Abstract
The literature has consistently reported no association between low to moderate alcohol consumption and pancreatic cancer; however, a few studies have shown that high levels of intake may increase risk. Most single studies have limited power to detect associations even in the highest alcohol intake categories or to examine associations by alcohol type. We analyzed these associations using 1,530 pancreatic cancer cases and 1,530 controls from the Pancreatic Cancer Cohort Consortium (PanScan) nested case–control study. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated using unconditional logistic regression, adjusting for potential confounders. We observed no significant overall association between total alcohol (ethanol) intake and pancreatic cancer risk (OR = 1.38, 95% CI = 0.86–2.23, for 60 or more g/day vs. >0 to <5 g/day). A statistically significant increase in risk was observed among men consuming 45 or more grams of alcohol from liquor per day (OR = 2.23, 95% CI = 1.02–4.87, compared to 0 g/day of alcohol from liquor, P-trend = 0.12), but not among women (OR = 1.35, 95% CI = 0.63–2.87, for 30 or more g/day of alcohol from liquor, compared to none). No associations were noted for wine or beer intake. Overall, no significant increase in risk was observed, but a small effect among heavy drinkers cannot be ruled out.
Keywords: Alcohol, Pancreatic cancer, Pooled analysis
Introduction
Worldwide, pancreatic cancer is the eighth leading cause of cancer death, and ~227,000 men and women died of this cancer in 2002 [1]. By decreasing exposure to risk factors of pancreatic cancer, it may be possible to reduce pancreatic cancer mortality, which thus far has been difficult to achieve given the lack of effective treatments, high fatality rates, and difficulty in detecting cancer at early stages. Currently established risk factors for pancreatic cancer include cigarette smoking, which is estimated to account for 20% of pancreatic cancers [2], type II diabetes [3], obesity [4], and chronic pancreatitis [5, 6].
As alcohol abuse is a cause of chronic pancreatitis, it has been postulated that excessive alcoholic intake could increase the risk of pancreatic cancer indirectly through this pathway. A large number of studies, including most cohort studies, have reported no association between alcohol intake and pancreatic cancer risk [7–15], while seven studies reported an elevated pancreatic cancer risk with high alcohol intake [16–22]. Increased risk of pancreatic cancer has also been observed among alcoholics [23–26]. Most cohort studies have limited power to detect associations in the highest categories of alcohol intake and to examine higher intakes of different alcoholic beverages. A recent pooled cohort analysis reported a statistically significant 22% increase in risk among those consuming 30 or more grams of alcohol per day compared to none [27]. To address these issues, we examined the association between alcohol intake and pancreatic cancer risk using the pooled nested case–control samples from 12 prospective cohort studies in the Pancreatic Cancer Cohort Consortium (PanScan).
Materials and methods
Study population
The PanScan study, which includes pancreatic cancer cases and controls pooled from 12 prospective cohort studies and one case–control study, was formed primarily to identify genome-wide associations (GWA) and to investigate gene–environment interactions. The cohorts included in PanScan were those participating in the NCI Cohort Consortium that were willing to participate in this effort. The main results for the GWA are published [28, 29]. The current analysis is based on the pooled case–control data set from the 12 prospective cohort studies (we excluded the case–control study given the different study design). The following cohorts contributed data and were included in this analysis: the Alpha-Tocopherol, Beta-Carotene Prevention Study (ATBC) [30], Clue II [31], Cancer Prevention Study II (CPS II) [32], European Prospective Investigation into Cancer and Nutrition (EPIC) [33], The New York University Women's Health Study (NYU-WHS) [34], The Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial (PLCO) [35], Shanghai Men's and Women's Health Study (SMWHS) [36, 37], the Women's Health Initiative (WHI) [38], the Nurses’ Health Study (NHS) [39], the Health Professionals Follow-up Study (HPFS) [40], the Women's Health Study (WHS) [41], and the Physicians’ Health Study (PHS) [42]. Details on the cases and controls included in PanScan from these cohorts are given in Table 1. A total of 1,530 cases and 1,530 controls were available for this analysis.
Table 1.
