Abstract
Activation of poly (ADP-ribose) polymerases contributes to ischemic damage by causing neuronal NAD+ depletion, release of apoptosis-inducing factor and consequent caspase-independent cell death. PARP-mediated cell death is sexually dimorphic, participating in ischemic damage in the male brain, but not the female brain. We tested the hypothesis that androgen signaling is required for this male-specific neuronal cell death pathway. We observed smaller damage following focal cerebral ischemia (MCAO) in male PARP-1 knockout mice compared to WT as well as decreased damage in male mice treated with the PARP inhibitor PJ34. Protection from ischemic damage provided by PJ-34 in WT mice is lost after removal of testicular androgens (CAST) and rescued by androgen replacement. CAST PARP-1 KO mice exhibit increased damage compared to intact male KO mice, an effect reversed by androgen replacement in an androgen receptor-dependent manner. Lastly, we observed that ischemia causes an increase in PARP-1 expression that is diminished in the absence of testicular androgens. Our data indicates that PARP-mediated neuronal cell death in the male brain requires intact androgen-androgen receptor signaling.
Keywords: cerebral ischemia, stroke, poly-adp ribose polymerase, androgen
INTRODUCTION
Poly (ADP-ribose) polymerases (PARP) are members of a family of enzymes that are particularly abundant in cell nuclei and can function as sensors of DNA damage. Poly(ADP-ribosyl)ation of proteins is a post-translational modification catalyzed by PARP and a key step in the regulation of multiple physiological cellular functions such as DNA repair, gene transcription, gene expression, cell cycle progression, cell death, chromatin function, and genomic stability (Pacher and Szabo, 2008). PARP-1 is the most abundant isoform of the PARP enzyme family and is an important regulator of neuronal cell death and cellular responses to DNA damage resulting from physiological circumstances as well as from neuronal injury (Eliasson et al., 1997). Activation of PARP-1 after ischemia-induced DNA damage is well recognized as a key factor in neuronal NAD+ depletion, mitochondrial release of apoptosis-inducing factor, and caspase-independent cell death (Jagtap and Szabo, 2005).
This PARP-1-initiated cell death pathway has been recently shown to be sexually dimorphic (McCullough et al., 2005; Lang and McCullough, 2008; Yuan et al., 2009)}. Genetic deletion or pharmacological inhibition of PARP improves brain outcomes from cerebral ischemia in males (Eliasson et al., 1997) but not in females regardless of ovarian hormone status (McCullough et al., 2005). Similar sex specificity has been reported in the neonatal brain treated with hypoxia-ischemia (Hagberg et al., 2004; Zhu et al., 2006). These initial studies suggested that PARP dependent, caspase independent, neuronal death pathway may be highly engaged in male ischemic brain, less so in the female. Subsequent work has confirmed and expanded our understanding that PARP signaling through apoptosis-inducing factor (AIF) is an important target in male cerebral ischemic pathology, while the intrinsic, caspase-dependent pathway is vital to female neuronal death (Lang and McCullough, 2008; Yuan et al., 2009).
The biological basis for PARP’s sexually dimorphic death signaling in cerebral ischemia is unclear. We hypothesized that the surprising specificity of this molecular mechanism is enabled by androgen availability and androgen receptor (AR) signaling in the male. The role of male sex steroids in ischemic sensitivity is relatively understudied. Male sex is a recognized risk factor for cerebrovascular disease and stroke (Foulkes et al., 1988), and male animals consistently exhibit greater damage following experimental ischemia (For recent reviews, see (Herson et al., 2009; Vagnerova et al., 2008; Hurn et al., 2005)). Consistent with the notion that androgens increase damage following cerebral ischemia, removal of endogenous testosterone by castration results in decreased ischemic damage in male rodents (Yang et al., 2002; Cheng et al., 2007). Importantly, infarct volume following middle cerebral artery occlusion (MCAO) increases in castrated males when testosterone is replaced (Hawk et al., 1998; Toung et al., 1998; Yang et al., 2002; Cheng et al., 2007). In the present study, we explored the interaction of male sex hormones and PARP-1 following focal cerebral ischemia. Our study breaks new ground by focusing on the role of androgens and AR in PARP-1 cell death signaling in vivo. Furthermore, since PARP inhibitors such as minocycline are currently in clinical trial, our study sheds new light on the effectiveness of these agents in male stroke patients and may serve as a prototype of sex-specific anti-ischemic treatments for brain.
