Abstract
Background
Changes in the myocardium in acute ischemia are dynamic and complex and the characteristics of myocardial tissue on cardiovascular magnetic resonance (CMR) in the acute setting are not fully defined. We investigated changes in edema and late gadolinium enhancement (LGE) with serial imaging early after acute MI, relating these to global and segmental myocardial function at 6 months.
Methods and Results
CMR scans were performed on 30 patients with ST elevation MI (STEMI) treated by primary PCI at each of 4 time points: 12-48 hours (24H); 5-7 days (1W); 14-17 days (2W); and 6 months (6M). All patients showed edema at 24H. The mean volume of edema (% LV) was 37 ± 16 at 24H and 39 ± 17 at 1W with a reduction to 24 ± 13 (P < 0.01) by 2W. Myocardial segments with edema also had increased signal on LGE at 24H (kappa = 0.77; P < 0.001). The volume of LGE decreased significantly between 24H and 6M (27 ± 15 % vs. 22 ± 12 %; P = 0.002). Of segments showing LGE at 24H, 50% showed resolution by six months. In segments with such a reduction in LGE, 65% also showed improved wall motion (P < 0.0001). The area of LGE measured at 6M correlated more strongly with troponin at 48h (r = 0.9; P < 0.01) than LGE at 24H (r = 0.7). The difference in LGE between 24H and 6M had profound effects on the calculation of salvage index (26 ± 21 % at 24H vs. 42 ± 23 % at 6M; P = 0.02).
Conclusions
Myocardial edema is maximal and constant over the first week post MI, providing a stable window for the retrospective evaluation of area at risk. By contrast, myocardial areas with high signal intensity in LGE images recede over time with corresponding recovery of function, indicating that acutely detected LGE does not necessarily equate with irreversible injury and may severely underestimate salvaged myocardium.
Keywords: myocardial salvage, myocardial edema, late gadolinium enhancement, magnetic resonance imaging, acute coronary syndrome
Cardiac magnetic resonance (CMR) is established as a key non-invasive modality for the evaluation of patients with stable coronary disease. It is the gold standard technique for the assessment of function (cine CMR) and the quantification of scarred myocardium in patients with previous myocardial infarction (MI) (late gadolinium enhancement, [LGE]).1
Recently, the development of T2-weighted (T2W) edema-sensitive sequences has enabled the identification of acutely ischemic myocardium, which is of particular interest in the evaluation of myocardial status in acute MI.2-5 By comparing the area ‘at risk’, determined by T2W imaging, and the final infarct size, obtained with LGE CMR imaging, myocardial salvage following treatment can be derived, and expressed as proportion of myocardial volume initially presumed at risk.6, 7 A number of clinical trials have used this measure, assessed in the acute setting, as a primary endpoint.8-10
However, to capitalize fully on these quantitative CMR techniques requires clear understanding of the dynamic features of each in the context of the rapidly changing pathological conditions that pertain in the myocardium after acute ischemic injury and reperfusion.11 Under these circumstances, there are profound changes in perfusion pressure; small vessel patency and permeability; pH; tissue water content and cellular composition.11 Over time, there is resorption of edema, resolution of inflammation and replacement of irreversibly injured myocytes with fibrous tissue.12 In the acute setting, although it is known that CMR edema imaging may identify both infarct and myocardium at risk and LGE overestimates the infarct zone, acute LGE is still considered a robust prognostic factor though whether it necessarily always reflects irreversible injury is still debated.13-15
Accordingly, we designed a detailed early time course CMR evaluation in patients within 48h of technically successful percutaneous coronary intervention (PCI) for acute MI. We quantified (1) myocardial edema at 4 time points post MI (12-48 hours; 5-7 days; 14-17 days and 6 months) and (2) characteristics of LGE early (12-48 hours) and late (6 months) after ischemic injury. We relate each of these parameters to temporally matched measurements of myocardial function at both global and segmental levels.
Our aims were to establish the longitudinal changes of myocardial edema by CMR and to determine the extent to which edema and LGE, obtained in the acute phase, predicted recovery of global and regional LV function at six months. Clarification of these acute dynamic changes of edema and LGE is crucial to the correct interpretation of CMR data in the acute setting and has important implications for the design and the calculation of sample size of clinical trials with myocardial salvage as endpoint.
Methods
Patient population
This prospective study was undertaken in a single tertiary centre. The study protocol was approved by the local ethics committee and all patients gave written informed consent. Patients with first ST segment elevation MI (STEMI) were eligible if the onset of symptoms had been < 12 hours before PCI and if they had ST-segment elevation of at least 0.1 mV in ≥ 2 contiguous limb leads or at least 0.2 mV ≥ 2 contiguous precordial leads. Patients with previous MI, previous revascularization procedure (coronary artery bypass grafts [CABG] or PCI), severe heart valve disease, known cardiomyopathy or hemodynamic instability lasting longer than 12 hours following revascularization were not enrolled. Further exclusion criteria were contraindications to CMR, including implanted pacemakers, defibrillators, or other metallic implanted devices and claustrophobia. Acute clinical management was at the discretion of the responsible physician, with the intention to reflect contemporary practice and guidelines (including use of aspiration catheters; glycoprotein IIb IIIa receptor inhibitors and high-dose clopidogrel loading). Blood samples were collected for Troponin I at the time of admission and every 12 hours post PCI.
CMR
Four separate 3Tesla CMR scans were performed on each patient at the following time points post-PCI: 12 - 48 hours (24H); 5 - 7 days (1W); 14 - 17 days (2W); and 6 months (6M). The first and last CMR scans assessed LV function, edema, and LGE. The second and third scans acquired function and edema imaging only (Figure 1). Identical short axis images at matching slice position with functional images were acquired using T2W and LGE imaging. Edema imaging was performed using a T2 prep-SSFP single shot sequence with coil signal intensity correction.3 A spine coil and a phased array 6-channel flexible surface coil were used. If necessary, shimming and center frequency adjustments were performed before T2W imaging to generate images free from off-resonance artifacts. LGE-CMR was performed with a T1-weighted segmented inversion-recovery gradient echo-phase sensitive-inversion recovery (GRE_PSIR) sequence 5 to 10 minutes after the administration of 0.1 mmol / kg contrast agent (Gadodiamide, Omniscan™, GE Healthcare, Amersham, UK). The inversion time was meticulously adjusted for optimal nulling of remote normal myocardium. (Refer to online supplement of acquisition parameters for both edema and LGE imaging)
Figure 1. Study protocol.