Cohort characteristics and median daily intakes of alcohol among controls for study on alcohol and pancreatic cancer: PanScan
| Cohort | Years of collection of alcohol data | Cases, n | Controls, n | Years of follow-up (median) | Baseline age range (years) | Baseline current drinkers, % | Median intake (10th and 90th percentiles) of alcohol (g/day) among controls who were drinkers |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Total alcohol intake | Alcohol from wine | Alcohol from beer | Alcohol from liquor | |||||||
| ATBC | 1985–1988 | 209 | 197 | 12 | 57–85 | 87.2 | 10.7 (1.7–41.2) | 0 (0–2.2) | 2.4 (0–14.2) | 5.4 (0–23.8) |
| Clue II | 1989 | 69 | 67 | 8 | 42–93 | 39.7 | 3.7 (0.8–49.5) | 0 (0–9.4) | 0.8 (0–30) | 0 (0–9.4) |
| CPS II | 1992–1993 | 154 | 155 | 10 | 64–90 | 59.9 | 6.6 (0.9–26.8) | 1.0 (0–14.1) | 0.9 (0–5.9) | 0.9 (0–16.9) |
| EPIC | 1992–2000 | 374 | 391 | 7 | 37–84 | 88.0 | 8.6 (0.8–47.2) | 5.0 (0.2–31.6) | 0.6 (0–18.0) | 0.2 (0–4.2) |
| HPFS | 1986 | 55 | 55 | 13 | 55–87 | 83.6 | 11.4 (1.0–38.4) | 0.8 (0–8.8) | 0.8 (0–10.2) | 2.0 (0–35.0) |
| NHS | 1980 | 88 | 88 | 23 | 47–80 | 73.9 | 6.6 (0.8–32.0) | 0.8 (0–11.0) | 0 (0–10.2) | 1.0 (0–14.0) |
| NYU-WHS | 1994–1997 | 10 | 12 | 12.5 | 48–81 | 40.9 | 9 (2.0–26.0) | 5.0 (0–10.0) | 0 (0–6.0) | 0 (0–20.0) |
| PHS I | 1982–1983 | 61 | 62 | 21 | 49–88 | 91.9 | 5.8 (5.8–40.6) | – | – | – |
| PLCO | 1993–2001 | 136 | 140 | 6 | 56–84 | 83.0 | 4.8 (0.4–44.8) | 0.2 (0–7.4) | 0.2 (0–16.8) | 0.4 (0–16.8) |
| SMWHS | 1996 (women) 2001 (men) |
78 | 79 | 3 | 43–77 | 6.4 | 12.4 (1.7–110.0)a | 1.7a | 12.5 (4.6–52.0)a | 10.3 (2.9–60.0)a |
| WHI | 1993–1998 | 256 | 244 | 4 | 53–88 | 67.4 | 3.4 (0.6–22.0) | 0.8 (0–13.8) | 0 (0–0.8) | 0 (0–8.2) |
| WHS | 1992–1993 | 40 | 40 | 10 | 47–82 | 58.8 | 5.6 (2.0–11.8) | 1.8 (0.8–5.6) | 1.0 (0–1.8) | 0 (0–6.4) |
| Pooled | 1,530 | 1,530 | 8 | 37–93 | 73.0 | 7.0 (0.8–40.6) | 0.9 (0–13.6) | 0.8 (0–12.0) | 0.8 (0–14.0) | |
Based on 8 observations for total alcohol, 1 observation for alcohol from wine, 4 observations for alcohol from beer, and 3 observations for alcohol from liquor
Case ascertainment and data collection
Cases included all incident primary pancreatic adeno-carcinoma (ICD-O-3 code C250-C259 or C25.0-C25.3, C25.7-C25.9). We excluded endocrine pancreatic tumors (C25.4, histology type, 8150, 8151, 8153, 8155, 8240, 8246). All cases were confirmed through cancer registries (ATBC, EPIC, HPFS, NHS, SMWHS, CPS II), death certificates (CPS II, EPIC, HPFS, NHS, NYUWHS, PHS-1, WHS), and/or review of medical records by medical personnel (ATBC [through 1999], EPIC, HPFS, NHS, NYU-WHS, PHS-1, PLCO, SMWHS, WHS).
Controls were frequency matched to cases (1:1 ratio) on calendar year of birth (±5 years), sex, race, and length of follow-up and were alive and cancer free on the incidence date of the matched case. Each cohort may have been matched additionally on other relevant factors such as age at baseline or age at blood draw (±5 years), date/time of day of blood draw, fasting blood draw, and smoking status.
Data on alcohol consumption, age, sex, cigarette smoking history, race, BMI, history of diabetes, folate intake, and other dietary data were obtained from each study contributing to PanScan. In addition, data on family history of pancreatic cancer were available for seven of the studies.