EXPERIMENTAL PROCEDURES
Animals
The present study was conducted in accordance with the National Institutes of Health guidelines for the care and use of animals in research and under protocols approved by the Oregon Health & Science University Animal Care and Use Committee. We used PARP-1 gene deficient (KO) male mice raised in our laboratory (homozygous breeding with intermittent confirmation of genotype, as described previously (Eliasson et al., 1997); animals bred to confluence on background strain [129S1/SvImJ]). 129S1/SvImJ mice were obtained from Jackson Laboratories to minimize strain and vendor variability and served as wild type (WT) controls. Male animals were randomized to groups of the same age and weight (22–30g).
Ischemic Model
Methods are as previously published in mouse (Vagnerova et al., 2006). Cerebral ischemia was induced by 90 minutes reversible middle cerebral artery occlusion (MCAO) via the intraluminal suture technique (6-0 monofilament nylon surgical suture with a heat-rounded tip) under isoflurane anesthesia. Adequacy of MCAO was confirmed by laser-Doppler flowmetry (LDF) measured over the ipsilateral parietal cortex and by neurological deficit scoring during continuous occlusion as follows: 0 = no deficit, 1 = failure to extend forelimb, 2 = circling, 3 = unilateral weakness, 4 = no spontaneous motor activity (Hurn and Macrae, 2000; Hurn et al., 1995). Only mice with clear neurological deficits (neurological deficit scoring ≥2) were included in the treatment groups. Physiological measurements were performed in separate cohorts (n = 3 per group), as previously described (Goyagi et al., 2001). In each animal, a femoral arterial catheter was placed for arterial blood pressure and blood gas measurement.
Gonadectomy and Hormones
Orchidectomy, as described previously, was performed under isoflurane anesthesia (Toung et al., 1998) 7 days prior to ischemia concurrent with administration of dihydrotestosterone (DHT)by subcutaneous implant technique (5 mg, continuous 21-day release implant, Innovative Research) (Hurn et al., 1995). AR antagonist flutamide (F) (5 mg, continuous 21-day release implant, Innovative Research) was implanted subcutaneously at the time of castration and DHT implantation. Sham castration was performed under anesthesia 7 days prior to MCAO.
Poly (ADP-Ribose) Polymerase Inhibition with PJ-34
Immediately before MCAO, 129S1/SvImJ mice (Taconic) were injected intraperitoneally with the PARP-1 inhibitor PJ-34 (10 mg/kg) or saline control (Garcia et al., 2001).
Imaging and Analysis
The brains were harvested at 24 hours post MCAO and sliced into five 2-mm thick coronal sections for staining with 1.2% triphenyl tetrazolium chloride (TTC) in saline (Takahashi et al., 1997). Infarction volume was measured by a blinded investigator using digital imaging and image analysis software (Sigma Scan Pro, Jandel). The area of infarct was measured on the rostral and caudal surfaces of each slice and numerically integrated across the thickness of the slice to obtain an estimate of infarct volume in each slice. Infarct volume of the total hemisphere, striatum, and cortex were measured. Volumes from all five slices were summed to calculate total infarct volume expressed as a percentage of contralateral structure volume. Infarct volume was corrected for edema by comparing the volume of ischemic to nonischemic hemispheres (Goyagi et al., 2001).
TaqMan real-time qPCR
Total RNA was obtained using RNeasy Mini kit (Qiagen, Valencia, CA) per manufacturer’s instructions. RNA concentration was determined by UV measurement; cDNA was reverse transcribed from 500 ng total RNA using the high capacity cDNA archive kit (Applied Biosystems, Foster City, CA); 50 ng cDNA was used for qPCR in a 96-well plate with a total volume of 50 μL. Each TaqMan reaction was performed in triplicate. Specific primer and probe sets for PARP were obtained from Applied Biosystems. 18S RNA levels were also determined to serve as internal control, and final results were expressed as the ratios of PARP-1 to 18S.
Statistical Analysis
All data are expressed as mean±SEM. Infarction volume and all densitometry measures were analyzed with one-way analysis of variance (ANOVA), and post hoc comparisons were made by Tukey’s test. Physiological and LDF values were analyzed by two-way ANOVA and post hoc Newman-Keuls to determine differences among treatment groups. The criterion for statistical significance was set at P < 0.05.