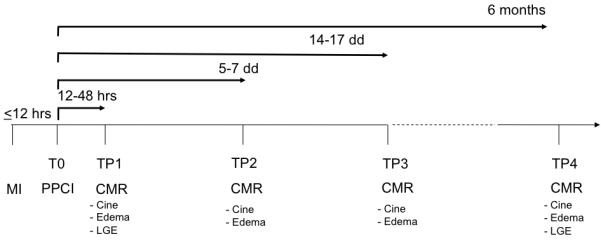
Imaging time points (TP) in relation to the acute myocardial infarction (MI) and primary percutaneous coronary intervention (PPCI). For each TP, the CMR data acquired is given. CMR = cardiovascular magnetic resonance; LGE = late gadolinium enhancement.
Post-processing analysis
Quantification of LV volumes and wall motion were performed as previously described.16, 17 For objective quantification of edema or LGE, a reference region of interest (ROI) was placed in remote myocardium. The signal intensity threshold indicating edema / LGE was imposed 2 standard deviations above the mean intensity of the reference ROI, as previously described.7 (online supplement)
Statistical Analyses
Values of continuous variables are expressed as mean (± SD). In order to investigate differences at multiple time points in LV volumes, EF, WMSI, myocardial volume of edema and LGE assessed by CMR, ANOVA analyses with adjustment for repeated measurements were performed. The Bonferroni procedure, where the overall Type I error (0.05) is distributed across multiple hypothesis tests, was used for post-hoc comparisons. For categorical variables, Wilcoxon signed-rank test was used to compare regional wall motion abnormalities with segmental scoring of edema and late gadolinium. A chi-squared test and a logistic regression model with a repeated-measures variable for the patient (to adjust for the non-independence of the data) were used to assess the relationship between the transmural extent of myocardial injury i.e. edema / LGE and improvement in regional function. The relationship between the improvement in wall motion along 6 months and the clinical and CMR characteristics were evaluated using linear regression analyses. Bland-Altman test for continuous T2W measurements and Cohen’s Kappa coefficient for T2W, LGE and wall motion abnormalities categorical measurements were performed to evaluate inter-observer variability for edema assessment and for transmurality grading and wall motion scoring respectively
All statistical tests were two-tailed, and all P values of less than 0.05 were considered statistically significant.
Results
Patient characteristics are given in Table 1. All patients presented with ST segment elevation on the ECG and were treated by primary PCI (except for 2 who were transferred from a different hospital and received thrombolysis and rescue PCI). All PCI took place within 12 hours of chest pain onset, with the mean time from onset of pain to balloon treatment 253 ± 150 minutes. Of 33 patients enrolled, 2 could not complete the first CMR examination due to claustrophobia, 1 patient was excluded due to urgent re-intervention, 2 did not undergo third scan due to technical difficulties or because of patient refusal (Figure online data supplement). The first CMR took place at 29 ± 10 hours from the PCI (24H), the second (1W) at 6 ± 1.4 days post PCI, the third (2W) at 16 ± 2.7 days post PCI and the fourth (6M) at 6.2 ± 1 months post PCI.
Table 1.
| Variable | Value (mean ± SD) |
|---|---|
| Age (yrs) | 56 ± 9 |
| Sex (M:F) | 26:4 |
| Ethnicity (Caucasian: Asian) | 29:1 |
| BMI | 29 ± 6 |
| Risk Factors [No (%)] | |
| Smoking | 16 (53) |
| Hypertension | 11 (37) |
| Diabetes | 3 (10) |
| Family history | 9 (30) |
| Hyperlipidemia | 11 (37) |
| Troponin I (12 hours post PCI) (mg / mL) | 40 ± 16 |
| Pain to balloon time (mins) | 253 ± 150 |
| Door to balloon Time (mins)[median (interquartiles)] | 44 (30; 62) |
| Culprit coronary artery [No (%)] | |
| LAD | 15 (50) |
| LCx | 2 (6) |
| RCA | 13 (43) |
| Number of vessels diseased [No (%)] | |
| 1 | 20 (67) |
| 2 | 6 (20) |
| 3 | 4 (13) |
| TIMI FLOW pre PCI [No (%)] | 0.6 ± 1 |
| 0 | 21 (70) |
| 1 | 3 (10) |
| 2 | 4 (13) |
| 3 | 2 (6) |
| TIMI FLOW post PCI [No (%)] | 2.5 ± 0.6 |
| 0 | 0 |
| 1 | 2 (6) |
| 2 | 10 (33) |
| 3 | 18 (60) |
| Medications during PCI [No (%)] | |
| GP IIb/IIIa inhibitor | 23 (76) |
| Clopidogrel | 30 (100) |
| Heparin | 28 (90) |
| Aspirin | 30 (100) |
| Concomitant medications on admission [No (%)] | |
| Beta-blockers | 1 (3) |
| Ace-inhibitors | 6 (20) |
| Statins | 4 (13) |
| Medications post infarct | |
| Beta-blockers | 28 (93) |
| Ace-inhibitors | 30 (100) |
| Statins | 30 (100) |
| Aspirin | 30 (100) |
| Diuretic | 3 (10) |
| Nitrates | 8 (27) |
CMR findings
The total number of successful scans was 112 (30 at 24H and 1W, 28 at 2W and 24 at 6M) and all were suitable for analysis. In segmental analyses, 8 segments at 24H and 12 at 2W showed off resonance artifacts in T2W images and were excluded from analyses at all TP (Table online data supplement). Both Bland-Altman (bias = 2 ± 10%) for continuous T2W measurements and Cohen’s Kappa coefficient (kappa = 0.8) for T2W, LGE and wall motion abnormalities categorical measurements indicated excellent levels of agreement for inter-observer variability assessments.