The Special Studies Institutional Review Board (SSIRB) of the National Cancer Institute approved the pooled Pan-Scan study. Each study was approved by its local IRB.
Measurement of alcohol exposure
Baseline alcohol consumption was obtained by questionnaire in each of the studies. For studies which did not provide intake in grams of ethanol per day, it was calculated for each alcoholic beverage (i.e., 13.72 g ethanol for 5 oz of wine; 12.96 g ethanol for 12 oz of beer; and 13.93 g ethanol for 1.5 oz of liquor) by multiplying these values to the reported consumption of these beverages (which reflects consumption over the preceding year). Total alcohol intake was created by calculating grams of ethanol per day across all alcoholic beverages. For Clue II, 12 g of ethanol per drink was used regardless of type of drink, given a precedent already set within this cohort. For PHS, intake of grams of ethanol (13.54 g of ethanol per drink) was computed from frequency data for overall alcohol intake as data on different types of alcoholic beverages were not available. For SMWHS, alcohol intake was measured in the Chinese measure liangs; intake of grams of ethanol per day was estimated by assuming 50 g/liang and multiplying by % of ethanol in different alcoholic beverages and frequency consumed (rice wine was included in the total alcohol variable but not in the liquor or wine variables).
Statistical analysis
We calculated odds ratios (OR) and 95% confidence intervals (95% CI) for pancreatic cancer risk using unconditional logistic regression. All models were adjusted for established risk factors of pancreatic cancer: age (continuous), cohort, sex, cigarette smoking history (never, former quit >10 years, former quit <10 years, current <20 cigarettes/day, current 20 cigarettes/day, current >20 cigarettes/day, unknown), race (Caucasian, Asian, other), BMI (continuous, kg/m2), and self-reported diabetes (yes, no, missing). For the total alcohol analysis, we used the lowest category of alcohol (>0 to <5 g/day) as the referent category as the nondrinker category is likely to include past drinkers who quit drinking and thereby could have an elevated risk. For the specific types of alcohol, we compared consumption of each type of alcohol to those who reported none for that specific alcohol type; nondrinkers were included in the model as a separate category. The PHS cohort and EPIC-Umea center were removed from the specific alcohol analyses as they did not have information on type of alcohol. In addition, in the analysis for the specific types of alcohol, we mutually adjusted for the other types of alcohol. We did not adjust for family history of pancreatic cancer as only seven cohorts had collected this information. We tested for trend among consumers of alcohol by entering the median value among controls for each category and modeling this as a continuous variable.
Heterogeneity in the risk estimates for our study was assessed using the Q statistic and the I2 statistic. We considered statistically significant heterogeneity at the P = 0.05 level of association. I2 was used because it describes the percentage of variability in point estimates that is due to heterogeneity rather than sampling error. A value of I2 of 50% or more was considered to be notably heterogeneous. To investigate whether one single study unduly influenced the pooled estimates, sensitivity analyses also were conducted to compare pooled risk estimates after systematically excluding each study in turn. Finally, we conducted stratified analyses by sex, smoking status, and BMI, as they are established risk factors of pancreatic cancer, as well as folate intake, since alcohol can influence folate metabolism and DNA methylation. Tests for interactions were conducted by including the cross-product terms for alcohol (continuous) and the stratified variables in the logistic regression models (using BMI ≤ 25 and >25; never, former, and current smoker; low and high folate based on median cutpoint).
Results
Total alcohol (ethanol) consumption and individual alcoholic beverage intake varied by cohort study (Table 1). The Shanghai cohort (SMWHS) had the lowest alcohol consumption with only 10% of the total population consuming any alcohol (most of the participants are women). In contrast, 92% of the Physicians’ Health Study (PHS) participants were current consumers of alcohol. In this pooled analysis, cases were more likely to have been smokers than controls (28.6 vs. 23.0%), to have a history of diabetes (10.4 vs. 6.8%), and to have a family history of pancreatic cancer (5.9 vs. 3.4% among the seven cohorts that provided these data). Dietary intake of folate, total and saturated fat were similar across cases and controls. BMI was significantly greater in cases than controls (P value = 0.02; data not shown).
Among controls (Table 2), men tended to drink more alcohol than women, and percent of current smokers and cigarette smoking intensity increased with increasing alcohol intake. Self-reported diabetics were less likely to consume alcohol. Total and saturated fat, and BMI, among controls, did not differ appreciably by alcoholic intake, but folate intake was lower with higher alcohol intake.
Table 2.