RESULTS
Infarct volumes of intact and castrated WT and PARP-1 KO male mice
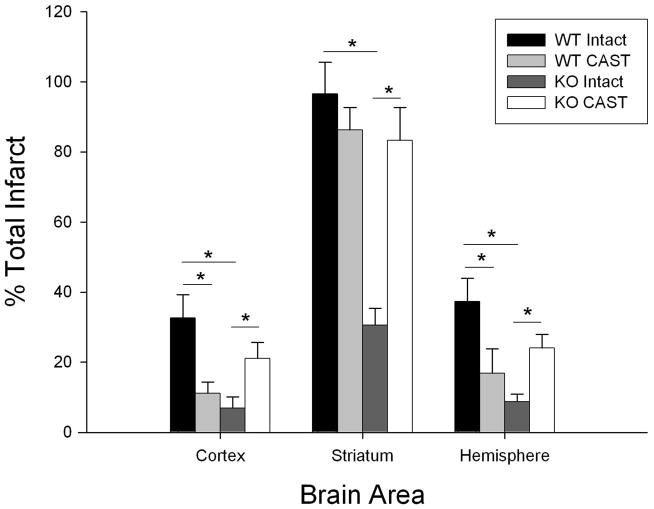
Gonadally intact and castrated male 129S1/SvImJ mice (WT) and PARP-1 KO (KO) mice were subjected to 90 minutes MCAO and infarct volume of cortex, striatum, and total (hemisphere) was analyzed. Castrated WT mice had significantly smaller total infarct volumes compared to intact WT male mice, consistent with our recently published results (Cheng et al.; Uchida et al.), 16.9% ± 2.1% (n=10) in CAST vs. 37.3% ± 6.9% (n=10) in intact. Similar findings were observed in cortical and striatal infarct volumes (cortex: 12.6% ± 3.2% in CAST vs. 32.6% ± 6.7% in intact; striatum: 86.2% ± 6.8% in CAST vs. 96.6% ± 9.4% in intact). In agreement with previous reports, infarct volume was significantly smaller in gonadally intact male KO mice compared to WT males (Figure 1). Surprisingly, the protection afforded by KO was lost following castration. In fact, CAST KO males exhibited significantly increased infarct volume compared to intact KO mice in cortex (23.4% ± 4.3% in CAST vs. 7.4% ± 3.2% in intact), striatum (85.1% ± 9.7% in CAST vs. 30.5% ± 5.0% in intact), and total infarction (24.1% ± 3.9% in CAST vs. 8.8% ± 2.2% in intact) (Figure 1).
Figure 1. Differential effects of castration on infarct size in WT and PARP-1 KO mice.
Infarct volumes in cortex, striatum, and total hemisphere (% contralateral structure = total infarct volume of ipsilateral structure/total volume contralateral structure) were assessed in intact and castrated WT (WT intact, n=10; WT CAST, n=10) and PARP-1 knockout mice (KO intact, n=10; KO CAST, n=10). Values are mean ± SEM. * p < 0.05.
