Abstract
Certain pathogens recruit host complement inhibitors such as factor H (fH) to evade the immune system. Microbial complement inhibitor-binding molecules can be promising vaccine targets by eliciting antibodies that neutralize this microbial defense mechanism. One such antigen, meningococcal fH-binding protein (fHbp), was used in clinical trials before the protein was discovered to bind fH. The potential effect of fH binding on vaccine immunogenicity had not been assessed in experimental animals because fHbp binds human fH specifically. In this study, we developed a human fH transgenic mouse model. Transgenic mice immunized with fHbp vaccine had 4- to 8-fold lower serum bactericidal antibody responses than control mice whose native fH did not bind the vaccine. In contrast, antibody responses were unimpaired in transgenic mice immunized with a control meningococcal group C polysaccharide-protein conjugate vaccine. In transgenic mice, immunization with an fH non-binding mutant of fHbp elicited antibodies with higher bactericidal activity than fHbp vaccination itself. Antibodies elicited by the mutant fHbp more effectively blocked fH binding to wild-type fHbp than antibodies elicited by fHbp that bound fH. Thus, a mutant fHbp vaccine that does not bind fH, but which retains immunogenicity, is predicted to be superior in humans than an fHbp vaccine that binds human fH. In the case of mutant fHbp vaccination, the resultant antibody responses may be directed more at epitopes in or near the fH-binding site, which result in greater complement-mediated serum bactericidal activity; these epitopes may be obscured when human fH is bound to the wild-type fHbp vaccine.
INTRODUCTION
Surface-exposed proteins from bacterial pathogens are potential vaccine candidates when they are targets of complement-dependent bactericidal or opsonophagocytic antibodies. Bacterial surface proteins that also bind host complement inhibitors (or complement down-regulators) are particularly attractive as vaccine candidates (1, 2) because antibodies directed against them may also block binding of the complement inhibitors. Binding of these inhibitors allows certain bacterial species to evade a host innate immune defense that would otherwise result in death of the organism. Down-regulation of complement activation occurs when the complement inhibitors are in close proximity to active complement components that are located nearby on the bacterial surface, thereby permitting the organism to disarm a key component(s) of innate host defense and cause disease. Factor H (fH) is one such important complement inhibitor. A soluble-phase inhibitor of the alternative pathway of complement, fH inhibits the assembly of an active C3 convertase by competing with factor B for C3b binding, accelerating the decay of the alternative pathway C3 convertase (C3b,Bb), while also acting as a cofactor in factor I-mediated cleavage of C3b to iC3b (3-7) . Recently, genetic variation in the human fH gene cluster was found to affect susceptibility to developing meningococcal disease (8).
The vaccine-potential of a number of microbial proteins that bind inhibitors of complement is an active area of investigation. These include, for example, M-protein (Streptococcus pyogenes) (9, 10), OspE (Borrelia burgdorferi) (11), PspC (Streptococcus pneumoniae) (12, 13), and GNA1870 (Neisseria meningitidis); the latter is also known as factor H-binding protein (fHbp) (14). A vaccine that elicits antibodies directed at bacterial ligands for complement inhibitors, such as fH, could confer protection by dual mechanisms: 1) by binding of the antibodies to the surface of the pathogen that result in direct activation of complement, and 2) by blocking binding of the complement inhibitor (for example fH), thereby enhancing susceptibility of the pathogen to complement-mediated bactericidal or opsonic activity.
N. meningitidis is a major cause of bacterial meningitis and sepsis worldwide. The organism binds both fH and C4BP to its surface (14-16), although maximal C4BP binding is observed only under conditions of low stringency, which may limit its physiological role (15). When fH is bound to the meningococcal cell surface, the ability of fH to down-regulate complement activation enables the organism to survive in human serum or blood (16-18). Ligands for fH binding to meningococci include a surface-exposed lipoprotein referred to as fHbp and a second recently described receptor protein, Neisserial surface protein A (NspA) (19). NspA may be important for evasion of complement-mediated killing by strains with low fHbp expression.
Recombinant fHbp antigens are part of two promising group B meningococcal vaccines that are in late-stage clinical development (20). These vaccines elicited serum bactericidal antibody responses in mice (21-23) and humans (24-26). It was only after fHbp vaccines had been developed and tested in clinical trials, was the antigen discovered to bind to fH (14). Furthermore, binding of fH to fHbp was found to be specific for human fH (27). A potential undesirable consequence of targeting an antigen that binds a human complement inhibitory protein is formation of a complex between the vaccine antigen and complement protein, which might interfere with antigen presentation and protective antibody responses.
The purpose of the present study was to investigate fHbp immunogenicity, under conditions where human fH was present; to accomplish this, we developed a human fH transgenic mouse model. We also investigated the immunogenicity of a newly identified mutant fHbp vaccine containing a single amino acid substitution that eliminated fH binding to mutant fHbp but which retained immunogenicity in wild-type mice. By eliminating fH binding, we hypothesized that in human fH transgenic mice, the mutant fHbp vaccine would elicit serum antibodies with greater potential for protection (e.g., greater bactericidal activity) than antibodies elicited by wild-type fHbp.
MATERIALS AND METHODS
Generation of human factor H transgenic mice
Full-length cDNA encoding human factor H (3.9 kbp) was subcloned into the EcoRI site of the expression vector pCAGGS (28). A cytomegalovirus (CMV) enhancer and chicken β-actin promoter sequences are located upstream of the EcoRI site in pCAGGS and a rabbit β-globin polyA sequence is located downstream of the EcoRI site. The resulting plasmid, pCAGGS-human fH, was digested with SalI and PstI to isolate the transgenic cassette fragment consisting of the CMV enhancer, the chicken β-actin promotor, the human fH cDNA and the rabbit β-globin poly(A) sequence. The isolated ~6 kb SalI and PstI fragment was purified for microinjection into mouse embryos from BALB/c mice. Mouse embryos were implanted into pseudo-pregnant female BALB/c mice (Charles River Breeding Laboratories) at the Transgenic Facility at University of Massachusetts Medical School. Human fH transgenic mice initially were identified by PCR analysis using genomic DNA prepared from mouse tails. A region inside human cfH was amplified by PCR using primers SCR7F 5′–CATCCTGGCTACGCTCTTCCAAAA–3′ and SCR8R 5′–ATCTAATTGATCCTGATGTTTCACC–3′ to yield a 232 bp product. Amplified products were electrophoretically resolved in 2% TAE agarose gels and were visualized by ethidium bromide staining under UV light. Expression of human fH in sera of pups was detected by Western blotting using affinity purified goat anti-human fH (Complement Technology Inc., Tyler, TX).
Serum human fH concentrations
To distinguish human from mouse fH, we used an fHbp capture ELISA that specifically binds human fH. Recombinant fHbp (2 μg/ml) in sterile PBS was adsorbed to wells of microtiter plates. After blocking with 1% BSA, dilutions of pre-immune mouse (wild-type or transgenic) or human sera were added. Bound human fH was detected using sheep anti-human fH (Lifespan Biosciences, Seattle, WA). Human fH concentrations were determined in comparison to dilutions of a human reference serum that contained 471 μg/ml of fH. Wild-type mice had no detectable serum human fH (<12 μg/ml). For comparison with humans, fH was measured in stored sera from 25 adult subjects in the San Francisco Bay area who had participated in a protocol approved by the Children’s Hospital Oakland IRB to screen normal sera as complement sources for serum bactericidal assays.