Selected characteristics of controls according to daily alcohol consumption: PanScan (n = 1,530)
| Characteristics | Alcohol consumption (g/day) |
||||
|---|---|---|---|---|---|
| Nondrinker | >0 to <5 | 5 to < 15 | 15 to < 30 | ≥30 | |
| Men, % | 31.0 | 39.1 | 56.5 | 58.4 | 76.3 |
| Age at reference diagnosis (years), mean ± SD | 70.0 ± 7.7 | 70.1 ± 8.0 | 69.6 ± 7.5 | 69.2 ± 8.1 | 67.4 ± 7.6 |
| Race | |||||
| Caucasian, % | 72.8 | 95.9 | 97.1 | 98.9 | 97.7 |
| African American, % | 4.6 | 1.6 | 0 | 0 | 0 |
| Asian, % | 20.8 | 1.4 | 1.5 | 1.1 | 2.3 |
| Other, % | 0.8 | 0.9 | 0 | 0 | 0 |
| Unknown, % | 1.0 | 0.2 | 1.5 | 0 | 0 |
| Smoking history | |||||
| Never smokers, % | 61.2 | 45.0 | 34.2 | 27.5 | 14.5 |
| Former smokers, % | 25.4 | 35.7 | 39.4 | 36.0 | 49.1 |
| Current smokers, % | 13.2 | 19.1 | 25.8 | 35.4 | 35.8 |
| Unknown, % | 0.3 | 0.2 | 0.6 | 1.1 | 0.6 |
| Smoking duration in smokers, mean ± SD | 27.7 ± 14.7 | 26.8 ± 14.6 | 29.4 ± 15.5 | 30.2 ± 13.4 | 29.4 ± 13.6 |
| No. of cigarettes per day in smokers, mean ± SD | 18.4 ± 14.0 | 16.2 ± 12.9 | 18.6 ± 12.9 | 17.3 ± 9.7 | 21.0 ± 14.7 |
| Body mass index (kg/m2), mean ± SD | 26.3 ± 4.8 | 26.4 ± 4.2 | 26.0 ± 4.6 | 25.7 ± 3.7 | 26.2 ± 3.6 |
| Family history of pancreatic cancer (1st degrees), %a | 2.3 | 1.4 | 0.9 | 0.6 | 1.7 |
| Self-reported diabetes, % | 12.4 | 6.1 | 3.8 | 3.4 | 3.5 |
| Dietary intake per day, mean ± SD | |||||
| Total fat (g/1,000 kcal) | 34.5 ± 12.7 | 38.6 ± 8.7 | 39.5 ± 8.4 | 38.7 ± 8.4 | 35.7 ± 8.1 |
| Saturated fat (g/1,000 kcal) | 11.7 ± 5.4 | 14.3 ± 4.6 | 14.9 ± 4.7 | 14.7 ± 4.8 | 13.0 ± 4.1 |
| Total folate intake (mg/1,000 kcal) | 288 ± 218 | 264 ± 193 | 227 ± 172 | 212 ± 152 | 180 ± 120 |
SD standard deviation
Information was available for ATBC, Clue II, CPS II, NHS, NYU-WHS, PLCO, and SMWHS
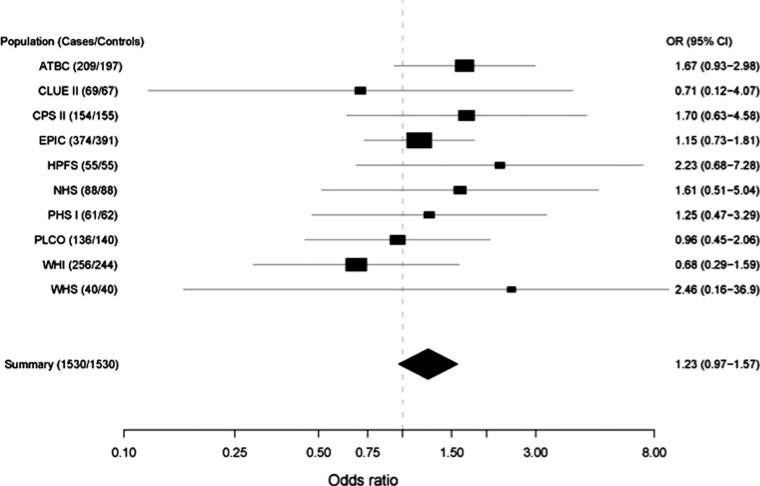
Figure 1 provides the study specific and pooled results for total alcohol intake based on the multivariate models (NYU-WHS and SMWHS are excluded from this plot because of insufficient numbers). Overall, 7 of 10 studies reported positive associations for total alcohol intake, while three were below unity. Studies with smaller numbers of cases and controls tended to show stronger associations, but these were all highly unstable estimates (NHS, HPFS, and WHS). The pooled OR for total alcohol intake comparing those drinking 30 or more grams of alcohol per day to those who drank some alcohol (>0 to <10 g/day) was not statistically significant (OR = 1.23, 95% CI = 0.97–1.57). There was no statistically significant heterogeneity between cohorts in this study population when evaluating total alcohol consumption in the pooled estimates (compared to referent, <10 g/day, P = 0.94 for nondrinkers; P = 0.89 for 10 < 30 g/day; P = 0.90 for >30 g/day).