Time course assessment of global left ventricular function and myocardial edema
All patients had positive findings for myocardial injury assessed by T2W imaging at 24H except one. In this particular case, the lesion as assessed by LGE and edema, was confined to the apical segment only. As indicated in Methods, the apical slices were excluded to avoid partial volume artifacts.
The mean volume of edema was stable over the first week (37 ± 16% of the LV myocardium at 24H and 39 ± 17 % at 1W) with a reduction by 2W (24 ± 13%; P < 0.01) and near resolution (7 ± 10%; P < 0.001) by 6M (Figure 2). The ejection fraction (EF) was well conserved (53 ± 9 %) at 24H and not significantly different at 6 months (59 ± 6 %; P = n.s.) (Table 2).
Figure 2. Time course of edema.
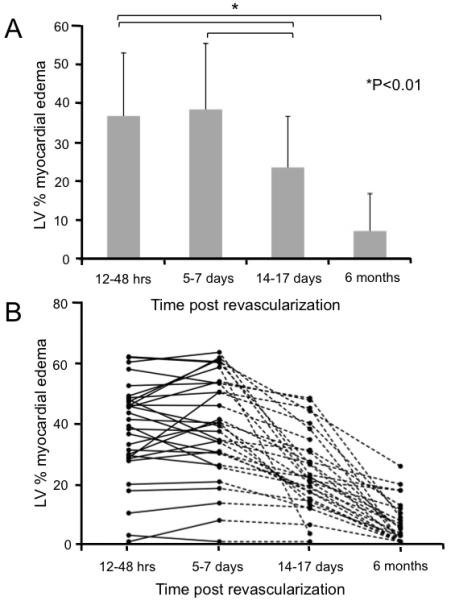
A Mean percentage of LV volume positive for myocardial edema at each time point. The volume of edema remained stable in the first week post event with a significant decrease at 15 -17 days with near resolution by 6 months. B. The time course of edema and resolution is given for each patient. There was a large range of LV % volume of edema (0 to 60%). This analysis on an individual level confirms the constancy of edema measured in the first 5-7 days, that is suggested in Panel A, with marked variation thereafter. Using the objective thresholding methods described in the text, a small number of patients had a substantial volume of apparent residual edema at 6 months.
Table 2.
| 24 hours (n=30) |
5-7 days (n=30) |
14-17 days (n=28) |
6 months (n=23) |
p-value | Post-HOC | |
|---|---|---|---|---|---|---|
| EF (%) | 53 ± 9 | 55 ± 10 | 54 ± 8 | 59 ± 6 | 0.9 | 1.0 |
| EDV (mL) | 144 ± 25 | 153 ± 33 | 156 ± 37 | 158 ± 22 | 0.2 | 1.0 (24H vs 1W, 24H vs 2W, 24H vs 6M ) 0.1 (24H vs 2W) 0.3 (24H, vs 6M) |
| ESV (mL) | 68 ± 22 | 72 ± 30 | 73 ± 27 | 57 ± 24 | 0.6 | 1.0 (24H vs 1W, 2W, 6M ) |
| SV | 76 ± 15 | 82 ± 14 | 82 ± 16 | 88 ± 17 | 0.3 | 1.0 (24H vs 1W, 24H vs 2W) 0.7(24H, vs 6M) |
| WMSI | 1.52 ± 0.3 | 1.4 ± 0.3 | 1.35 ± 0.3 | 1.36 ± 0.3 | <0.001 | <0.01 (24H vs 1W, 24Hvs 2W, 24H vs 6M) |
| EDEMA (LV%) |
37 ± 16 | 39 ± 17 | 24 ± 13 | 6 ± 9 | <0.001 | 0.001 (24H vs 2W) 0.001 (1W vs 2W) 0.001 (24H,2,3 vs 6M) |
| LGE (LV%) | 27 ± 15 | 21 ± 11 | 0.002 | 0.002 |
Time course assessment of edema and LGE on a segmental level: relations to wall motion (24H-2W)
At 24H, 151 out of 428 (35 %) segments were positive for myocardial edema; 133 / 428 (31%) showed LGE, and 139 / 428 (32%) segments showed abnormal function (Table online data supplement). Segments with evidence of injury assessed by T2W or LGE imaging and those with wall motion abnormalities were co-localized (location agreement, Kappa = 0.77; P < 0.001) with 120 / 133 (90%) showing all of edema, LGE and wall motion abnormalities. Similarly, of 277 segments with no edema, 257 (93%) showed normal wall motion, while of those with no LGE, 264 (89%) showed no myocardial edema either. Only 31 / 428 (7%) of segments were positive for edema in the absence of LGE.
In order to assess whether or not the extent of the acute myocardial injury determines regional dysfunction, we examined the relationship between within-segment injury (% segment affected by edema or LGE) and the presence or absence of wall motion impairment in that segment. At 24H, the proportion of segments with wall motion impairment increased in relation to the extent of both myocardial edema (P < 0.01; Figure 3A) and LGE (P < 0.01; Figure 3B). Of the injured segments (those having either edema or positive for LGE), 75 (17%) had improved motion in the first 2 weeks. This was associated with an improvement in WMSI (from 1.52 ± 0.3 at 24H hrs to 1.3 ± 0.3 at 2W, P < 0.01) (Table 2).
Figure 3. Impaired segments (% affected) as a function of the extent of segment positive for (A) edema and (B) LGE at 24H.