Effect of androgen/androgen receptor signaling on infarct volumes in WT and PARP-1 KO mice
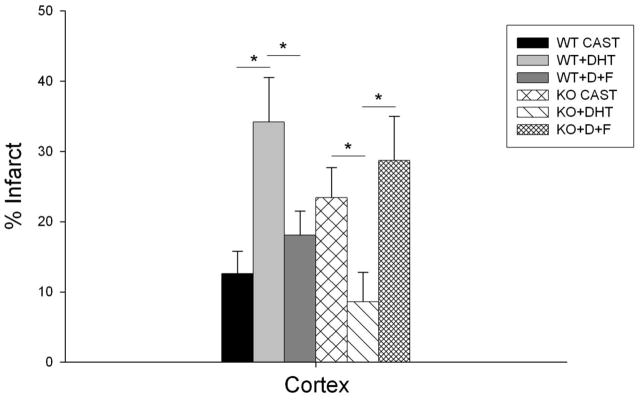
In order to assess the role of androgen receptor signaling, castrated WT and KO mice were implanted with DHT, a potent androgen receptor agonist that cannot be aromatized to estrogen. Compared to untreated WT castrates, mice implanted with DHT (5 mg) had significantly larger infarct volumes in cortex (34.2% ± 6.3% in DHT implanted vs. 12.6% ± 3.2% in CAST; n=11, P<0.05) and in hemisphere (40.2% ± 6.7% in DHT implanted vs. 16.9% ± 2.1% in CAST; P<0.05) (Figure 2). In contrast, androgen replacement renewed protection in KO castrates. Compared to untreated castrates, KO mice implanted with DHT had significantly smaller infarct volumes in cortex (8.6% ± 4.2% in DHT implanted vs. 23.4% ± 4.3% in CAST; n=10, P<0.05) and in hemisphere (16.6% ± 6.3% in DHT implanted vs. 24.1% ± 3.9% in CAST; P<0.05). Similar findings were observed in striatum, although did not reach statistical significance (data not shown). To further examine the effect of androgen/androgen receptor (AR) interaction on the PARP-1 cell death pathway, we treated DHT implanted mice with the AR antagonist flutamide (F). Infarction size was increased in PARP-1 KO male mice implanted with DHT and equimolar flutamide compared to KO mice implanted with DHT alone. Infarct volume was significantly increased in cortex (28.7% ± 6.3% in D+F implanted vs. 8.6% ± 4.2% in DHT implanted; n=10, P<0.05) and in total hemisphere (41.3% ± 7.8% in DHT+F implanted vs. 16.6% ± 6.3% in DHT implanted; P<0.05). In WT males treated with DHT+F, infarct volume was significantly reduced in cortex (18.1% ± 3.4% in DHT+F implanted vs. 34.2% ± 6.3% in DHT implanted; n=11, P<0.05), and in total hemisphere (25.8% ± 4.6% in DHT+F implanted vs. 40.2% ± 6.7% in DHT implanted; P<0.05) (Figure 2).
Figure 2. Effects of DHT and androgen receptor antagonist flutamide on infarct size in WT and PARP-1 KO mice.
Infarct volume in cortex (% contralateral cortex = total infarct volume of ipsilateral cortex/total volume contralateral cortex) was assessed in vehicle-treated castrated (WT CAST, n=10; KO CAST, n=10) and in castrated mice implanted with 5 mg DHT (WT+DHT, n=11; KO+DHT, n=10) or 5 mg DHT and 5 mg flutamide (WT+D+F, n=11; KO+D+F, n=10). Values are mean ± SEM. * p < 0.05.
Infarct volume in WT intact males treated with PARP-1 inhibitor PJ 34
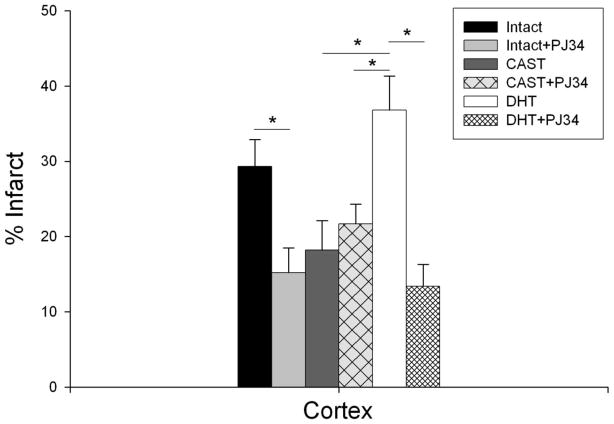
When we blocked PARP-1 signaling in WT intact males with the well studied pharmacological inhibitor PJ34, results were consistent with our findings in PARP-1 KO males, i.e., infarct volume was significantly reduced in cortex (15.2% ± 3.3% in PJ34 treated mice vs. 29.3% ± 3.6% in vehicle treated; n=10, P<0.05) (Figure 3). PJ34 did not alter cortical infarct volume when administered to WT castrated males (21.7% ± 2.6% in CAST+PJ34 vs. 18.2% ± 3.9% in CAST+Vehicle; n=10). However, DHT replacement rescued the protective effect of PJ34; cortical infarct volume was significantly decreased in DHT+PJ34 group compared to DHT alone (13.4% ± 2.9% DHT+PJ34 vs. 36.8% ± 4.5% DHT; n=10, P<0.05) (Figure 3). Similar findings were observed in striatal and total infarct volumes (data not shown).
Figure 3. Effects of PARP inhibitor PJ34 in combination with DHT on infarct size in WT mice.
