF (treadmill test duration in minutes).
CRF, cardiorespiratory fitness.
We additionally analyzed the association of muscular strength and risks of all-cause mortality separately for men aged <60 years (n=1323, 88%) and ≥ 60 years (n=183, 12%), as well as for men with normal weight (n=479, 32%) and overweight (n=1027, 68%), in both cases using the fully adjusted model (except age in the analysis stratified by age, and body mass index in the analysis stratified by weight status). There was no interaction between muscular strength and age (P=0.28) either between muscular strength and weight status (P=0.36) in predicting risk of mortality. Among participants aged ≥ 60, those in the upper third of muscular strength had a 56% lower risk of death when compared with those in the lower third (hazard ratio=0.44; 95% CI=0.20–0.98).
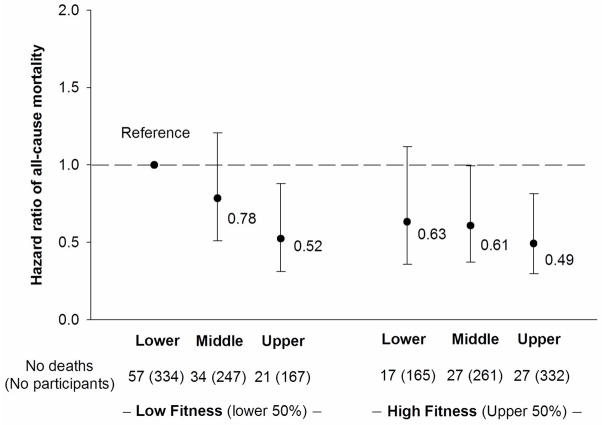
Figure 1 shows all-cause mortality risks across combined categories of muscular strength (thirds) and CRF (low and high fitness). There was no significant interaction between muscular strength and CRF in predicting risk of mortality (P=0.46). Those participants in the low fitness group and upper third of muscular strength had a 48% lower risk of mortality (0.52; 95% CI=0.31–0.82) compared with the reference group (low fitness, lower third of muscular strength). Among participants in the high fitness group, men in middle and upper thirds of muscular strength had 39% (0.61; 95% CI=0.37–0.995) and 51% (0.49; 95% CI=0.30–0.82) lower risks of mortality respectively, compared with the reference group.
Figure 1.
Combined association of muscular strength (thirds) and cardiorespiratory fitness (low fitness, high fitness) with hazard ratio of all-cause mortality after adjustment for age, physical activity, current smoking, alcohol intake, body mass index, systolic and diastolic blood pressure, total cholesterol, diabetes, abnormal electrocardiogram, and family history of cardiovascular disease. Error bars represent 95% confidence interval.
DISCUSSION
The primary finding of this study is that a high level of muscular strength was significantly associated with a lower risk of all-cause mortality in hypertensive men, after controlling for potential confounders including CRF. The combined analysis showed that hypertensive men with high levels of both muscular strength and CRF presented the lowest risk of all-cause mortality. This finding extends our previous observation that hypertensive men with at least moderate CRF have a lower risk of mortality (4,5,31). The present study suggests that a high level of muscular strength also provides an additional protective effect, and the reduction in mortality can be higher when hypertensive men have a high level of both muscular strength and CRF.
Several prospective studies have shown that muscular strength is inversely associated with all-cause and cancer mortality (15–23), suggesting the important role of muscular health in the prevention of chronic disease (9). Our study overcomes some limitations of previous reports, such the use of relatively small muscle groups (mainly handgrip test) (15,16,21,32–35), short term follow-ups (4–6 years) (32–35), the inclusion of only older adults (≥65 years) (32,34,35), or the absence of CRF (15,21,32–34). In our study, muscular strength and CRF were moderately correlated (age adjusted partial r=0.42), suggesting that the association between muscular strength and risk of death works at least partially through different mechanisms than that associated with the protective effect of CRF. The apparent protective effect of muscular strength against risk of death might be due to muscular strength in itself, to respiratory muscular strength and pulmonary function (36), to muscle fiber type or configuration, or as a consequence of regular physical exercise, specifically resistance exercise. We have previously reported a strong and positive association between the frequency of self reported resistance exercise and maximal muscular strength in men enrolled in the ACLS (37). Results from intervention studies indicate that resistance training enhances muscular strength and endurance, muscle mass, functional capacity, daily physical activity, risk profile for CVD, and quality of life (10), all which are well known predictors of mortality risk. The benefits of resistance training are evident in men and women, young adults and older people, in normal weight, overweight and obese people, and in people with or without CVD, including hypertension (7,10).