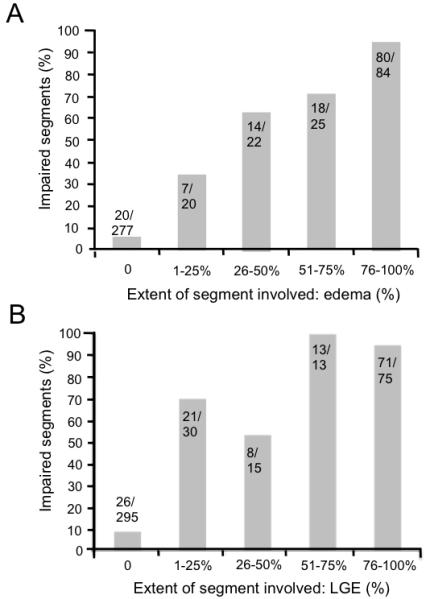
Segments with acute myocardial injury as identified either by presence of late gadolinium (LGE) and/or edema. For both edema and LGE, the probability of segmental dysfunction increased with the fraction of segment affected. For unaffected segments, as expected, a large majority showed normal function.
LGE volume reduction over 6 months
The volume of LGE decreased significantly between the first time point (27 ± 15% of LV myocardial volume) and the last time point at 6M (22 ± 12%, P = 0.002) (Figure 4A). Furthermore, on a patient by patient basis, there was considerable variation in the extent of reduction in LGE. The reduction in LGE from acute to 6M was up to 68%, with 46% of patients showing some reduction in LGE at 6 months. LGE was also reduced when analyzed at segmental level. Out of the 336 segments analyzed at 6M, 108 had been positive for LGE at 24H of which 54 (50%) showed resolution of LGE at 6M(representative examples are shown in Figure 5).
Figure 4. (A) Myocardial LGE early (12-48hrs) vs. late (6 months) and (B) wall motion improvement abnormalities.
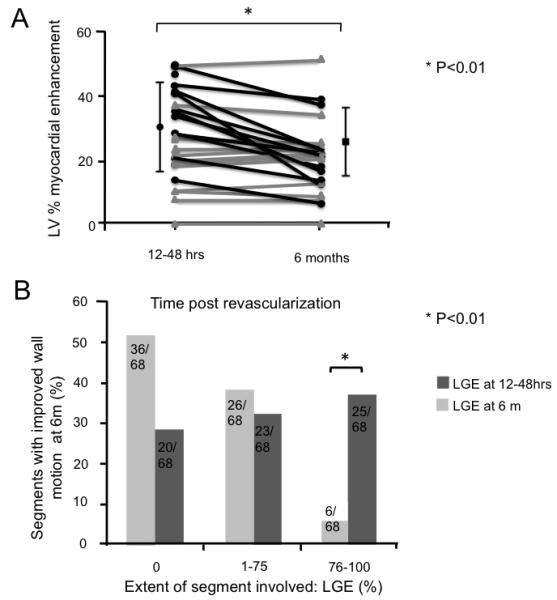
A. The myocardial volume (mean % ± SD) positive for LGE, decreased significantly from 12 - 48 hrs to 6 months. The change in extent of LGE is also shown for each patient. Patients with unchanged LGE at 6 months are shown in red while those with decreased LGE in black. Eleven patients out of 24 (46%) who underwent CMR at 6 months time, showed a reduction in LGE volume (of 38 ± 14%). In the remaining 13 patients, no reduction in size of the LGE volume was identified (23% ± 14% vs. 23% ± 14% respectively). B. Segments with improved function at six months (n = 68) broken down by extent of LGE within that segment LGE (none; 1-75% = partial thickness and 76-100% = full thickness) at both 24H and 6M. Across categories, the extent of LGE, measured at 12 - 48 hours (dark bars) was a poor predictor of functional recovery. Significantly, even segments showing full thickness LGE were associated with functional recovery. By contrast, LGE extent at 6 months (light bars) was strongly inversely correlated with improved function in that segment.
Figure 5. Representative CMR images.
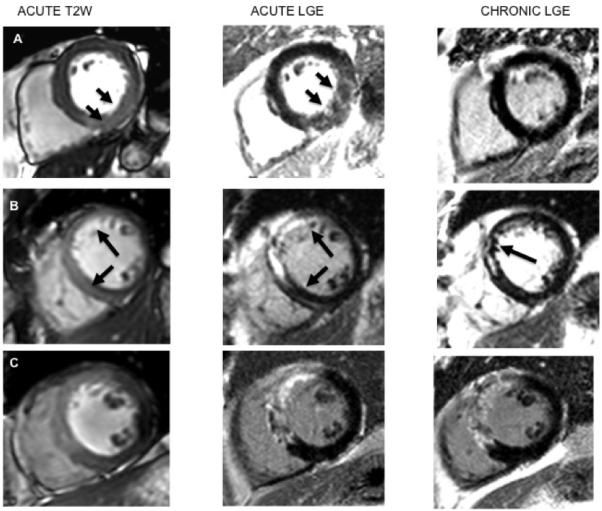
Edema images (left column), acute LGE images (center), chronic LGE (right column) are displayed. Three separate patients (rows A to C) representing alternative patterns of acute vs. chronic CMR features are shown. A. Edema is present in inferior wall; the appearance of LGE is unambiguous as high signal zone that corresponds to the area of edema, but there is no LGE present at 6 months. B. T2W image, on the left, shows edema in the anterior wall; the acute LGE shows compact enhancement, which is reduced in size by 6 months. C. The edema imaging confirms acute injury. In this example LGE present early persists without significant alteration to the 6 month time point.
Recovery of function accompanies resolution of LGE
We next examined whether resolution of LGE could be associated with recovery of function in affected segments. Out of 336 segments analyzed at 6M, 79 had shown abnormal function at 24H of which 68 (86%) showed recovery of function at 6M.
Importantly, of those segments that showed an improvement in LGE at 6 months 35 / 54 (65%) also showed an improvement in wall motion (P < 0.0001) indicating that LGE at 24H did not necessarily signify irreversible myocardial injury.