Recombinant fHbp
The gene encoding fHbp ID 1 (http://pubmlst.org/Neisseria/fHbp) was subcloned into expression plasmid pET21b as described previously (21-23). A R41S substitution was introduced by changing the codon AGG to AGC in the fHbp gene using the QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) and the manufacturer’s protocol. Recombinant fHbp and R41S mutant proteins were purified by Ni2+ affinity chromatography as previously described (22, 29). Molecular weight and purity were assessed by SDS-PAGE (NuPAGE, Invitrogen, Carlsbad, CA), and the thermal stability was monitored by differential scanning calorimetry (VP-DSC, Microcal, Northampton, MA), which was performed as previously described (30). Binding of human fH and anti-fHbp MAbs (31, 32) to recombinant fHbp were measured by ELISA as described previously (22, 29). Surface plasmon resonance experiments were performed by immobilizing purified human fH (Complement Technology Inc., Tyler TX; 2400 or 4000 response units) on a CM5 chip (GE Healthcare, Piscataway, NJ) via amine coupling. Binding of soluble fHbp to immobilized fH was measured at concentrations ranging from 0.016 to 5 μM using a Biacore X / 100 instrument (GE Healthcare, Piscataway, NJ).
Mouse immunogenicity
The immunogenicity of fHbp and R41S mutant fHbp vaccines was evaluated in six- to eight-week old BALB/c wild-type or human fH transgenic mice. Three doses of fHbp vaccine containing 20 μg of fHbp adsorbed with 600 μg of aluminum hydroxide were administered i.p. at three-week intervals. Control immunization was performed using the same schedule with a meningococcal group C conjugate vaccine (Meningitec; Wyeth, Montreal, Canada). Each dose contained 2 μg of polysaccharide and 3 μg of CRM197 diphtheria toxoid protein adsorbed with 100 μg of aluminum phosphate. The Institutional Animal Care and Use Committees at the University of Massachusetts Medical School and Children’s Hospital Oakland Research Institute approved the respective protocols performed at each study site.
Measurement of serum antibody responses
Serum IgG anti-fHbp antibody responses were measured by ELISA as described previously (30). IgG anti-CRM and anticapsular antibody responses were measured using diphtheria CRM197 (List Biological Laboratories, Campbell, CA) and group C meningococcal polysaccharide conjugated to adipic dihydrazide (33) as the antigens on the plate, respectively. Serum bactericidal antibody responses were measured using log-phase bacteria and human serum depleted of IgG as a complement source as previously described (34). Briefly, a 1-ml aliquot of human serum was passed over a protein G column (1 ml HiTrap Protein G, GE Life Sciences, Piscataway, NJ) equilibrated with PBS and the flow-through fraction was collected. The IgG-depleted fraction exhibited a >95% decrease in IgG concentration measured by ELISA and ~30% decrease in hemolytic complement activity measured with the EZ Complement CH50 test kit (Diamedix Corp., Miami, FL), which resulted, in part, from dilution of the serum sample. To compensate for the lower CH50 activity, 12 μl of the IgG-depleted serum was added to the 40 μl (30%) bactericidal reaction instead of 8 μl (20%) of non-depleted serum. Bactericidal responses to fHbp vaccines were determined using group B strain H44/76 (B:15:P1.7,16; ST-32), which originally was a case isolate from an epidemic in Norway(35), and was obtained from Novartis Vaccines (referred to as 44/76-SL (36)). This strain is a relatively high expresser of fHbp (37). Bactericidal responses to the group C polysaccharide protein conjugate vaccine were measured using group C strain 4243 (C:2a:P1.5,2; ST-11) (38), which is an invasive clinical isolate from Dallas County, TX (39, 40).
Anti-fHbp antibody inhibition of human fH binding to fHbp
Inhibition of human fH binding to fHbp by serum anti-fHbp antibodies was measured by ELISA (41). fHbp was affixed to microtiter wells as described above. To compare inhibition of fH binding to fHbp by antibodies raised against fHbp (itself) or the R41S mutant vaccines, we tested individual sera from vaccinated transgenic mice (N=11 per vaccine group) at four dilutions (1:100, 1:400, 1:1600 and 1:6400); each dilution was tested in the presence of 5% IgG-depleted human serum (pool of three individuals) as a source of exogenous human fH. After incubation for 2 h at room temperature, wells were washed and bound human fH was detected with sheep anti-human fH (1:7000; Lifespan Biosciences, Seattle, WA) followed by donkey anti-sheep IgG conjugated to alkaline phosphatase (1:5000; Sigma-Aldrich, St. Louis, MO). The percent inhibition by different dilutions of the immune sera was calculated by comparison with the amount of human fH bound in the absence of the mouse serum.
Statistical analyses
Two-tailed Student’s t tests were used to compare reciprocal geometric mean titers (GMT) of serum antibody responses between two independent groups of mice. An exception was in Study 2, where we used a one-tailed t test to examine whether anti-fHbp antibody responses of transgenic mice immunized with the wild-type fHbp vaccine were not lower than immunized wild-type mice, which was justified based on the lower responses in transgenic mice observed in Study 1. General linear regression models were used to test whether the type of fHbp vaccine and human fH concentrations affected serum bactericidal antibody responses. In addition, we calculated the Pearson correlation coefficients between log10 serum bactericidal titers and log10 human fH concentrations of transgenic mice immunized with the fHbp that bound human fH and the mutant vaccine that did not bind human fH. The test of equality of two Pearson correlation coefficients drawn from the two different samples was performed as described (42). To meet normality assumption, both serum bactericidal antibody measurements and fH concentrations were log10 transformed in regression and correlation analyses. A two-tailed P value of less than or equal to 0.05 was considered statistically significant.
RESULTS
A mutant fHbp with a single amino acid substitution, R41S, does not bind human fH
Based on a published crystal structure of fHbp bound to a fragment of fH (43), the arginine residue at position 41 forms a charged hydrogen-bond with fH (R41, Figure 1A). We predicted that fH binding might be eliminated if the arginine were replaced by serine (S41, lower right inset panel). Because most of the important fHbp epitopes that elicit bactericidal antibody have been reported to be in the region encompassing residues 100 to 255 (44), we speculated that the R41S substitution would not decrease serum bactericidal antibody responses to the mutant fHbp. The purified wild-type and R41S mutant proteins showed similar purity and molecular weight by SDS-PAGE (Figure 1B).
Figure 1.
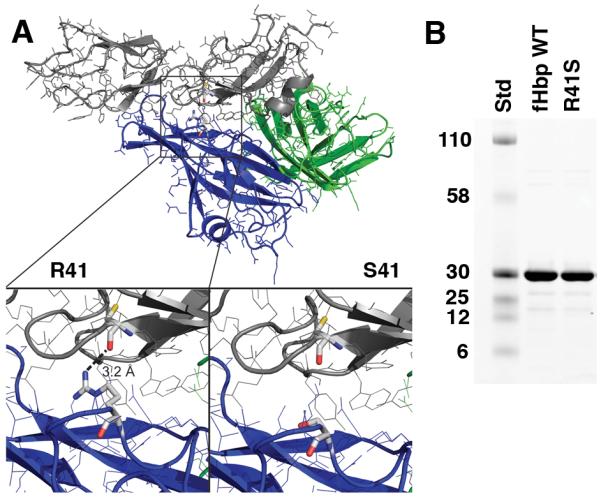
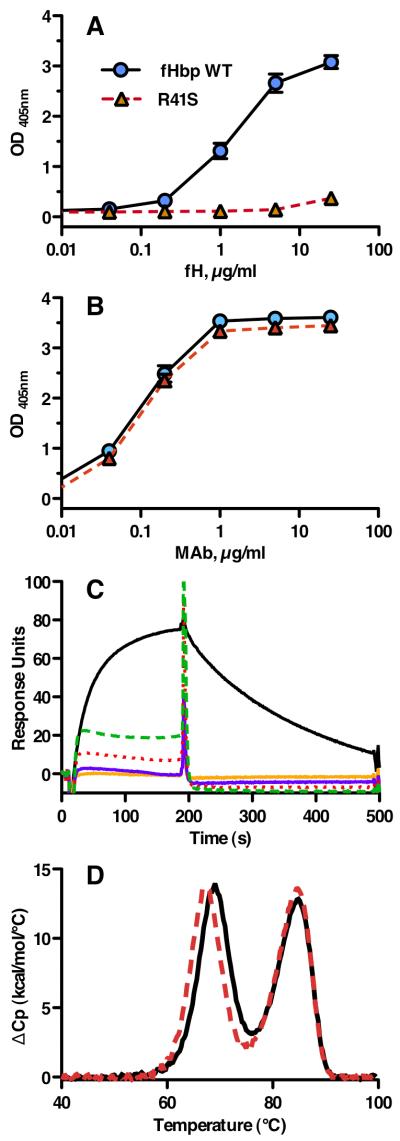
A, Structural model of fHbp bound to a fragment of fH based on published atomic coordinates (43). The blue and green ribbons represent the respective N- and C-terminal domains of the fHbp molecule. The gray ribbon represents the sixth and seventh short consensus repeat domains of human fH previously shown to mediate the interaction of human fH and fHbp (43). The arginine residue at position 41 formed a charged H-bond with fH (left lower inset), which was predicted to be eliminated when arginine was replaced by serine (right lower inset). B, SDS-PAGE of fHbp. Wild-type (WT) fHbp and R41S mutant fHbp (5 μg/lane) were separated on 4-12% polyacyrlamide gradient gel (NuPAGE, Invitrogen, Carlsbad, CA) and stained with Coomassie blue. The molecular weights (in kDa) of the protein standards (Std) are labeled on the left.