Fig. 1.
Cohort-specific risk estimates for pancreatic cancer for daily consumption of ≥30 g alcohol compared to >0 to <10 g alcohol. Adjusted for age (continuous), sex (in sex combined models), race (Caucasian, Asian, other), smoking status (never, former smokers quit ≥10 years, former smokers quit <10 years, current smokers <20 cigarettes/day, current smokers = 20 cigarettes/day, current smokers >20 cigarettes/day, unknown), diabetes (yes, no, missing), and BMI (continuous). Solid squares represent the odds ratios. Horizontal lines represent 95% confidence intervals. Solid diamond presents the summary odds ratio and its 95% confidence interval. Test for heterogeneity (P = 0.20, I2 < 50%). NYU-WHS and SMWHS are excluded from this plot because of insufficient numbers
Unadjusted and adjusted pooled odds ratios and confidence intervals are presented in Table 3. Overall, total alcohol was not associated with a significant increase in risk of pancreatic cancer; controlling for smoking in the adjusted model attenuated the association in the top category of total alcohol intake. Similar associations were observed for men and women for total alcohol intake. In a sensitivity analysis, we observed a substantially lower pooled estimate for the top category of alcohol intake after removing the ATBC study (OR = 1.12, 95% CI = 0.66–1.90, comparing 60 or more g/day vs. >0 to <5 g/day), and a substantially higher pooled estimate after removing the EPIC study (OR = 2.30, 95% CI = 1.13–4.68, comparing 60 or more g/day vs. >0 to <5 g/day). Exclusion of other studies did not influence the pooled estimates.
Table 3.
Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for alcohol consumption in relation to pancreatic cancer risk: PanScan
| Men |
Women |
Men and women combined |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases/controls, n | OR (95% CI)a | OR (95% CI)b | Cases/controls, n | OR (95% CI)a | OR (95% CI)b | Cases/controls, n | OR (95% CI)a | OR (95% CI)b | |
| 730/725 | 800/805 | 1,530/1,530 | |||||||
| Total alcohol (g/day) | |||||||||
| 0 | 127/122 | 1.24 (0.87–1.76) | 1.23 (0.86–1.75) | 306/272 | 1.23 (0.95–1.59) | 1.21 (0.93–1.59) | 433/394 | 1.24 (1.01–1.53) | 1.19 (0.97–1.48) |
| >0 to <5 | 146/172 | 1.00 | 1.00 | 254/268 | 1.00 | 1.00 | 400/440 | 1.00 | 1.00 |
| 5 to <10 | 134/123 | 1.31 (0.93–1.85) | 1.31 (0.92–1.87) | 68/105 | 0.68 (0.48–0.97) | 0.71 (0.50–1.01) | 202/228 | 0.96 (0.76–1.23) | 1.00 (0.78–1.28) |
| 10 to <15 | 59/72 | 0.96 (0.63–1.45) | 0.94 (0.62–1.43) | 59/45 | 1.40 (0.91–2.15) | 1.49 (0.97–2.31) | 118/117 | 1.11 (0.83–1.49) | 1.15 (0.85–1.54) |
