In the past few years, several major studies have been launched aimed at predicting who will develop Alzheimer’s disease (AD) with the ultimate goal of providing a platform for therapeutic intervention with disease modifying therapies. Many of these studies have been designed to evaluate the role of neuroimaging and chemical biomarkers in assessing and predicting progression in cognitively normal individuals and subjects with mild cognitive impairment (MCI). Mild cognitive impairment refers to the intermediate clinical state in which individuals have an acquired memory impairment beyond what would expected for age, yet they do not meet criteria for dementia.(1) This research is aimed at characterizing and hopefully treating individuals in the pre-dementia phase of AD.
In 2004, the North American Alzheimer’s Disease Neuroimaging Initiative (ADNI) was launched to evaluate the roles of MRI, FDG PET, cerebrospinal fluid (CSF) and amyloid imaging with other biomarkers in predicting clinical progression.(2) The ADNI is a public-private partnership sponsored by the National Institute on Aging and the Foundation for the National Institutes of Health. All of the data generated by ADNI are publically available on the ADNI website (http://www.adni-info.org/index). An Australian project with some similar goals antedated ADNI and since ADNI’s inception parallel efforts have been launched in Japan and Europe. As data have been generated from ADNI, a theoretical framework for the cascade of events leading to clinical impairment in AD has been proposed (Figure). This figure represents an hypothetical sequence of events initiated by amyloid deposition in the brain followed by related pathological events resulting in neuronal degeneration and ultimately cognitive impairment. This theoretical model needs to be validated; however, it does offer insight into the roles that different imaging measures and biomarkers may play at various points in the development of AD.
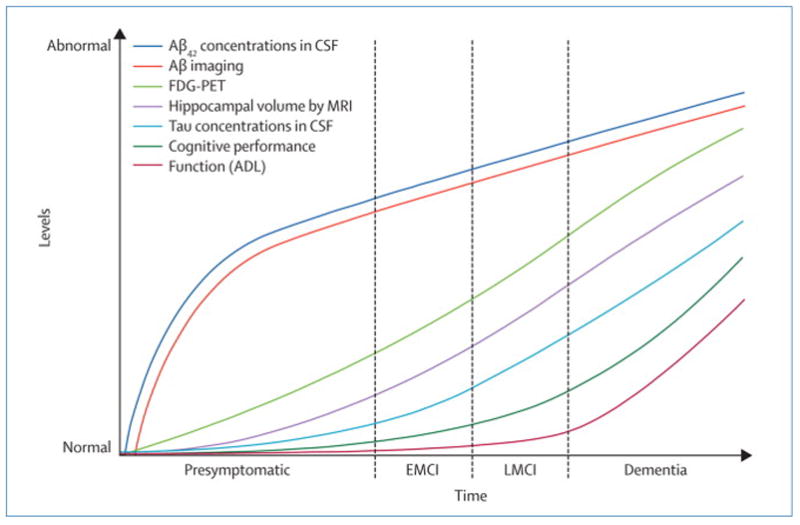
Hypothetical progression of pathological and clinical events with associated detection techniques, imaging or biomarkers, that lead to Alzheimer’s disease. All measures are portrayed with increasing degrees of abnormality on the ordinate. EMCI: early MCI; LMCI: late MCI; CSF: cerebrospinal fluid; FDG PET: fluorodeoxyglucose positron emission tomography; MRI: magnetic resonance imaging; ADL: activities of daily living.
Several important studies relating to this model were published in 2009. The ADNI team, led by investigators at the University of California, Berkeley, assessed the potential complementary roles of amyloid imaging (11C-PiB), hippocampal volume measured on MRI and episodic memory.(3) They demonstrated that Aβ deposition may be the primary event in the cascade and is followed by Aβ-induced hippocampal atrophy which then leads to a decline in episodic memory. Investigators at the Mayo Clinic and ADNI assessed serial changes in imaging markers over 12 months and demonstrated that global levels of amyloid deposition, as documented by 11C-PiB, do not change over one year in cognitively normal, amnestic MCI and AD subjects, but an MRI measure of ventricular volume changes increased in a regular fashion from normal cognition to MCI and, ultimately, to AD, supporting the contention portrayed in the Figure that various imaging measures may be differentially sensitive to change at different points on the clinical spectrum.(4)
Several research groups have been evaluating the role of CSF biomarkers in predicting clinical progression. The ADNI team led by investigators from the University of Pennsylvania documented that CSF Aβ42, total tau and genetic status, possession of Apolipoprotein E ε4 alleles, provided the best assessment of mild AD cross-sectionally, and the total tau/Aβ42 ratio predicted progression from MCI to AD.(5) Another large multicenter study of 750 MCI subjects from 12 European and U.S. centers demonstrated that CSF Aβ42, total tau and phospho-tau could identify MCI subjects progressing to AD with high accuracy, but they cautioned that there was large inter-site variability in the measurements, calling for an international standardization effort.(6)
A Netherlands led group of investigators evaluated very mildly impaired subjects in the European multi-center study DESCRIPA and demonstrated that a pattern of CSF findings was useful in predicting which subjects with only a subjective memory impairment might be at risk of progressing to AD.(7) This study also corroborated the role of CSF markers in predicting which subjects with various subtypes of MCI were likely to progress. Finally, two recent large genome-wide association studies have shown that a combination of genetic markers may be able to predict an individual’s susceptibility to developing AD in the future, indicating that genetic status is likely to play a role in predicting clinical outcome.(8, 9) These studies identified three new genetic risk factors for late onset AD in addition to Apolipoprotein E. Both of these studies involved thousands of subjects and suggested evidence that CLU (clusterin), PICALM (clathrin assembly protein gene) and CR1 (complement component, 3b/4b, receptor 1) may be involved in amyloid B clearance from the brain.
Taken together, these studies imply that a combination of genetic susceptibilities, neuroimaging measures and biomarkers may prove very useful at predicting one’s likelihood of developing AD. At present this approach is most useful in minimally symptomatic individuals such as those with MCI. Ultimately, the knowledge acquired about the role of imaging and biomarkers at the MCI stage may be applicable to clinically asymptomatic persons allowing for possible intervention with disease-modifying therapies when they are developed. The field of AD research is progressing at a rapid pace, and with the burgeoning crisis of the aging of many societies, research targeted at prevention or delay in onset is imperative.
RCP serves as the Chair of the Safety Monitoring Committee for Elan Pharmaceuticals and Chair of the Safety Monitoring Committee Wyeth Pharmaceuticals and as a consultant for Elan Pharmaceuticals and GE Healthcare. He is supported by grants from the National Institute on Aging: P50 AG16574, U01 AG06786, U01 AG24904.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersen R, Knopman D, Boeve B, Geda Y, Ivnik R, Smith G, et al. Mild Cognitive Impairment: Ten Years Later. Archives of Neurology. 2009 doi: 10.1001/archneurol.2009.266. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC, Aisen PS, Beckett LA, Donahue MJ, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical Characterization. Neurology. 2009 doi: 10.1212/WNL.0b013e3181cb3e25. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009 May;132(Pt 5):1310–23. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack C, Jr, Low V, Weigand S, Wiste H, Senjem M, Knopman D, et al. Serial PiB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009 Apr;65(4):403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009 Jul 22;302(4):385–93. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 7.Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009 Jul;8(7):619–27. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 8.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009 Oct;41(10):1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009 Oct;41(10):1094–9. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]