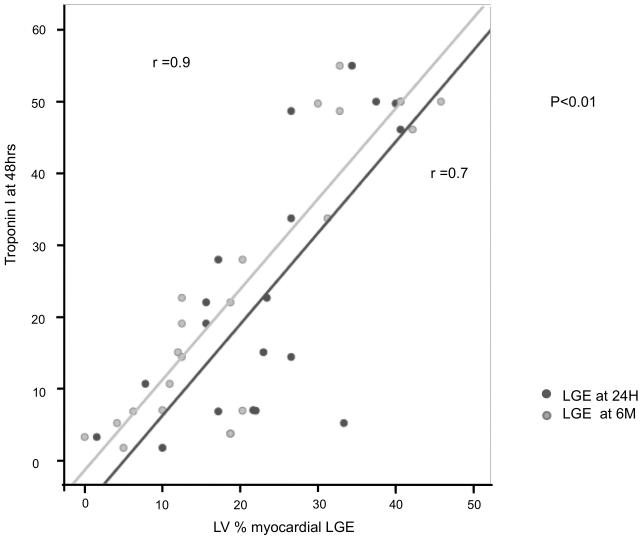
Conversely, only 8% (6/68) of segments with persistent transmural LGE at 6M had improved function (P < 0.01) (Figure 4B). Furthermore, on multivariate analysis the only CMR measure that was predictive of functional recovery was the infarct size determined by LGE CMR (% LVscore) at 6M (P = 0.015). The importance of CMR assessment of infarct size at 6 months is further emphasized by the strong correlation between the infarct size (% LVscore) assessed at 6 months and troponin I, assessed at 48 hrs (r = 0.9; P < 0.01); while the equivalent relationship with LGE measured at 24H was relatively weak (r = 0.7, P < 0.01; Figure 6).
Figure 6. Relationship between the extent of late gadolinium (LGE) and the Troponin I at 48 hrs.
The correlation between the LV % of LGE (% LVscore) assessed at 6 months and troponin I assessed at 48 hrs (r = 0.9; P < 0.01) is shown in comparison to the equivalent relationship with LGE measured at 12 - 48 hours (r = 0.7, P < 0.01).
Effects of timing of LGE measurement on the calculation of salvage index
Calculation of myocardial salvage [index] depends both on (a) the acutely determined area at risk (T2W image) and (b) the final infarct size (LGE). Given the variation in LGE described above, we calculated the effect of its assessment early (24H) vs. late (6M) on the estimation of salvage. Depending on the imaging time for LGE, the salvaged myocardium index was substantially different: 26 ± 21% at 24H vs. 42 ± 23% at 6M (P = 0.02). Thus, LGE timing critically determines the calculated salvage index.
Discussion
Optimal application and accurate interpretation of CMR in acute myocardial ischemia require that the nature and patterns of change of CMR features are fully defined. We report the first detailed time course study that examines early changes in myocardial function, edema and LGE in patients treated with primary PCI for STEMI, and which relates these early features to late measures of infarct size and functional recovery at global and segmental levels.
The principal findings are that: (1) edema was present in virtually all cases; the volume of edema remained unchanged over the first week but decreased significantly by 15 days. (2) A large majority of segments that were positive for edema also showed evidence of LGE, assessed at 24H. (3) In 46% of the patients, LGE present on early scans had diminished in size by 6 months and, in some cases, dramatically. (4) Acute LGE was a weak predictor of functional recovery compared to chronic LGE, as even segments with transmural LGE at 24H, showed an improvement in contractility at 6 months. (5) The reduction in LGE at the later time had a profound effect on the calculation of salvage index, which varied by up to ~60%, depending on the time point used. These findings have important implications for the timing and interpretation of CMR after acute STEMI, including in the estimation of viable vs. infarcted tissue and on the design and implementation of clinical trials that use salvage index as an end point.
Acute myocardial ischemia-reperfusion is a dynamic process, involving a complex cascade of intracellular and interstitial changes that affect: oxygenation and pH in the myocardium; intravascular pressure, small vessel permeability and patency; tissue water content, inflammatory cell infiltration and local hemorrhage.18 These changes potentially alter both the inherent MR characteristics of the tissue and the delivery, distribution and removal of exogenous contrast agents, compared to the stable setting.19 Specifically, the kinetics of gadolinium distribution and precise tissue specificity in acute infarction are not fully understood13-15 and the potential for accumulation of LGE even in viable myocardium has not been excluded.
Myocardial edema
Both intracellular edema due to decoupling of water molecules from proteins20 and/or tissue accumulation of water leads to a prolongation of T221 that appears as transmural bright signal on T2W images.22 In dogs, myocardial edema has been demonstrated after 30 minutes ischemia.5 Previous reports have identified persistence of edema signal for at least 12 days post infarction,3, 6 but the timing of maximal myocardial edema and its early evolution had not been defined. This information is important in order to establish the optimal imaging window for the assessment of salvaged myocardium6, 7 and for estimation of prognosis.23, 24 We demonstrate that edema is maximal and constant during the first week post MI, reducing thereafter. Therefore, the window for retrospective quantification of myocardium ‘at risk’ is up to 7 days, with a risk of underestimation after that time.