Infarct volume in cortex (% contralateral cortex = total infarct volume of ipsilateral cortex/total volume contralateral cortex) was assessed in intact mice treated with vehicle or 10 mg/kg PJ34 (Intact, n=11; Intact+PJ34, n=10) and castrated mice treated with vehicle or 10 mg/kg PJ34 (CAST, n=10; CAST+PJ34, n=10) or 5 mg DHT implant (DHT, n=10) or 10 mg/kg PJ34 and 5 mg DHT implant (DHT+PJ34, n=10). Values are mean ± SEM. * p < 0.05.
Intra-ischemic physiological function and hormone levels
To evaluate the physiological effects of androgen removal and replacement, we measured blood pressure and arterial blood gases during MCAO in intact and castrated WT and KO mice. There were no differences between genotypes or treatment; mean arterial blood pressure, blood gases, and glucose remained within physiological limits in all groups (Table 1). In addition, intra-occlusion cortical cerebral blood flow (CBF), assessed by laser Doppler flowmetry (LDF), was similar in all groups. Total and free serum testosterone levels (data not shown) revealed no significant differences between intact groups (KO vs. WT) and no differences between castrated groups (KO CAST vs. WT CAST).
Table 1.
Physiological data during MCAO.
| Groups | WT | KO | WT/C | KO/C | WT/C/D/F | KO/C/D/F |
|---|---|---|---|---|---|---|
| Temp. | 36.2±0.3 | 36.1±0.6 | 36±0.6 | 36.7±0.3 | 36.6±0.8 | 36.5±0.6 |
| MAP (mm Hg) | 74±6 | 79±7 | 72±3 | 69±5 | 73±8 | 74±2 |
| LDF (%baseline) | 67±3 | 73±7 | 81±6 | 69±10 | 65±12 | 71±5 |
| pH | 7.31±0.03 | 7.31±0.02 | 7.30±0.02 | 7.32±0.02 | 7.39±0.01 | 7.32±0.02 |
| PCO2 (mm Hg) | 42.5±3 | 38.4±1.5 | 44.5±3.4 | 41±0.6 | 36.5±3.1 | 42.8±8.6 |
| pO2 (mm Hg) | 137.9±8 | 132.5±10 | 97.8±6 | 111.8±1.2 | 164±5.1 | 161.3±1.5 |
| Hb | 13.2±0.5 | 13±0.4 | 12±0.3 | 13±0.3 | 11.6±1.1 | 12±0.7 |
| Glu (mg/dL) | 110.5±10 | 119±30.3 | 140±11 | 127±15 | 130±17.6 | 121±33 |
No differences were seen in physiological variables between PARP-1 KO and WT male mice under baseline (not shown) and ischemic conditions. Measured variables were not different among treatment groups, ie, castration, DHT implantation, flutamide implantation. All measurements are within physiological range, and all data are reported as mean±SEM. No statistically significant differences among groups were observed using one way ANOVA. WT, intact WT; KO, intact KO; WT/C, castrated WT; KO/C, castrated KO; WT/C/D/F, castrated WT implanted with DHT+Flutamide; KO/C/D/F, castrated KO implanted with DHT+Flutamide. Temp, rectal temperature; MAP, mean arterial pressure; LDF, laser Doppler flow; pCO2, carbon dioxide partial pressure; pO2, oxygen partial pressure; Hb, hemoglobin; Glu, plasma glucose.
Effect of ischemia and androgens on PARP-1 transcription
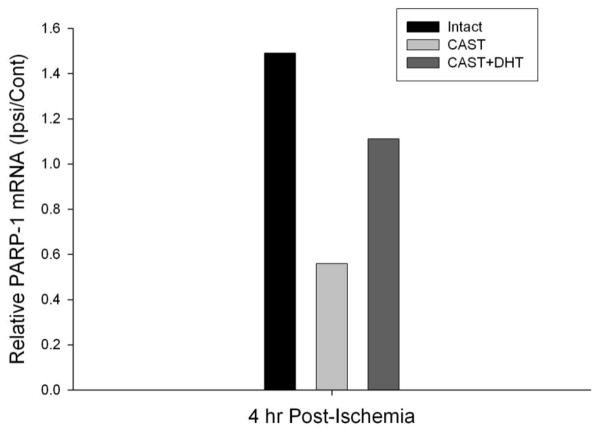
We used quantitative real time RT-PCR (qPCR) to measure the effect of ischemia and androgens on PARP-1 mRNA levels in the cerebral cortex of WT male mice. We observed an approximately 50% increase (as compared to non-ischemic hemisphere) in PARP-1 mRNA level 4 h after MCAO in the ipsilateral cortex of WT intact males (Figure 4). In contrast, the PARP-1 mRNA level declined 4 h after MCAO in WT castrates. DHT (5 mg) implantation partially reversed the effect of castration (Figure 4). Importantly, under basal conditions (no ischemia), no difference in PARP-1 mRNA was observed in WT intact mice compared to castrates with or without DHT (5 mg) treatment.