By ELISA, the R41S mutant did not bind human fH (Figure 2A), but a control anti-fHbp MAb, JAR 4 (31, 32), bound almost identically to both the mutant fHbp and wild-type fHbp (Figure 2B). This control indicated that comparable agmounts of the respective proteins were adsorbed to the wells of the microtiter plate. Further, the R41S substitution did not affect expression of the epitope recognized by JAR 4, which involved residues 25-27 and 57-59 in the N-terminal domain (31), which was proximal to the amino acid substitution. By ELISA, the wild type and R41S mutant fHbp also showed similar respective concentration-dependent binding with three other bactericidal anti-fHbp MAbs. These included MAb502 and JAR 1, which reacted with epitopes on the C-terminal domain of the protein (45) and JAR 5, which recognized epitopes involving amino acid residues 121 and 122 (31, 41) (data not shown).
Figure 2.
Characterization of fHbp vaccine antigens. A, By ELISA, the fHbp WT (blue circles) bound fH, whereas the R41S substitution (red triangles, dashed line) eliminated binding of soluble human fH to solid-phase mutant fHbp. B, Binding of anti-fHbp MAb JAR 4 to solid-phase wild-type or R41S mutant fHbp indicated that similar amounts of the two proteins were adsorbed to the wells of the microtiter plate and that a conformational epitope in the N-terminal domain was retained. Symbols are the same as used in panel A. The data in panels A and B represent the mean and SE of three to six independent measurements. For data points without apparent error bars, the variability was too small to be evident. C, Binding of soluble fHbp to immobilized human fH as measured by surface plasmon resonance. Human fH (4000 response units) was coupled to the biosensor chip; the WT fHbp (0.5 μM, solid black line) showed +77.4 response units while 0.5 (solid orange line) or 1.0 μM (solid purple line) of the mutant R41S fHbp showed no binding (−1.0 and −0.8, respectively). Non-specific binding of the mutant fHbp is evident at 2.5 (red dotted line) or 5.0 (green dashed line) μM. D, Thermal stability of WT (solid black line) and R41S (dashed red line) proteins measured by differential scanning calorimetry. Protein solutions (0.5 mg/ml) were in PBS and the scan rate was 60 °C/h. Reference buffer data were subtracted as a baseline and the data were normalized based on the calculated molecular weight of the recombinant fHbp (27.7 kDa). The lower and higher temperature transitions correspond to the unfolding of the N- and C-terminal domains, respectively (46).
In surface plasmon resonance experiments, we tested binding of soluble fHbp to immobilized human fH, which was performed as described in the Methods. In the first experiment, 2400 response units (RU) of purified human fH were coupled to the biosensor chip; the R41S mutant protein (0.5 μM) showed no binding with fH (−0.6 response units) compared with +22.5 RU with 0.5 μM of the respective native fHbp antigen. As previously reported, the equilibrium dissociation constant was 45 ± 9 nM (18). In the second experiment, 4000 RU of purified human fH were coupled to the biosensor chip; binding of soluble WT fHbp (0.5 μM) showed +77.4 RU while 0.5 or 1.0 μM of the mutant R41S fHbp showed no binding (−1.0 or −0.8, respectively). When the concentration of R41S was increased to 2.5 or 5.0 μM, some binding with the mutant was evident (Figure 2C), which appeared to be non-specific based on the shape of the association signal and rapid dissociation.
To test the structural integrity of the mutant protein, we determined its thermal stability by differential scanning calorimetry (Figure 2D). As previously described (30, 46), the wild-type fHbp unfolded with two transitions, with midpoints (Tm) at 69.0 and 84.1 °C (black solid line), which corresponded to the respective N- and C-terminal domains. The R41S mutant unfolded with two transitions, centered at 67.9 and 84.7 °C (red dashed line). The first transition with the mutant protein was lower than that with the WT protein (ΔTm = −1.1 °C), which was consistent with the location of the amino acid substitution in the N-terminal domain.
The R41S mutant fHbp has preserved immunogenicity in wild-type mice
In wild-type mice whose serum fH did not bind to either vaccine antigen, the wild-type and R41S mutant fHbp vaccines elicited similar serum bactericidal antibody responses (reciprocal geometric mean titers (GMT) of 77 and 71, respectively; P=0.32 by t test) (Initial study, Figure 3). In contrast, a separate mutant fHbp vaccine, E218A/E239A, which previously had been reported not to bind to fH (43) and to have impaired immunogenicity in wildtype mice (30), elicited significantly lower bactericidal titers (reciprocal GMT of 9 vs. 77 for the wild-type fHbp, P=0.01 by t test) (Initial study, Figure 3). These results confirmed diminished antibody responses from possible loss of epitopes or minor destabilization of the C-terminal domain of the molecule that contained the E218A and E239A substitutions, which is in the region of the molecule reported to contain the majority of the epitopes responsible for generating bactericidal antibodies (44). In contrast, the antibody responses of the wild-type mice to the R41S mutant fHbp vaccine indicated that substitution of serine for arginine in the N-terminal domain did not decrease immunogenicity. The similar bactericidal antibody responses to the wild-type and R41S mutant fHbp vaccines were replicated in an independent study (115 vs. 83, P=0.90) (replicate study, Figure 3).
Figure 3.
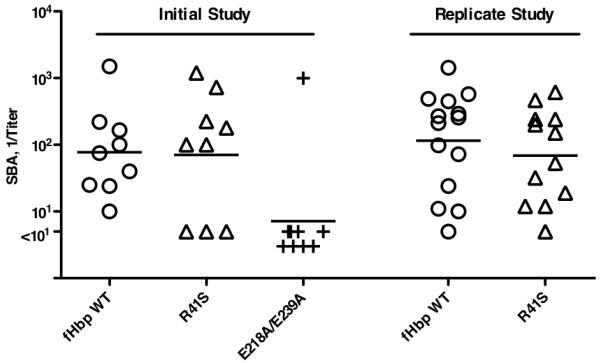
Serum bactericidal responses of wild-type mice to fHbp vaccines. Titers of individual mice immunized with different vaccines are represented by symbols (fHbp WT vaccine, circles; R41S mutant vaccine, triangles; and E218A/E239A mutant vaccine, + symbols). The geometric mean titers are indicated by horizontal lines. In an initial study (left), wild-type mice were immunized with three doses of fHbp WT or R41S mutant vaccines. As a control, a group of mice were immunized with a second mutant fHbp vaccine, E218A/E239A, which in previous studies showed lack of fH binding but had impaired immunogenicity in wild-type mice (30). Mice immunized with the R41S mutant developed similar geometric mean bactericidal titers as those immunized with the wild-type fHbp (71 vs. 77, respectively, P=0.32), whereas mice immunized with the E218A/E239A mutant had a GMT of 9 (P=0.01, compared to wild-type fHbp vaccine). The similar immunogenicity of the wild-type fHbp and R41S vaccines was confirmed in a replicate study, which is shown on the right (GMTs of 115 vs. 83, respectively, P=0.90).