Late gadolinium enhancement
Late gadolinium enhancement occurs following myocardial infarction. In the context of established MI and scar formation, the area of myocardial fibrosis is very closely linked to the extent and distribution of LGE.1, 12 Furthermore, the extent of LGE can be used to predict recovery of myocardial function after revascularization,1, 25 with the transmural segmental extent of LGE / scar inversely correlating with the probability of functional recovery. Reduction in LGE has been previously reported from the acute phase to a chronic stage, and attributed to “shrinking of the scar”, together with compensatory hypertrophy of adjacent myocardium.26, 27
Although LGE occurs in context of acute MI, this cannot reflect tissue fibrosis, which takes several weeks to develop. In a dog model, Fieno et al showed that fibrosis increased 11-fold between 3 and 60 days after permanent coronary artery occlusion.28 Conversely, in a model of acute MI, LGE can be positive within 1 hour of injury.29 Given the variant tissue composition between acute and chronic states, it cannot be assumed that the presence of LGE necessarily always implies irreversible injury. We found that the overall volume of LGE decreased by 22% between the first time point and six months and, that on a patient by patient basis, there was considerable variation in the extent of reduction in LGE. Importantly, of those myocardial segments showing reduction in LGE at 6 months, 68% also showed an improvement in wall motion (P < 0.0001). Using a technique based on differential signal intensity threshold analysis, Yan et al identified an peri-infarct ‘border zone’ on the periphery of the LGE area.30 However, recovery confined to a putative ‘border zone’ is unlikely to account for the observations in the current study, since 51% of segments showing improved function had transmural (or near-transmural) LGE extent when imaged < 48 hours after primary PCI. These findings indicate that LGE within this time frame does not necessarily reflect irreversible injury. Our findings do not identify the tissue correlate of LGE at early time points, but do stress the important possibility of resolution, even of transmural LGE, in association with recovery of myocardial function in that area.
LGE and recovery of function
The ability of LGE obtained in the acute setting to predict later functional recovery is contentious.31-34 Choi et al found that improvement in segmental contractile function between < 7 days post infarct and 8-12 weeks later was inversely related to the transmural extent of infarction on the first scan.32 That work differs from the current study in several important respects. Most importantly, the second CMR scan did not include assessment of LGE, making it impossible to know if LGE had changed between scans. While early assessment of LGE will, on average, indicate injured tissues, our work demonstrates that LGE early after an event does not always reflect irreversibly injured, non-viable tissue, and even transmural LGE early after acute ischemia can be associated with recovery of function in that segment. In keeping with these findings, Beek et al reported that 25 % of segments with transmural hyperenhancement 7 ± 3 days post MI had the potential for functional improvement after 13 weeks.33
Our findings of resolving LGE are further consistent with a recent clinical study showing that LGE diminishes within one week of acute MI.35 The authors speculated that reduction in LGE may have reflected initial LGE in reversibly injured myocardium. By incorporating edema imaging, to demonstrate the extent of the ischemia zone, and combining this with (a) early and late phase LGE and (b) functional assessment at a segmental level, the current study demonstrates conclusively that this is indeed the case — and that early LGE does not always lead to late scar. Our findings cannot determine mechanisms of ‘shrinkage’ of the LGE area or whether there is hypertrophy of adjacent viable myocardium in the long term36, 37 which may contribute to recovery of function at a later stage.
Salvage index
The difference between volume of myocardium at risk and of that eventually infarcted gives a measure of myocardial salvage that can be indexed to the area at risk to provide the salvage index.6 In clinical trials, this indexed measure should reduce the inter-patient variability associated with measures of absolute infarct size, with a consequent reduction in sample size38 needed to assess therapies intended to reduce infarct size.9
Our data suggest that, given the tendency to reduction in LGE over time, studies incorporating imaging time points as early as 12 hours23 will markedly overestimate the area of irreversible injury in some patients. Based on our findings the magnitude of the underestimate of salvage that could be introduced in assessment < 48 hours post MI (vs. 6 months) is 38 % ± 14%, with important implications for trial design and implementation.
Study limitations
Our data suggest almost complete resolution of edema at 6 months. However, in small number of cases persistently increased T2 signal intensity was identified at 6 months. From experimental studies, resolution of myocardial edema occurs within 2 months of acute MI.39 While accumulation of lipid may occur in the very early phases post acute MI,40 this would not contribute significantly to T2 relaxation properties. A further possibility may relate to the threshold for edema quantification. While efforts were made to impose a recognized and objective segmentation protocol, it is possible that an alternative segmentation strategy or the measurement of absolute myocardial relaxivity values may provide additional benefit. The use of new CMR techniques as T2 mapping should provide a better understanding of tissue composition both in acute stage and chromic stage; however further research will be needed to validate these techniques. The patients in this study were subject to an intensive sequence of MR scans, including 3 CMR scans within 15 days of acute MI. For reasons of safety, gadolinium was not given at each time point, but reserved for the first and last. For this reason, we were unable to define the precise time course of resolution of LGE. Although LGE resolution was associated with improved myocardial segmental function, we did not undertake any additional evaluation of myocardial viability, specifically no comparative study using positron emission tomography (PET), nor, in this clinical study, were histological samples available to define precisely the tissue correlates during each MR time point. Finally, the patients included in the study were mostly white and men and therefore further investigation will be needed in order to establish potential application of these data to those of other race and gender.
Conclusions
Edema of the myocardium occurs in almost all cases of STEMI and primary PCI. It is maximal and constant over the first week post MI, providing a stable window for the retrospective evaluation of area at risk. By contrast, LGE, while present early in acute MI, recedes over time and acutely detected LGE does not necessarily equate with irreversible injury. These findings have important implications for the interpretation of CMR early after acute MI and the design of clinical research studies using CMR-derived infarction or salvage index as endpoints.
CLINICAL PERSPECTIVE.
Late gadolinium enhancement (LGE) and edema imaging are used to assess acute myocardial injury, area at risk and salvaged myocardium post reperfusion. Late gadolinium enhancement (LGE) is currently considered the gold standard for myocardial infarct visualization both in acute and chronic myocardial infarction and an accurate predictor of recovery of wall motion post revascularization. The present study shows that CMR features of acute myocardial infarction are dynamic and change for both LGE and edema. Following revascularization, edema is shown to peak within the first week post reperfusion. LGE performed in the first 24H does not necessarily indicate irreversible injury; our results show that 51 % of the segments with transmural LGE at 24H post reperfusion recovered function at 6 months. A detailed knowledge of the early dynamic changes of both LGE and edema imaging is crucial in assessing final infarct size and myocardium salvage, especially when designing clinical trials using CMR.
Supplementary Material
Acknowledgments
The authors thank the staff of the Heart Centre at John Radcliffe Hospital and OCMR for their support and help. The authors gratefully acknowledge technical advice from Dr. A. Arai, NIH, Bethesda, US.