Figure 4. Effect of androgen removal and replacement on PARP-1 mRNA following ischemia.
Relative PARP-1 mRNA was assessed using quantitative real-time RT-PCR from ipsilateral and contralateral cortex, each normalized to 18S RNA. Cortical RNA was collected 4 hours after reperfusion in intact male mice (Intact, n=3), castrated mice (CAST, n=3), and castrated mice implanted with 5 mg DHT (CAST+DHT, n=3).
Discussion
This study provided three important findings. First, removal of testicular androgens prevents the protection from ischemic damage in male brain following pharmacological inhibition of PARP and increases damage in PARP-1 KO mice, indicative of reversal of neuroprotection following gene deletion. Second, DHT replacement rescues this protection in WT and PARP-1 KO mice in an androgen receptor-mediated mechanism. Lastly, we found that ischemia causes increase in expression of PARP-1 mRNA and that removal of testicular androgens decreases PARP-1 mRNA after ischemia. Therefore, we conclude that PARP-1 activation requires androgen background to be a key step in ischemic cell death and that androgen/PARP-1 interactions may be a crucial mechanism that defines male ischemia-sensitive phenotype.
The present study is in agreement with previous studies demonstrating that PARP inhibition confers protection in male mice in ischemic stroke models (Hagberg et al., 2004; McCullough et al., 2005; Yuan et al., 2009; Szabo et al., 2006) and in rodent models of shock or inflammation (Jagtap and Szabo, 2005; Szabo et al., 2006). In contrast, multiple studies in a variety of injury paradigms have demonstrated that PARP inhibitors or genetic deletion do not improve outcome in female animals (Lang and McCullough, 2008; McCullough et al., 2005; Yuan et al., 2009). While a great deal of research has focused on gender differences in PARP-mediated ischemic damage, surprisingly, to date there are no reports of the role of androgens in PARP-mediated ischemic damage in male brain. Consistent with multiple prior studies, we observed that removal of endogenous androgens by castration of male mice decreases infarct volume (Cheng et al., 2007; Yang et al., 2002; Hawk et al., 1998; Toung et al., 1998). In addition, our data demonstrated for the first time that the benefit to male mice lacking the PARP-1 gene is lost following castration, consistent with the notion that androgens are required for the benefit provided by PARP-1 gene deletion. The increased ischemic damage observed in castrated PARP-1 KO mice is indicative of a reversal of the neuroprotection afforded by PARP-1 gene deletion in intact animals, rather than a non-specific effect due to magnitude of damage. Similarly, we interpret the decrease in infarct volume observed following DHT replacement in PARP-1 KO mice to indicate re-instated protection. Alternatively, it is possible that PARP-1 KO reveals a novel protective pathway mediated by DHT in the absence of the PARP-1 cell death pathway. This observation is not completely inconsistent with our previous work indicating that DHT is capable of both protection and exacerbation of infarct volume following MCAO, depending on dose. Intact WT mice have significantly reduced infarct volume following treatment with the PARP inhibitor PJ-34. In contrast, PJ-34 has no effect on infarct volume in castrated WT mice. We interpret these data toto indicate that the protection provided by the pharmacological inhibitor PJ-34 requires the presence of endogenous androgens and is lost in castrated male mice. Moreover, we observed that the benefit of PARP-1 gene deficiency and PJ-34 to male mice was rescued following androgen repletion, in an androgen receptor-dependent manner, further strengthening our conclusion that endogenous androgens are specifically required to facilitate the role of PARP-1 in ischemic damage.