Generation of human fH transgenic mice
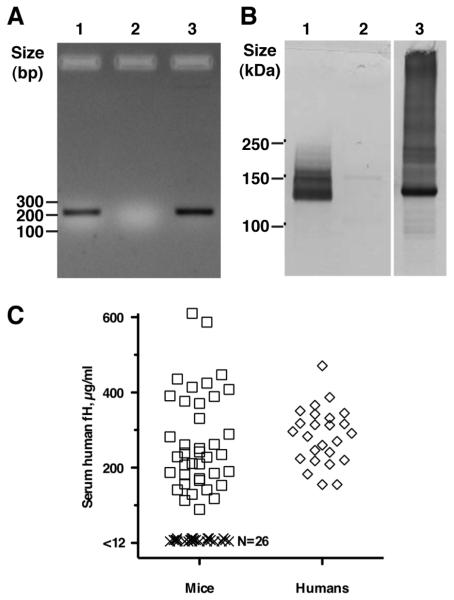
To test the effect of human fH on the immunogenicity of fHbp vaccines, we generated human fH transgenic mice by microinjection of embryos with a PCR product containing the human fH cDNA (Figure 4A), which was performed as described in Methods. Transgenic mice containing human fH in their sera were identified by Western blotting (Figure 4B). We quantified human fH in transgenic mice using a fHbp capture ELISA (Figure 4C). Wild-type mice had no detectable serum human fH (<12 μg/ml). The mean ± SD concentration of human fH in the sera of the transgenic mice was 268 ± 128 μg/ml. The mean fH concentration ± SD in the human sera was 284 ± 75 μg/ml. The respective means were not significantly different (P=0.57), but the variance of the fH concentrations of the human sera was lower than in transgenic mice (F=2.89; P=0.008).
Figure 4.
Generation of human factor H transgenic mice. A. Identification of human factor H transgenic mice by PCR analysis of genomic DNA from tails. Amplified product (232 bp) was from human factor H transgenic mice (lane 1), wild-type mice (lane 2), and plasmid containing human factor H cDNA (lane 3). B. Human factor H detected in sera from transgenic mice. Human fH visualized by Western blotting. Serum from BALB/c mice that expressed human fH (Lane 1), wild-type BALB/c mice negative for human fH (Lane 2), and normal human serum (Lane 3) were separated on a 4-12% polyacrylamide gel by electrophoresis; proteins were transferred to a PVDF membrane. Membranes were probed with affinity isolated goat anti-human fH (Complement Technology Inc., Tyler, TX) that recognized human, but not mouse, fH (48). C. Human serum fH quantification by ELISA. Each individual serum sample is represented by a single data point of the mean concentration from two to three independent measurements. Positive mice (square symbols, N=39) had human fH concentrations >90 μg/ml and negative mice (X symbols, N=26) had concentrations <12 μg/ml, which was the lower limit of detection in the assay. For comparison, concentrations of human fH were measured in stored sera from 25 healthy adult humans (diamond symbols).
Binding of human fH decreases the immunogenicity of fHbp vaccine
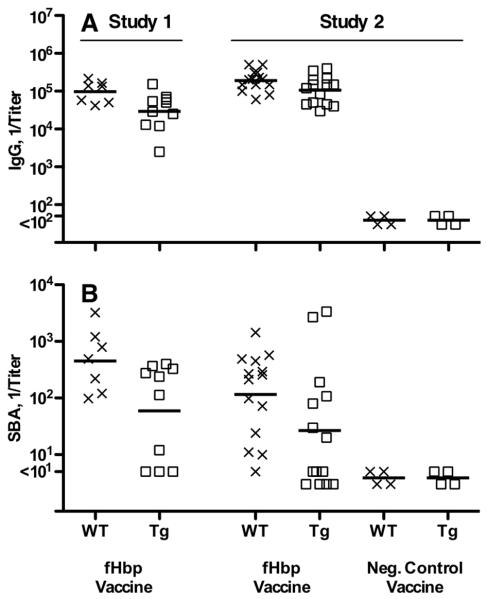
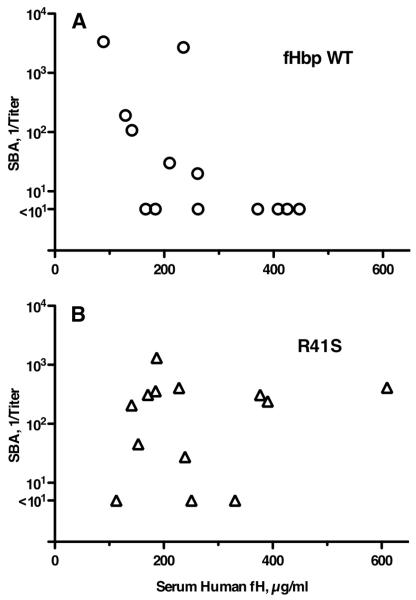
In two studies, human fH transgenic or wild-type mice were immunized with a recombinant fHbp vaccine that bound human fH. Three weeks after the third injection of vaccine, the serum IgG anti-fHbp antibody responses of the transgenic mice were lower than the wild-type mice (Study 1, reciprocal GMT of 30,000 vs. 97,000, P=0.03; Study 2, reciprocal GMT of 107,000 vs. 190,000 (P=0.025) (Figure 5A). In Study 1, the serum bactericidal antibody responses of the transgenic mice were 8-fold lower than the wild-type mice whose fH did not bind the vaccine (reciprocal GMT of 59 vs. 453 in wild-type mice, P=0.03) (Figure 5B). In Study 2, transgenic mice immunized with the fHbp vaccine that bound human fH again had lower serum bactericidal antibody responses (reciprocal GMT of 31 vs. 115 in wild-type mice, P=0.05). In Study 1, serum human fH concentrations were stratified as being <12 μg/ml or >80 μg/ml, whereas in Study 2 we measured the actual serum concentration of human fH in the pre-immune serum of each animal. There was an inverse correlation between the serum human fH concentrations of the transgenic mice and serum bactericidal antibody responses to the fHbp vaccine that bound human fH (Figure 6A; Pearson correlation coefficient, r= −0.65; P=0.02). In other words, the higher was the serum human fH concentration, the lower the bactericidal titer. Collectively the data indicated that binding of human fH to the fHbp vaccine impaired protective antibody responses.
Figure 5.
Serum antibody responses of wild-type (WT, X symbols) or human fH transgenic (Tg, square symbols) mice immunized with a fHbp vaccine that bound human fH. A, IgG anti-fHbp responses. Titers of individual mice are represented by the respective symbols and the geometric mean titers are indicated by horizontal lines. The symbols for mice immunized with negative control vaccines are titers of serum pools, each from 3-4 mice. B, Serum bactericidal responses measured with human complement against group B strain H44/76. Symbols represent titers of individual mice as in Panel A. Comparing respective IgG GMT of fHbp immunized WT and Tg mice, Study 1, P=0.03; Study 2, P=0.025. Comparing respective SBA GMT of fHbp immunized WT and Tg mice, Study 1, P=0.03; Study 2, P=0.05.