To the best of our knowledge, this is the first study that relates muscular strength and mortality in the high risk population of hypertensive men. Being hypertensive is associated with an increased incidence of all-cause and CVD mortality (1). However, this and other studies show that lifestyle factors such as diet (38), regular physical activity (7), CRF (4,5,31) and now muscular strength can be effective in the management of the higher risk in hypertensive patients. According to our results, hypertensive men should follow the recommendations for resistance exercise not only to reduce resting blood pressure (11,12), but also for reducing mortality risk. In fact, the Physical Activity Guidelines for Americans (8) encourage adults to perform muscle-strengthening activities that involve major muscle groups on 2 or more days a week, supervised by a health-care provider in the case of people with chronic medical condition.
The results of the present study, however, should be interpreted with caution. The small number of deaths prevents a firm conclusion and does not allow for the examination of the relation between muscular strength and disease specific mortality risk in hypertensive men. Studies involving larger number of deaths should analyze the potential association between muscular strength and CVD mortality in hypertensive people. The composition of the study sample by well educated white men of middle and upper socioeconomic status, and the impossibility of performing a parallel analysis on women, need also to be considered. However, the homogeneity of our study group on socioeconomic factors enhances internal validity of our findings because it reduces the likelihood of confounding by these characteristics. In addition, men in the ACLS cohort are very similar on key clinical measures such as lipids, glucose, and blood pressure to participants in other large epidemiological studies in the US (39). We are not aware of biological reasons to believe that the benefits of muscular strength would be different in hypertensive people from other ethnic or socioeconomic groups. Prospective studies among diverse populations and among women are needed.
As another potential limitation, we did not have sufficient information on diet or medication usage/adherence to include in our analysis, which may have biased the results through residual confounding. Hypertensive people on medication therapy might be more health conscious and have healthier lifestyle habits. However, it seems unlikely that these factors would account for all of the observed association between muscular strength and mortality. Future studies should include such information whenever possible. In addition, as we only have baseline data on muscular strength and CRF, we do not know whether changes in any of these variables occurred during follow-up and how this might have influenced the results. It is possible that many men with hypertension were treated at some point in the follow-up interval, and others may have experienced increases/decreases in the above exposures.
A major strength of this study was the inclusion of objective and standardized maximal tests for muscular strength (upper and lower body) and CRF using highly reliable measurement protocols in a relatively large cohort of hypertensive men with extensive follow-up. Undetected subclinical disease is always a concern in any observational study, but it is less likely to have occurred in our cohort because of the comprehensive physical examination and the clinical assessment completed by each participant. Moreover, participants were healthy enough to achieve at least 85% of age-predicted maximal heart rate during the treadmill test. In addition, excluding deaths during the first three years of follow-up did not alter the results in the current study.
In conclusion, this study found that high levels of muscular strength are associated with a lower risk for all-cause mortality in men with diagnosed hypertension, and this is in addition to the protective effect provided by CRF. Hypertensive men should follow current physical activity guidelines and engage in muscle-strengthening activities that involve major muscle groups, not only to reduce resting blood pressure but also to potentially reduce long-term mortality risk. Hypertensive men can attain even greater reduction in mortality risk if they maintain high levels of both muscular strength and CRF.
Acknowledgments
This work was supported by National Institutes of Health grants AG06945, HL62508 and R21DK088195 (to X Sui from the National Institute of Diabetes and Digestive and Kidney Diseases), and in part by an unrestricted research grant from the Coca-Cola Company; Spanish Ministry of Education (AP-2005-4358; EX-2008-0641); the Swedish Heart-Lung Foundation (20090635), and the Swedish Council for Working Life and Social Research (FAS).
The authors thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management.