The mechanism underlying PARP-mediated ischemic damage remains a subject of intense research and debate. One prevailing theory is that cell death occurs due to depletion of ATP for NAD+ synthesis and inhibition of mitochondrial function. In addition, specific signaling pathways have been identified in brain that elucidate the molecular consequences of PARP-activation after injury, including translocation of apoptosis-inducing factor (AIF) from the mitochondria to the nucleus, formation of PAR (poly[ADP-ribose] polymer), a product of PARP activation that is directly toxic to neurons, excessive expression of pro-inflammatory mediators, and reduced expression of prosurvival factors (Jagtap and Szabo, 2005; Pacher and Szabo, 2008; Moroni, 2008; Haddad et al., 2006; Hamby et al., 2007). In addition, data from in vivo and in vitro studies demonstrate that ischemic cell death pathways are fundamentally different in male and female brain (for review, see Herson et al., 2009; Vagnerova et al., 2008). Females appear to be sensitive to caspase-mediated cell death, whereas cell death in males is triggered by caspase-independent pathways involving AIF and PARP activation (Lang and McCullough, 2008; Li et al., 2005; Li and McCullough, 2009; Yuan et al., 2009). We observed an increase in PARP-1 transcription following ischemia in the intact male brain that was dramatically minimized in castrated male mice, indicating the possibility that male-specific ischemic damage is regulated by expression and activity of PARP-1. Interestingly, a very recent report demonstrated that PAR accumulates to a greater extent in male brain than female brain after cerebral ischemia (Yuan et al., 2009). Therefore, our data is consistent with the hypothesis that PARP-1 activation may be a key “switch point” in determining the mode of cell death.
Although the effects of testosterone on stroke risk and stroke outcome are not as well understood as the effects of estrogen, studies have shown that male sex steroids can influence cell survival in brain following ischemia. Androgens provide protection in primary neuronal cultures after oxidative stress, β-amyloid toxicity, and serum deprivation (Ahlbom et al., 1999; Hammond et al., 2001; Zhang et al., 2004; Ahlbom et al., 2001) but can also amplify excitotoxicity (Caruso et al., 2004). Our recent studies demonstrate that androgen replacement in castrated males yielding high but physiological circulating steroid levels exacerbates ischemic damage, while very low levels decrease damage (Uchida et al., 2009; Cheng et al., 2007). Further, we have observed that DHT, the primary intracellular androgen, enhances transcription of inflammatory genes as well as genes functionally associated with apoptosis, maintenance of the extracellular matrix, dysregulation of the blood brain barrier, kinase signaling, and metabolism (Cheng et al., 2007). This study is the first to investigate the interaction between androgen signaling and PARP-1. Our data demonstrated that the androgen/androgen receptor (AR) signaling pathway is required for PARP-1 to mediate ischemic injury outcomes. The AR acts as a classical steroid receptor, initiating genomic mechanisms for androgen-responsive genes, potentially increasing or decreasing gene products involved in cell survival. Castration and DHT replacement had no effect on PARP-1 transcription in non-ischemic mice. Interestingly, our data revealed that AR alters transcription of PARP-1 in the context of ischemia, enabling ischemia-induced increase in PARP-1 mRNA. The molecular mechanism underlying the complex interaction between AR, PARP-1, and ischemia is of great interest for future investigations.
In conclusion, removal of endogenous androgens reversed the protection observed with pharmacological inhibition or genetic deletion of PARP-1 following experimental stroke in male mice, suggesting a fundamental interaction between androgens and PARP-1. Our data further implicates that androgen-AR signaling is an essential component of the PARP-1-mediated cell death pathway following ischemia in male mice. Lastly, castration reversed the effects of ischemia on PARP-1 transcription; and androgen replacement restored the phenotype, indicating the need for further studies of the complex interaction between androgen receptor signaling and PARP-1 following cerebral ischemia.
Acknowledgments
The authors thank Ms. Kathy Gage, Grant and Publications Writer for the Department of Anesthesiology and Perioperative Medicine, OHSU, for her outstanding editorial work in the preparation of this manuscript. The authors would also like to acknowledge the excellent service and care provided by the Department of Anesthesiology and Perioperative Medicine Mouse Colony Core, which oversaw management of the PARP-1 KO mouse breeding colony. Work supported by NIH grants PO1NS49210 and the Bugher Foundation in collaboration with the American Heart Association.