Figure 6.
Relationship of serum group B bactericidal antibody (SBA) titers and serum human fH concentrations of transgenic mice. A, Tg mice immunized with the fHbp wild-type (WT, circular symbols) vaccine that bound fH (r= −0.65, P = 0.02, Pearson correlation coefficient between log10 SBA and log10 fH). B, Tg mice immunized with the fHbp R41S mutant vaccine (triangular symbols) that did not bind fH (r= +0.17, P= 0.58). The respective r values were significantly different (P=0.03, performed as described (42)).
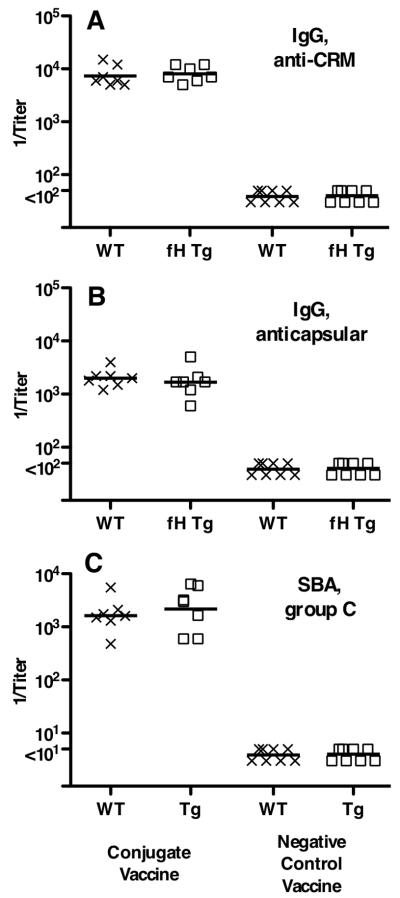
Study 1 did not include a non-fHbp control vaccine. Therefore, we were uncertain whether the lower immunogenicity of the fHbp vaccine in the transgenic mice resulted from binding of the vaccine antigen with human fH, or whether there might have been lower serum antibody responses of the transgenic animals to vaccine antigens in general. In Study 2, we included groups of transgenic and wild-type mice immunized with a control meningococcal group C polysaccharide-CRM197 conjugate vaccine. The respective serum IgG anti-CRM and anticapsular antibody titers, and serum bactericidal antibody responses of the transgenic mice immunized with the meningococcal conjugate vaccine were not significantly different from those of the wild-type mice (Figure 7). These results indicated that the transgenic mice responded normally to the control vaccine, and that the lower responses of transgenic mice to the fHbp vaccine resulted from human fH binding to the vaccine.
Figure 7.
Serum antibody responses of wild-type (WT, X symbols) and human fH transgenic (fH Tg, square symbols) mice immunized in Study 2 with a control meningococcal group C polysaccharide-diphtheria CRM197 conjugate vaccine. A, IgG anti-diphtheria CRM197 antibody titers (IgG anti-CRM). B, IgG group C anticapsular antibody titers. C, serum bactericidal titers (SBA) against group C strain 4243. Each symbol represents the titer of an individual mouse. Horizontal lines represent reciprocal geometric mean titers. Negative controls included serum pools from wild-type (N=8) or transgenic mice (N=8) immunized with adjuvant only or an irrelevant antigen vaccine.
A mutant fHbp that does not bind human fH has increased immunogenicity in human fH transgenic mice
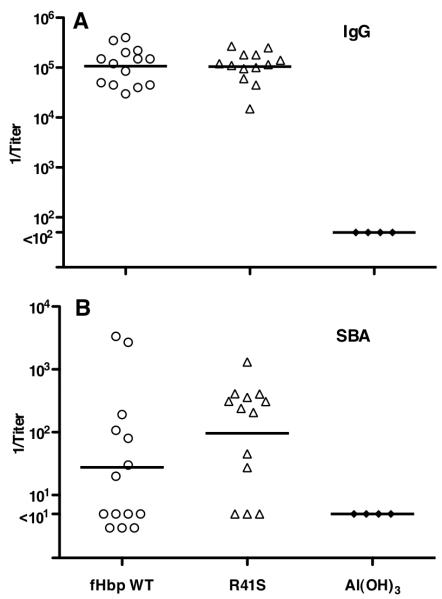
In Study 2, human fH transgenic mice were immunized with the R41S mutant fHbp that did not bind fH. The IgG anti-fHbp titers were not significantly different than those of control transgenic mice immunized with the WT fHbp that bound fH (GMT of 1:106,000 vs. 1:105,000, Figure 8A). The serum bactericidal responses to the mutant R41S fHbp vaccine were ~3-fold higher than those to the fHbp vaccine that bound fH (Figure 8B) but the difference was not statistically significant (GMT of 96 vs. 31, P=0.11 by t test). This analysis however, did not take into consideration a possible confounding effect of the human fH serum concentrations of the transgenic mice on serum anti-fHbp bactericidal responses. In transgenic mice immunized with the mutant fHbp that did not bind fH, there was no significant correlation between the serum bactericidal antibody responses and serum human fH concentrations (r = +0.17; P=0.58, Figure 6B), whereas as described above, there was an inverse correlation with the bactericidal titers elicited by the wild-type vaccine that bound fH (r= −0.65; P=0.02, Figure 6A). The respective correlation coefficients for the two vaccines were significantly different from each other (P=0.03).
Figure 8.
Serum antibody responses of human fH transgenic mice immunized in Study 2 with a mutant fHbp (R41S) vaccine that did not bind fH. A, IgG anti-fHbp responses. Titers of individual mice are represented by symbols (control fHbp WT vaccine, circles; R41S mutant vaccine, triangles; Al(OH)3 control, filled diamonds). The geometric mean titers are indicated by horizontal lines. The symbols for mice immunized with negative control vaccine are from testing serum pools (each pool from 3-4 individual mice). Comparing respective IgG GMT of wild-type fHbp and R41S mutant vaccine groups, P=0.96. B, Serum bactericidal responses measured with human complement against group B strain H44/76. Comparing respective SBA GMT of wild-type fHbp and R41S mutant vaccine groups, P=0.11. When the analysis of the SBA responses was adjusted by general linear regression for the confounding inverse effect of serum human fH concentrations on SBA responses of Tg mice immunized with the fHbp that bound fH, the higher responses to the mutant R41S vaccine were significant (P=0.018, See Figure 9 and text).
By general linear regression there was a significant interaction between the type of fHbp vaccine and the human fH concentration on the bactericidal response (likelihood ratio test, P=0.018). To determine if the type of fHbp vaccine (fHbp wild-type or R41S mutant) affected the serum bactericidal antibody responses of the transgenic mice, we used the regression model to estimate ratios of the reciprocal serum bactericidal GMT of transgenic mice immunized with the R41S mutant vaccine over those of transgenic mice immunized with the fHbp vaccine that bound human fH at various serum human fH concentrations (Figure 9). While there were no significant differences in bactericidal responses when serum human fH concentrations were low (<250 μg/ml), the bactericidal responses to the R41S mutant vaccine were significantly higher when the serum fH concentrations were higher than 250 μg/ml (P<0.05), or higher than 316 μg/ml (P<0.01). Since many humans have fH concentrations in this range (Figure 4C), the results suggested that mutant fHbp molecules that do not bind fH might be superior vaccines in humans.
Figure 9.