Sources of Funding The study was funded by the Oxford Comprehensive Biomedical Research Center, NIHR funding scheme. RC is a Wellcome Trust Senior Research Fellow in Clinical Science. SN and RC acknowledge the support of the BHF Centre of Research Excellence, Oxford.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 2.Aletras AH, Tilak GS, Natanzon A, Hsu L-Y, Gonzalez FM, Hoyt RF, Jr., Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with t2-weighted cardiac magnetic resonance imaging: Histopathological and displacement encoding with stimulated echoes (dense) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 3.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared ssfp improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cury RC, Shash K, Nagurney JT, Rosito G, Shapiro MD, Nomura CH, Abbara S, Bamberg F, Ferencik M, Schmidt EJ, Brown DF, Hoffmann U, Brady TJ. Cardiac magnetic resonance with t2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118:837–844. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG. Edema as a very early marker for acute myocardial ischemia: A cardiovascular magnetic resonance study. J Am Coll Cardiol. 2009;53:1194–1201. doi: 10.1016/j.jacc.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–1587. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Berry C, Kellman P, Mancini C, Chen MY, Bandettini WP, Lowrey T, Hsu LY, Aletras AH, Arai AE. Magnetic resonance imaging delineates the ischemic area-at-risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3:527–535. doi: 10.1161/CIRCIMAGING.109.900761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francone M, Bucciarelli-Ducci C, Carbone I, Canali E, Scardala R, Calabrese FA, Sardella G, Mancone M, Catalano C, Fedele F, Passariello R, Bogaert J, Agati L. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with st-segment elevation myocardial infarction: Insight from cardiovascular magnetic resonance. Journal of the American College of Cardiology. 2009;54:2145–2153. doi: 10.1016/j.jacc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Larose E, Tizon-Marcos H, RodÈs-Cabau J, Rinfret S, DÈry J-P, Nguyen CM, Gleeton O, Boudreault J-R, Roy L, NoÎl B, Proulx G, Rouleau J, Barbeau G, LarochelliËre RD, Bertrand OF. Improving myocardial salvage in late presentation acute st-elevation myocardial infarction with proximal embolic protection. Catheterization and Cardiovascular Interventions. 2010;76:461–470. doi: 10.1002/ccd.22588. [DOI] [PubMed] [Google Scholar]
- 10.Ortiz PÈrez JT, Lee DC, Meyers SN, Davidson CJ, Bonow RO, Wu E. Determinants of myocardial salvage during acute myocardial infarction: Evaluation with a combined angiographic and cmr myocardial salvage index. JACC: Cardiovascular Imaging. 2010;3:491–500. doi: 10.1016/j.jcmg.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Buja LM. Myocardial ischemia and reperfusion injury. Cardiovascular Pathology. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen E-L, Simonetti O, Bundy J, Finn JP, Klocke FJ, Judd RM. Relationship of mri delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 13.de Roos A, Doornbos J, van der Wall E, van Voorthuisen A. Mr imaging of acute myocardial infarction: Value of gd-dtpa. Am. J. Roentgenol. 1988;150:531–534. doi: 10.2214/ajr.150.3.531. [DOI] [PubMed] [Google Scholar]
- 14.Saeed M, Bremerich J, Wendland MF, Wyttenbach R, Weinmann HJ, Higgins CB. Reperfused myocardial infarction as seen with use of necrosis-specific versus standard extracellular mr contrast media in rats. Radiology. 1999;213:247–257. doi: 10.1148/radiology.213.1.r99se30247. [DOI] [PubMed] [Google Scholar]
- 15.Klein C, Schmal TR, Nekolla SG, Schnackenburg B, Fleck E, Nagel E. Mechanism of late gadolinium enhancement in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2007;9:653–658. doi: 10.1080/10976640601105614. [DOI] [PubMed] [Google Scholar]
- 16.Karamitsos TD, Hudsmith LE, Selvanayagam JB, Neubauer S, Francis JM. Operator induced variability in left ventricular measurements with cardiovascular magnetic resonance is improved after training. J Cardiovasc Magn Reson. 2007;9:777–783. doi: 10.1080/10976640701545073. [DOI] [PubMed] [Google Scholar]
- 17.Dall’Armellina E, Morgan TM, Mandapaka S, Ntim W, Carr JJ, Hamilton CA, Hoyle J, Clark H, Clark P, Link KM, Case D, Hundley WG. Prediction of cardiac events in patients with reduced left ventricular ejection fraction with dobutamine cardiovascular magnetic resonance assessment of wall motion score index. Journal of the American College of Cardiology. 2008;52:279–286. doi: 10.1016/j.jacc.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Gaal WJ, Banning AP. Percutaneous coronary intervention and the no-reflow phenomenon. Expert Rev Cardiovasc Ther. 2007;5:715–731. doi: 10.1586/14779072.5.4.715. [DOI] [PubMed] [Google Scholar]
- 19.Lima JAC, Judd RM, Bazille A, Schulman SP, Atalar E, Zerhouni EA. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced mri : Potential mechanisms. Circulation. 1995;92:1117–1125. doi: 10.1161/01.cir.92.5.1117. [DOI] [PubMed] [Google Scholar]
- 20.Kuntz ID, Jr., Brassfield TS, Law GD, Purcell GV. Hydration of macromolecules. Science. 1969;163:1329–1331. doi: 10.1126/science.163.3873.1329. [DOI] [PubMed] [Google Scholar]
- 21.Friedrich MG. Myocardial edema--a new clinical entity. Nat Rev Cardiol. 2010;7:292–296. doi: 10.1038/nrcardio.2010.28. [DOI] [PubMed] [Google Scholar]