LIST OF ABBREVIATIONS
- AIF
apoptosis inducing factor
- AR
androgen receptor
- CAST
castrated
- cDNA
complementary deoxyribonucleic acid
- DHT
dihydrotestosterone
- DNA
deoxyribonucleic acid
- F
flutamide
- KO
knockout
- LDF
laser-Doppler flowmetry
- MCAO
middle cerebral artery occlusion
- mRNA
messenger ribonucleic acid
- NAD
nicotinamide adenine dinucleotide
- PARP
poly (ADP-ribose) polymerase
- PJ34
N-(6-Oxo-5,6-dihydrophenanthridin-2-yl)-(N,N-dimethylamino)acetamide hydrochloride
- qPCR
quantitative polymerase chain reaction
- RNA
ribonucleic acid
- RT-PCR
reverse transcriptase-polymerase chain reaction
- TTC
triphenyl tetrazolium chloride
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur J Neurosci. 1999;11:1285–1291. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Caruso A, Di GGV, Castiglione M, Marinelli F, Tomassini V, Pozzilli C, Caricasole A, Bruno V, Caciagli F, Moretti A, Nicoletti F, Melchiorri D. Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J Neurochem. 2004;88:1179–1185. doi: 10.1046/j.1471-4159.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27:1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19:547–554. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- Garcia SF, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabo C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- Goyagi T, Goto S, Bhardwaj A, Dawson VL, Hurn PD, Kirsch JR. Neuroprotective effect of sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is linked to reduced neuronal nitric oxide production. Stroke. 2001;32:1613–1620. doi: 10.1161/01.str.32.7.1613. [DOI] [PubMed] [Google Scholar]
- Haddad M, Rhinn H, Bloquel C, Coqueran B, Szabo C, Plotkine M, Scherman D, Margaill I. Anti-inflammatory effects of PJ34, a poly(ADP-ribose) polymerase inhibitor, in transient focal cerebral ischemia in mice. Br J Pharmacol. 2006;149:23–30. doi: 10.1038/sj.bjp.0706837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, Johnston MV. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90:1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- Hamby AM, Suh SW, Kauppinen TM, Swanson RA. Use of a poly(ADP-ribose) polymerase inhibitor to suppress inflammation and neuronal death after cerebral ischemia-reperfusion. Stroke. 2007;38:632–636. doi: 10.1161/01.STR.0000250742.61241.79. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796:296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin Reprod Med. 2009a;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurn PD, Littleton-Kearney MT, Kirsch JR, Dharmarajan AM, Traystman RJ. Postischemic cerebral blood flow recovery in the female: effect of 17 beta-estradiol. J Cereb Blood Flow Metab. 1995;15:666–672. doi: 10.1038/jcbfm.1995.82. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Vannucci SJ, Hagberg H. Adult or perinatal brain injury: does sex matter? Stroke. 2005;36:193–195. doi: 10.1161/01.STR.0000153064.41332.f6. [DOI] [PubMed] [Google Scholar]
- Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? J Transl Med. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Ann Neurol. 2005;58:317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29:670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Simon NG, Wang Y, Hu S. Neural androgen receptor regulation: effects of androgen and antiandrogen. J Neurobiol. 1999;41:505–512. doi: 10.1002/(sici)1097-4695(199912)41:4<505::aid-neu6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- Moroni F. Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage. Curr Opin Pharmacol. 2008;8:96–103. doi: 10.1016/j.coph.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C, Pacher P, Swanson RA. Novel modulators of poly(ADP-ribose) polymerase. Trends Pharmacol Sci. 2006;27:626–630. doi: 10.1016/j.tips.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Traystman RJ, Hashimoto K, London ED, Kirsch JR. Postischemic brain injury is affected stereospecifically by pentazocine in rats. Anesth Analg. 1997;85:353–357. doi: 10.1097/00000539-199708000-00020. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnerova K, Hurn PD, Bhardwaj A, Kirsch JR. Sigma 1 receptor agonists act as neuroprotective drugs through inhibition of inducible nitric oxide synthase. Anesth Analg. 2006;103:430–4. doi: 10.1213/01.ane.0000226133.85114.91. table. [DOI] [PubMed] [Google Scholar]
- Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107:201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol. 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD. Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J Neurosci. 2004;24:5315–5321. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006;96:1016–1027. doi: 10.1111/j.1471-4159.2005.03639.x. [DOI] [PubMed] [Google Scholar]