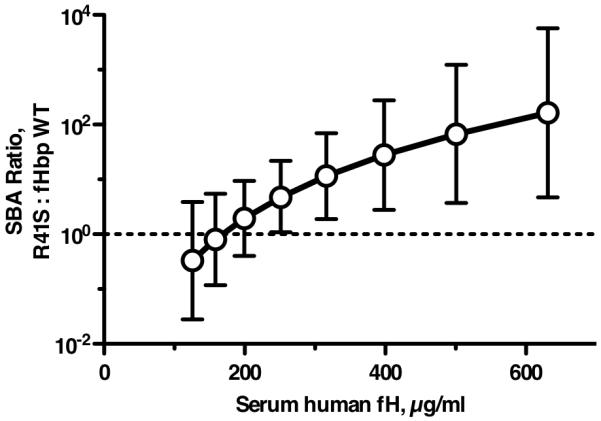
Effect of serum human fH concentrations on ratio of bactericidal antibody responses elicited by R41S mutant fHbp vaccine versus wild-type fHbp vaccine. The respective GMT ratios (R41S mutant : fHbp wild-type) were estimated from the general linear regression model (see text), which showed that the effect of fHbp vaccine type differed by serum fH concentration on bactericidal titer (P=0.018). Based on the regression model, the ratios of the geometric mean bactericidal responses of the group immunized with R41S fHbp vaccine over that of the group immunized with wild-type fHbp vaccine were significantly greater than 1 (in favor of the mutant fHbp vaccine) for all human fH concentrations >250 μg/ml (P<0.05), and for >316 μg/ml (P<0.01).
Antibodies elicited by the R41S mutant fHbp vaccine have superior inhibition of binding of fH to the nominal vaccine, fHbp
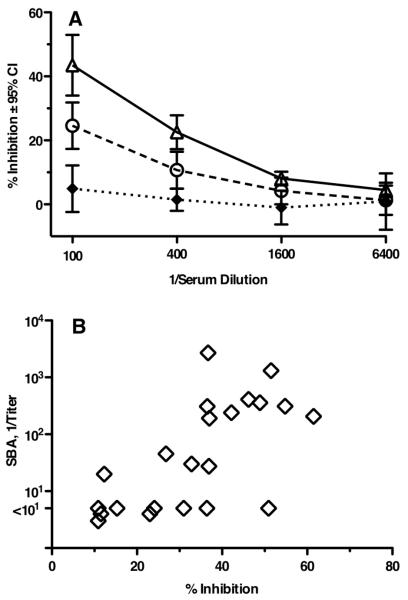
Binding of human fH to a fHbp vaccine may occlude epitopes on the vaccine antigen that may be necessary to interact with immune cells that influence production of anti-fHbp antibodies with functional activity. To test this hypothesis, we measured inhibition of binding of fH to fHbp by different dilutions of post-immune sera from transgenic mice (Figure 10A). At 1:100 and 1:400 dilutions, the sera from mice immunized with the fHbp R41S mutant showed greater inhibitory activity than sera from mice immunized with the fHbp that directly bound fH (P<0.03). This greater inhibition in the R41S mutant vaccine group was observed despite the fact that the IgG anti-fHbp antibody responses of the transgenic mice immunized with the two vaccines were similar (Figure 8A). Collectively, these results indicated qualitative differences in the antibody repertoire elicited by each of the two vaccines. Further, the antibodies capable of inhibiting binding of fH appeared to be more protective based on a significant correlation between percent inhibition of fH binding to fHbp and serum bactericidal titer (r=0.69, P=0.004, Figure 10B).
Figure 10.
A, Inhibition of binding human fH to fHbp by immune sera as measured by ELISA. Dilutions of individual sera from transgenic mice (N=11 per vaccine group) immunized with wild-type (circular symbols) or R41S mutant (triangular symbols) fHbp vaccines were tested for their ability to inhibit binding of fH present in 5% IgG-depleted human serum (used as a source of human fH) to immobilized wild-type fHbp. At 1:100 and 1:400 dilutions, there was significantly greater inhibitory activity in the R41S mutant fHbp vaccine group (open triangles) than mice given the fHbp vaccine that bound fH (open circles, P<0.03). Filled diamonds represent inhibition of serum pools from transgenic mice immunized with aluminum adjuvant alone (N=4 pools). B, Correlation between fH inhibition and serum bactericidal activity of individual sera. Data are from sera of individual transgenic mice immunized with wild-type or R41S mutant fHbp vaccines (r = +0.69, P=0.0004).
DISCUSSION
In this study, we developed a human fH transgenic mouse model with the intent to investigate the role of this complement down-regulator on infections unique to humans where fH has been proposed to play a role (27, 47, 48). Human fH, whose amino acid sequence and structure varies from mouse fH, binds uniquely to Neisseria spp.; mouse fH does not. We carried this principle further and investigated the effect of immunization with the Neisseria meningitidis outer membrane protein vaccine candidate, fHbp. To our knowledge, this study represents the first report of expression in a mouse of full-length human fH to permit fH direct binding, via short consensus repeat (SCR) sequences 6 and 7 (49), to a vaccine component made from N. meningitidis. Although the alternative pathway inhibitory action of human fH was not directly investigated in these studies, human fH is known to regulate the non-human alternative pathway of complement similar to its action on the respective human pathway (27, 48).
The respective serum antibody responses of the fH transgenic and wild-type mice to the control group C meningococcal conjugate vaccine were similar. These data indicated that the presence of the transgene and/or human fH in the transgenic mice did not impair serum IgG antibody responses to a control capsular polysaccharide or carrier protein antigen, or complement-mediated bactericidal activity of the elicited anticapsular antibodies. The effect of human fH on decreasing immunogenicity of the fHbp vaccine in the human fH transgenic mice, therefore, was specific for the fHbp vaccine antigen, and was observed in two independent studies. Moreover, there was an inverse correlation between the serum bactericidal antibody responses in the transgenic mice immunized with the vaccine that bound human fH and the serum human fH concentrations of individual mice (Figure 6A), which strengthened the case for a causal relationship between human fH and decreased fHbp vaccine immunogenicity. Finally, a mutant fHbp vaccine that did not bind fH, but which, importantly, retained immunogenicity in wild-type mice, elicited higher serum bactericidal antibody titers in transgenic mice than the fHbp vaccine that bound fH. Collectively, these data indicated that binding of the vaccine antigen to this complement protein, fH, impaired the development of protective antibody responses.
The possibility that a mutant fHbp molecule may be a superior vaccine candidate if it did not bind human fH had been suggested by Meri et al. (2) and Schneider et al. (43). Based on the crystal structure of fHbp binding to a fragment of fH, Schneider et al. identified two fHbp glutamate residues that were important for fH binding (at positions E218 and E239 based on the numbering of the mature fHbp beginning with the lipidated cysteine residue (22, 50)). Factor H binding was eliminated when alanine was substituted at these two positions (43). In a subsequent study, an E218A/E239A mutant fHbp vaccine elicited lower serum bactericidal antibody responses in wild-type BALB/c and CD-1 mice compared to a native fHbp vaccine (30), a finding that we confirmed in this report. As noted above, residues 218 and 239 are in the region of the fHbp molecule that is also important for eliciting bactericidal antibodies (44). Thus, some mutations that eliminate fH binding may occur as a result of structural changes and/or alterations of electrostatic charges of the molecule that decrease protective antibody responses.