- 22.Karolle BL, Carlson RE, Aisen AM, Buda AJ. Transmural distribution of myocardial edema by nmr relaxometry following myocardial ischemia and reperfusion. Am Heart J. 1991;122:655–664. doi: 10.1016/0002-8703(91)90508-f. [DOI] [PubMed] [Google Scholar]
- 23.Larose E, RodÈs-Cabau J, Pibarot P, Rinfret S, Proulx G, Nguyen CM, DÈry J-P, Gleeton O, Roy L, NoÎl B, Barbeau G, Rouleau J, Boudreault J-R, Amyot M, De LarochelliËre R, Bertrand OF. Predicting late myocardial recovery and outcomes in the early hours of st-segment elevation myocardial infarction: Traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. Journal of the American College of Cardiology. 2010;55:2459–2469. doi: 10.1016/j.jacc.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Raman SV, Simonetti OP, Winner MW, Iii, Dickerson JA, He X, Mazzaferri EL, Jr, Ambrosio G. Cardiac magnetic resonance with edema imaging identifies myocardium at risk and predicts worse outcome in patients with non-st-segment elevation acute coronary syndrome. Journal of the American College of Cardiology. 2010;55:2480–2488. doi: 10.1016/j.jacc.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvanayagam JB, Kardos A, Francis JM, Wiesmann F, Petersen SE, Taggart DP, Neubauer S. Value of delayed-enhancement cardiovascular magnetic resonance imaging in predicting myocardial viability after surgical revascularization. Circulation. 2004;110:1535–1541. doi: 10.1161/01.CIR.0000142045.22628.74. [DOI] [PubMed] [Google Scholar]
- 26.Ingkanisorn WP, Rhoads KL, Aletras AH, Kellman P, Arai AE. Gadolinium delayed enhancement cardiovascular magnetic resonance correlates with clinical measures of myocardial infarction. J Am Coll Cardiol. 2004;43:2253–2259. doi: 10.1016/j.jacc.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa Y, Sakuma H, Suzawa N, Kitagawa K, Makino K, Hirano T, Takeda K. Late gadolinium-enhanced magnetic resonance imaging in acute and chronic myocardial infarction. Improved prediction of regional myocardial contraction in the chronic state by measuring thickness of nonenhanced myocardium. J Am Coll Cardiol. 2005;45:901–909. doi: 10.1016/j.jacc.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 28.Fieno DS, Hillenbrand HB, Rehwald WG, Harris KR, Decker RS, Parker MA, Klocke FJ, Kim RJ, Judd RM. Infarct resorption, compensatory hypertrophy, and differing patterns of ventricular remodeling following myocardial infarctions of varying size. J Am Coll Cardiol. 2004;43:2124–2131. doi: 10.1016/j.jacc.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 29.Schulz-Menger J, Gross M, Messroghli D, Uhlich F, Dietz R, Friedrich MG. Cardiovascular magnetic resonance ofacute myocardial infarction at a very early stage. Journal of the American College of Cardiology. 2003;42:513–518. doi: 10.1016/s0735-1097(03)00717-4. [DOI] [PubMed] [Google Scholar]
- 30.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 31.Gerber BL, Garot J, Bluemke DA, Wu KC, Lima JA. Accuracy of contrast-enhanced magnetic resonance imaging in predicting improvement of regional myocardial function in patients after acute myocardial infarction. Circulation. 2002;106:1083–1089. doi: 10.1161/01.cir.0000027818.15792.1e. [DOI] [PubMed] [Google Scholar]
- 32.Choi KM, Kim RJ, Gubernikoff G, Vargas JD, Parker M, Judd RM. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation. 2001;104:1101–1107. doi: 10.1161/hc3501.096798. [DOI] [PubMed] [Google Scholar]
- 33.Beek AM, Kühl HP, Bondarenko O, Twisk JWR, Hofman MBM, van Dockum WG, Visser CA, van Rossum AC. Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. Journal of the American College of Cardiology. 2003;42:895–901. doi: 10.1016/s0735-1097(03)00835-0. [DOI] [PubMed] [Google Scholar]
- 34.Rogers WJ, Jr., Kramer CM, Geskin G, Hu YL, Theobald TM, Vido DA, Petruolo S, Reichek N. Early contrast-enhanced mri predicts late functional recovery after reperfused myocardial infarction. Circulation. 1999;99:744–750. doi: 10.1161/01.cir.99.6.744. [DOI] [PubMed] [Google Scholar]
- 35.Engblom H. Rapid initial reduction of hyperenhanced myocardium after reperfused first myocardial infarction suggests recovery of the peri-infarction zone: One year follow up by mri. Circulation cardiovascular imaging. 2009;2:47–55. doi: 10.1161/CIRCIMAGING.108.802199. [DOI] [PubMed] [Google Scholar]
- 36.Rehwald WG, Fieno DS, Chen E-L, Kim RJ, Judd RM. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation. 2002;105:224–229. doi: 10.1161/hc0202.102016. [DOI] [PubMed] [Google Scholar]
- 37.Holmes JW, Yamashita H, Waldman LK, Covell JW. Scar remodeling and transmural deformation after infarction in the pig. Circulation. 1994;90:411–420. doi: 10.1161/01.cir.90.1.411. [DOI] [PubMed] [Google Scholar]
- 38.Klocke FJ. Cardiac magnetic resonance measurements of area at risk and infarct size in ischemic syndromes. J Am Coll Cardiol. 2010;55:2489–2490. doi: 10.1016/j.jacc.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 39.Reimer KA, Jennings RB. The changing anatomic reference base of evolving myocardial infarction. Underestimation of myocardial collateral blood flow and overestimation of experimental anatomic infarct size due to tissue edema, hemorrhage and acute inflammation. Circulation. 1979;60:866–876. doi: 10.1161/01.cir.60.4.866. [DOI] [PubMed] [Google Scholar]
- 40.Bilheimer DW, Buja LM, Parkey RW, Bonte FJ, Willerson JT. Fatty acid accumulation and abnormal lipid deposition in peripheral and border zones of experimental myocardial infarcts. J Nucl Med. 1978;19:276–283. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.