Abbreviations list
- ACLS
Aerobics Center Longitudinal Study
- CI
confidence interval
- CRF
cardiorespiratory fitness
- CVD
cardiovascular disease
- 1-RM
one repetition maximum
Footnotes
The authors declare no relationship with industry that is associated with this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–8. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–32. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 4.Blair SN, Kohl HW, 3rd, Barlow CE, Gibbons LW. Physical fitness and all-cause mortality in hypertensive men. Ann Med. 1991;23:307–12. doi: 10.3109/07853899109148065. [DOI] [PubMed] [Google Scholar]
- 5.Church TS, Kampert JB, Gibbons LW, Barlow CE, Blair SN. Usefulness of cardiorespiratory fitness as a predictor of all-cause and cardiovascular disease mortality in men with systemic hypertension. Am J Cardiol. 2001;88:651–6. doi: 10.1016/s0002-9149(01)01808-2. [DOI] [PubMed] [Google Scholar]
- 6.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness and risk of nonfatal cardiovascular disease in women and men with hypertension. Am J Hypertens. 2007;20:608–15. doi: 10.1016/j.amjhyper.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–53. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- 8.US Dept of Health and Human Services. Physical Activity Guidelines for Americans. Washington, DC: 2008. p. 2008. [Google Scholar]
- 9.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–82. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 10.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–84. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen VA, Fagard RH. Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23:251–9. doi: 10.1097/00004872-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kelley GA, Kelley KS. Progressive resistance exercise and resting blood pressure: A meta-analysis of randomized controlled trials. Hypertension. 2000;35:838–43. doi: 10.1161/01.hyp.35.3.838. [DOI] [PubMed] [Google Scholar]
- 13.Stewart KJ, Bacher AC, Turner KL, et al. Effect of exercise on blood pressure in older persons: a randomized controlled trial. Arch Intern Med. 2005;165:756–62. doi: 10.1001/archinte.165.7.756. [DOI] [PubMed] [Google Scholar]
- 14.Maslow AL, Sui X, Colabianchi N, Hussey J, Blair SN. Muscular strength and incident hypertension in normotensive and prehypertensive men. Med Sci Sports Exerc. 2010;42:288–95. doi: 10.1249/MSS.0b013e3181b2f0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rantanen T, Harris T, Leveille SG, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55:M168–73. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 16.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57:B359–65. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 17.Katzmarzyk PT, Craig CL. Musculoskeletal fitness and risk of mortality. Med Sci Sports Exerc. 2002;34:740–4. doi: 10.1097/00005768-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 18.FitzGerald SJ, Barlow CE, Kampert JB, Morrow JR, Jackson AW, Blair SN. Muscular fitness and all-cause mortality: prospective observations. J Phys Act Health. 2004;1:7–18. [Google Scholar]
- 19.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 20.Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–20. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int J Epidemiol. 2007;36:228–35. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz JR, Sui X, Lobelo F, et al. Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol Biomarkers Prev. 2009;18:1468–76. doi: 10.1158/1055-9965.EPI-08-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 25.Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005;37:1849–55. doi: 10.1249/01.mss.0000175865.17614.74. [DOI] [PubMed] [Google Scholar]
- 26.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 27.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10:675–88. [PubMed] [Google Scholar]
- 28.Pollock ML, Bohannon RL, Cooper KH, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976;92:39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 29.American College of Sports Medicine. ACSM’s Guidelines For Exercise Testing And Prescription 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 30.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119:837–9. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 31.McAuley PA, Sui X, Church TS, Hardin JW, Myers JN, Blair SN. The joint effects of cardiorespiratory fitness and adiposity on mortality risk in men with hypertension. Am J Hypertens. 2009;22:1062–9. doi: 10.1038/ajh.2009.122. [DOI] [PubMed] [Google Scholar]
- 32.Phillips P. Grip strength, mental performance and nutritional status as indicators of mortality risk among female geriatric patients. Age Ageing. 1986;15:53–6. doi: 10.1093/ageing/15.1.53. [DOI] [PubMed] [Google Scholar]
- 33.Fujita Y, Nakamura Y, Hiraoka J, et al. Physical-strength tests and mortality among visitors to health-promotion centers in Japan. J Clin Epidemiol. 1995;48:1349–59. doi: 10.1016/0895-4356(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 34.Rantanen T, Era P, Heikkinen E. Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc. 1997;45:1439–45. doi: 10.1111/j.1532-5415.1997.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 35.Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75–84-year-old people. Age Ageing. 1995;24:468–73. doi: 10.1093/ageing/24.6.468. [DOI] [PubMed] [Google Scholar]
- 36.Buchman AS, Boyle PA, Wilson RS, Gu L, Bienias JL, Bennett DA. Pulmonary function, muscle strength and mortality in old age. Mech Ageing Dev. 2008;129:625–31. doi: 10.1016/j.mad.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jurca R, Lamonte MJ, Church TS, et al. Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc. 2004;36:1301–7. doi: 10.1249/01.mss.0000135780.88930.a9. [DOI] [PubMed] [Google Scholar]
- 38.O’Shaughnessy KM. Role of diet in hypertension management. Curr Hypertens Rep. 2006;8:292–7. doi: 10.1007/s11906-006-0067-y. [DOI] [PubMed] [Google Scholar]
- 39.Barlow CE, LaMonte MJ, Fitzgerald SJ, Kampert JB, Perrin JL, Blair SN. Cardiorespiratory fitness is an independent predictor of hypertension incidence among initially normotensive healthy women. Am J Epidemiol. 2006;163:142–50. doi: 10.1093/aje/kwj019. [DOI] [PubMed] [Google Scholar]