Several lines of evidence indicate that the fHbp R41S mutant vaccine described in the present study, which does not bind human fH, may be a superior fHbp vaccine for humans. In two studies in wild-type mice the respective serum bactericidal antibody responses elicited by the R41S mutant or wild-type fHbp vaccines were not significantly different. These data indicated that the epitopes required for eliciting bactericidal antibodies were preserved in the R41S mutant when tested in a mouse model in the absence of human fH binding to either vaccine. Second, there were no significant differences in the respective serum bactericidal antibody responses to the two fHbp vaccines in human fH transgenic mice with low human fH concentrations (Figure 9), compared to elicitation of significantly higher serum bactericidal responses only by the mutant fHbp vaccine when serum human fH concentrations were high, but in a range present in many humans (Figure 4C). Third, despite having elicited similar serum IgG anti-fHbp titers in transgenic mice (Figure 8A), the R41S mutant fHbp vaccine elicited antibodies with greater fH blocking activity than antibodies elicited by the fHbp vaccine that bound fH (Figure 10A). We propose that antibodies elicited by the mutant “fHbp” vaccine that was no longer able to bind fH were directed at surface-exposed epitopes near the fH binding site, thereby inhibiting fH binding and contributing to higher serum bactericidal titers. In support of this hypothesis was a significant correlation between percent inhibition of fH binding to fHbp and serum bactericidal titer (r=0.69, P=0.004, Figure 10B).
Collectively our data indicate that binding of the human complement regulatory protein, fH, to vaccine antigens can decrease protective antibody responses and that a mutant antigen freed of fH binding, in this case by replacing a large, positively charged amino acid (arginine, pI=11.15) with a smaller, more neutral one (serine, pI=5.68), may be potentially a superior immunogen in humans. Mutant fHbp vaccines that do not bind fH may also avoid the theoretical safety risk of exposure of the host to neo-antigens, in this case in the form of an fH-fHbp immune complex, that may elicit auto-reactive antibodies to fH or fH bound to cell surfaces. Our findings, therefore, may have broad implications in the development of vaccines against microbes that bind other host molecules as well.
ACKNOWLEDGEMENTS
We thank Dr. Barbara Brogioni (Novartis Vaccines, Siena, Italy) for conducting the surface plasmon resonance binding experiments, and Dr. Anthony Schryvers (University of Calgary) for providing the group C meningococcal conjugate vaccine.
1Funding Statement: This work was supported in part by Public Health Service grants R01 AI 046464 and AI 082263 (to D.M.G.), AI 032725 and 084048 (to P.A.R.), AI 054544 (to S.R.), and AI 070955 (to P.T.B.) from the National Institute of Allergy and Infectious Diseases, NIH. The work at Children’s Hospital Oakland Research Institute was performed in a facility funded by Research Facilities Improvement Program grant C06 RR 016226 from the National Center for Research Resources, NIH.
2Abbrviations
- fH
factor H
- fHbp
factor H-binding protein
- Tg
transgenic
- WT
wild-type
REFERENCES
- 1.Serruto D, Rappuoli R, Scarselli M, Gros P, van Strijp JA. Molecular mechanisms of complement evasion: learning from staphylococci and meningococci. Nat Rev Microbiol. 2010;8:393–399. doi: 10.1038/nrmicro2366. [DOI] [PubMed] [Google Scholar]
- 2.Meri S, Jordens M, Jarva H. Microbial complement inhibitors as vaccines. Vaccine. 2008;26(Suppl 8):I113–117. doi: 10.1016/j.vaccine.2008.11.058. [DOI] [PubMed] [Google Scholar]
- 3.Fearon DT, Austen KF. Activation of the alternative complement pathway due to resistance of zymosan-bound amplification convertase to endogenous regulatory mechanisms. Proc Natl Acad Sci U S A. 1977;74:1683–1687. doi: 10.1073/pnas.74.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiler JM, Daha MR, Austen KF, Fearon DT. Control of the amplification convertase of complement by the plasma protein beta1H. Proc Natl Acad Sci U S A. 1976;73:3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J Exp Med. 1977;146:257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sim RB, Twose TM, Paterson DS, Sim E. The covalent-binding reaction of complement component C3. Biochem J. 1981;193:115–127. doi: 10.1042/bj1930115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whaley K, Ruddy S. Modulation of the alternative complement pathways by beta 1 H globulin. J Exp Med. 1976;144:1147–1163. doi: 10.1084/jem.144.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davila S, Wright VJ, Khor CC, Sim KS, Binder A, Breunis WB, Inwald D, Nadel S, Betts H, Carrol ED, de Groot R, Hermans PW, Hazelzet J, Emonts M, Lim CC, Kuijpers TW, Martinon-Torres F, Salas A, Zenz W, Levin M, Hibberd ML. Genome-wide association study identifies variants in the CFH region associated with host susceptibility to meningococcal disease. Nat Genet. 2010;42:772–776. doi: 10.1038/ng.640. [DOI] [PubMed] [Google Scholar]
- 9.Horstmann RD, Sievertsen HJ, Knobloch J, Fischetti VA. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci U S A. 1988;85:1657–1661. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischetti VA, Horstmann RD, Pancholi V. Location of the complement factor H binding site on streptococcal M6 protein. Infect Immun. 1995;63:149–153. doi: 10.1128/iai.63.1.149-153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellwage J, Meri T, Heikkila T, Alitalo A, Panelius J, Lahdenne P, Seppala IJ, Meri S. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem. 2001;276:8427–8435. doi: 10.1074/jbc.M007994200. [DOI] [PubMed] [Google Scholar]
- 12.Jarva H, Janulczyk R, Hellwage J, Zipfel PF, Bjorck L, Meri S. Streptococcus pneumoniae evades complement attack and opsonophagocytosis by expressing the pspC locus-encoded Hic protein that binds to short consensus repeats 8-11 of factor H. J Immunol. 2002;168:1886–1894. doi: 10.4049/jimmunol.168.4.1886. [DOI] [PubMed] [Google Scholar]
- 13.Iannelli F, Oggioni MR, Pozzi G. Allelic variation in the highly polymorphic locus pspC of Streptococcus pneumoniae. Gene. 2002;284:63–71. doi: 10.1016/s0378-1119(01)00896-4. [DOI] [PubMed] [Google Scholar]
- 14.Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, Ngampasutadol J, Vogel U, Granoff DM, Ram S. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarva H, Ram S, Vogel U, Blom AM, Meri S. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J Immunol. 2005;174:6299–6307. doi: 10.4049/jimmunol.174.10.6299. [DOI] [PubMed] [Google Scholar]
- 16.Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, Tang CM. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176:7566–7575. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 17.Welsch JA, Ram S, Koeberling O, Granoff DM. Complement-dependent synergistic bactericidal activity of antibodies against factor H-binding protein, a sparsely distributed meningococcal vaccine antigen. J Infect Dis. 2008;197:1053–1061. doi: 10.1086/528994. [DOI] [PubMed] [Google Scholar]
- 18.Dunphy KY, Beernink PT, Brogioni B, Granoff DM. Effect of factor H-binding protein sequence variation on factor H binding and survival of Neisseria meningitidis in human blood. Infect Immun. 2011;79:353–359. doi: 10.1128/IAI.00849-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement. PLoS Pathog. 2010;6:e1001027. doi: 10.1371/journal.ppat.1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granoff DM. Review of meningococcal group B vaccines. Clin Infect Dis. 2010;50(Suppl 2):S54–65. doi: 10.1086/648966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, Ooi P, Smith RP, Weise P, Wetherell M, Xie X, Zagursky R, Zhang Y, Zlotnick GW. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun. 2004;72:2088–2100. doi: 10.1128/IAI.72.4.2088-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masignani V, Comanducci M, Giuliani MM, Bambini S, Adu-Bobie J, Arico B, Brunelli B, Pieri A, Santini L, Savino S, Serruto D, Litt D, Kroll S, Welsch JA, Granoff DM, Rappuoli R, Pizza M. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. J Exp Med. 2003;197:789–799. doi: 10.1084/jem.20021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giuliani MM, Adu-Bobie J, Comanducci M, Arico B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, Cartocci E, Ciucchi L, Di Marcello F, Ferlicca F, Galli B, Luzzi E, Masignani V, Serruto D, Veggi D, Contorni M, Morandi M, Bartalesi A, Cinotti V, Mannucci D, Titta F, Ovidi E, Welsch JA, Granoff D, Rappuoli R, Pizza M. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plested JS, Welsch JA, Granoff DM. Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity. Clin Vaccine Immunol. 2009;16:785–791. doi: 10.1128/CVI.00007-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E, Toneatto D, Pollard AJ. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J. 2010;29:e71–79. doi: 10.1097/INF.0b013e3181f59f6d. [DOI] [PubMed] [Google Scholar]
- 26.Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, Oster P, Miller E, Pollard AJ. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51:1127–1137. doi: 10.1086/656741. [DOI] [PubMed] [Google Scholar]
- 27.Granoff DM, Welsch JA, Ram S. Binding of complement factor H (fH) to Neisseria meningitidis is specific for human fH and inhibits complement activation by rat and rabbit sera. Infect Immun. 2009;77:764–769. doi: 10.1128/IAI.01191-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 29.Beernink PT, Granoff DM. Bactericidal antibody responses induced by meningococcal recombinant chimeric factor H-binding protein vaccines. Infect Immun. 2008;76:2568–2575. doi: 10.1128/IAI.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beernink PT, Shaughnessy J, Ram S, Granoff DM. Impaired immunogenicity of a meningococcal factor H-binding protein vaccine engineered to eliminate factor h binding. Clin Vaccine Immunol. 2010;17:1074–1078. doi: 10.1128/CVI.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beernink PT, Lopasso C, Angiolillo A, Felici F, Granoff D. A region of the N-terminal domain of meningococcal factor H-binding protein that elicits bactericidal antibody across antigenic variant groups. Mol Immunol. 2009;46:1647–1653. doi: 10.1016/j.molimm.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welsch JA, Rossi R, Comanducci M, Granoff DM. Protective activity of monoclonal antibodies to genome-derived neisserial antigen 1870, a Neisseria meningitidis candidate vaccine. J Immunol. 2004;172:5606–5615. doi: 10.4049/jimmunol.172.9.5606. [DOI] [PubMed] [Google Scholar]
- 33.Granoff DM, Maslanka SE, Carlone GM, Plikaytis BD, Santos GF, Mokatrin A, Raff HV. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin Diagn Lab Immunol. 1998;5:479–485. doi: 10.1128/cdli.5.4.479-485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beernink PT, Caugant DA, Welsch JA, Koeberling O, Granoff DM. Meningococcal factor H-binding protein variants expressed by epidemic capsular group A, W-135, and X strains from Africa. J Infect Dis. 2009;199:1360–1368. doi: 10.1086/597806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Froholm LO, Caugant DA, Holten E, Hoiby EA, Rosenqvist E, Wedege E. Meningococcal strains isolated from teenage patients during the serogroup B vaccination trial in Norway: serotyping, serosubtyping, immunotyping and clonal analysis. NIPH Ann. 1991;14:139–143. [PubMed] [Google Scholar]
- 36.Borrow R, Aaberge IS, Santos GR, Eudely TL, Oster P, Glennie A, Findlow J, Hoiby EA, Rosenqvist E, Balmer P, Martin D. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup B, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin Diagn Lab Immunol. 2005;12:970–976. doi: 10.1128/CDLI.12.8.970-976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pajon R, Beernink PT, Harrison LH, Granoff DM. Frequency of factor H-binding protein modular groups and susceptibility to cross-reactive bactericidal activity in invasive meningococcal isolates. Vaccine. 2010;28:2122–2129. doi: 10.1016/j.vaccine.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsch JA, Granoff D. Naturally acquired passive protective activity against Neisseria meningitidis Group C in the absence of serum bactericidal activity. Infect Immun. 2004;72:5903–5909. doi: 10.1128/IAI.72.10.5903-5909.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris SL, King WJ, Ferris W, Granoff DM. Age-related disparity in functional activities of human group C serum anticapsular antibodies elicited by meningococcal polysaccharide vaccine. Infect Immun. 2003;71:275–286. doi: 10.1128/IAI.71.1.275-286.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastor P, Medley FB, Murphy TV. Meningococcal disease in Dallas County, Texas: results of a six-year population-based study. Pediatr Infect Dis J. 2000;19:324–328. doi: 10.1097/00006454-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Beernink PT, Welsch JA, Bar-Lev M, Koeberling O, Comanducci M, Granoff DM. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate, factor H-binding protein. Infect Immun. 2008;76:4232–4240. doi: 10.1128/IAI.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, N.J.: 1983. [Google Scholar]
- 43.Schneider MC, Prosser BE, Caesar JJ, Kugelberg E, Li S, Zhang Q, Quoraishi S, Lovett JE, Deane JE, Sim RB, Roversi P, Johnson S, Tang CM, Lea SM. Neisseria meningitidis recruits factor H using protein mimicry of host carbohydrates. Nature. 2009;458:890–893. doi: 10.1038/nature07769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giuliani MM, Santini L, Brunelli B, Biolchi A, Arico B, Di Marcello F, Cartocci E, Comanducci M, Masignani V, Lozzi L, Savino S, Scarselli M, Rappuoli R, Pizza M. The region comprising amino acids 100 to 255 of Neisseria meningitidis lipoprotein GNA 1870 elicits bactericidal antibodies. Infect Immun. 2005;73:1151–1160. doi: 10.1128/IAI.73.2.1151-1160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scarselli M, Cantini F, Santini L, Veggi D, Dragonetti S, Donati C, Savino S, Giuliani MM, Comanducci M, Di Marcello F, Romagnoli G, Pizza M, Banci L, Rappuoli R. Epitope mapping of a bactericidal monoclonal antibody against the factor H binding protein of Neisseria meningitidis. J Mol Biol. 2009;386:97–108. doi: 10.1016/j.jmb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Mascioni A, Bentley BE, Camarda R, Dilts DA, Fink P, Gusarova V, Hoiseth SK, Jacob J, Lin SL, Malakian K, McNeil LK, Mininni T, Moy F, Murphy E, Novikova E, Sigethy S, Wen Y, Zlotnick GW, Tsao DH. Structural basis for the immunogenic properties of the meningococcal vaccine candidate LP2086. J Biol Chem. 2009;284:8738–8746. doi: 10.1074/jbc.M808831200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu L, Ma Z, Jokiranta TS, Whitney AR, DeLeo FR, Zhang JR. Species-specific interaction of Streptococcus pneumoniae with human complement factor H. J Immunol. 2008;181:7138–7146. doi: 10.4049/jimmunol.181.10.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ngampasutadol J, Ram S, Gulati S, Agarwal S, Li C, Visintin A, Monks B, Madico G, Rice PA. Human factor H interacts selectively with Neisseria gonorrhoeae and results in species-specific complement evasion. J Immunol. 2008;180:3426–3435. doi: 10.4049/jimmunol.180.5.3426. [DOI] [PubMed] [Google Scholar]
- 49.Shaughnessy J, Lewis LA, Jarva H, Ram S. Functional comparison of the binding of factor H short consensus repeat 6 (SCR 6) to factor H binding protein from Neisseria meningitidis and the binding of factor H SCR 18 to 20 to Neisseria gonorrhoeae porin. Infect Immun. 2009;77:2094–2103. doi: 10.1128/IAI.01561-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, Ambrose K, Borrow R, Findlow J, Taha MK, Deghmane AE, Kriz P, Musilek M, Kalmusova J, Caugant DA, Alvestad T, Mayer LW, Sacchi CT, Wang X, Martin D, von Gottberg A, du Plessis M, Klugman KP, Anderson AS, Jansen KU, Zlotnick GW, Hoiseth SK. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis. 2009;200:379–389. doi: 10.1086/600141. [DOI] [PubMed] [Google Scholar]