| 15 to <30 | 112/104 | 1.25 (0.88–1.77) | 1.29 (0.90–1.84) | 61/74 | 0.85 (0.58–1.25) | 0.87 (0.59–1.29) | 173/178 | 1.05 (0.81–1.35) | 1.08 (0.83–1.40) |
| 30 to <45 | 81/62 | 1.57 (1.04–2.37) | 1.66 (1.08–2.53) | 38/31 | 1.24 (0.75–2.06) | 1.17 (0.69–1.96) | 119/93 | 1.37 (1.00–1.88) | 1.36 (0.99–1.88) |
| 45 to <60 | 31/39 | 0.91 (0.54–1.54) | 0.91 (0.53–1.56) | 14/10c | 1.45 (0.63–3.33) | 1.32 (0.56–3.09) | 38/45 | 0.90 (0.57–1.42) | 0.86 (0.54–1.37) |
| ≥60 | 40/31 | 1.54 (0.91–2.60) | 1.50 (0.88–2.57) | – | – | – | 47/35 | 1.47 (0.92–2.34) | 1.38 (0.86–2.23) |
| P-trend | 0.11 | 0.11 | 0.30 | 0.41 | 0.06 | 0.11 | |||
| ORcontd | 1.04 (0.98–1.11) | 1.03 (0.97–1.11) | 1.07 (0.93–1.24) | 1.06 (0.91–1.23) | 1.04 (0.98–1.11) | 1.03 (0.97–1.10) | |||
| Alcohol from beer (g/day) | |||||||||
| 0e | 91/96 | 1.00 | 1.00 | 296/281 | 1.00 | 1.00 | 387/377 | 1.00 | 1.00 |
| >0 to <5 | 224/231 | 1.01 (0.71–1.43) | 0.98 (0.69–1.39) | 137/180 | 0.69 (0.52–0.92) | 0.70 (0.52–0.93) | 361/411 | 0.83 (0.67–1.03) | 0.82 (0.66–1.02) |
| 5 to <10 | 85/78 | 1.10 (0.72–1.69) | 1.13 (0.73–1.74) | 20/25 | 0.73 (0.40–1.35) | 0.78 (0.42–1.45) | 105/103 | 0.94 (0.68–1.30) | 0.97 (0.70–1.34) |
| 10 to <15 | 36/42 | 0.89 (0.52–1.51) | 0.86 (0.50–1.47) | 22/14c | 1.43 (0.71–2.87) | 1.44 (0.70–2.99) | 45/50 | 0.84 (0.54–1.31) | 0.82 (0.52–1.28) |
| 15 to <30 | 37/32 | 1.16 (0.66–2.05) | 1.17 (0.65–2.08) | – | – | – | 38/33 | 1.03 (0.62–1.72) | 1.09 (0.65–1.83) |
| 30 to <45 | 26/18 | 1.47 (0.75–2.89) | 1.69 (0.83–3.42) | – | – | – | 35/22 | 1.44 (0.82–2.53) | 1.58 (0.88–2.84) |
| >45 | 17/20 | 0.86 (0.42–1.76) | 0.78 (0.37–1.64) | – | – | – | 20/21 | 0.87 (0.46–1.66) | 0.79 (0.41–1.54) |
| P-trend | 0.59 | 0.60 | 0.36 | 0.48 | 0.29 | 0.38 | |||
| ORcontd | 1.02 (0.93–1.13) | 1.01 (0.91–1.12) | 1.33 (0.93–1.91) | 1.30 (0.89–1.88) | 1.04 (0.95–1.14) | 1.03 (0.93–1.13) | |||
| Alcohol from wine (g/day) | |||||||||
| 0e | 178/204 | 1.00 | 1.00 | 71/54 | 1.00 | 1.00 | 249/258 | 1.00 | 1.00 |
| >0 to <5 | 206/188 | 1.47 (1.06–2.03) | 1.49 (1.07–2.06) | 255/285 | 0.69 (0.47–1.03) | 0.79 (0.52–1.20) | 461/473 | 1.09 (0.85–1.39) | 1.17 (0.91–1.49) |
| 5 to <10 | 57/44 | 1.74 (1.08–2.81) | 1.76 (1.08–2.88) | 70/76 | 0.70 (0.43–1.15) | 0.84 (0.51–1.39) | 127/120 | 1.17 (0.84–1.62) | 1.28 (0.91–1.79) |
| 10 to <15 | 25/31 | 1.13 (0.61–2.08) | 1.20 (0.65–2.23) | 39/50 | 0.59 (0.34–1.03) | 0.74 (0.41–1.31) | 64/81 | 0.87 (0.59–1.30) | 1.02 (0.68–1.53) |
| 15 to <30 | 20/17 | 1.50 (0.74–3.06) | 1.57 (0.76–3.23) | 24/21 | 0.86 (0.43–1.73) | 0.99 (0.49–2.03) | 44/38 | 1.25 (0.76–2.03) | 1.39 (0.85–2.28) |
| 30 to <45 | 16/16 | 1.48 (0.69–3.19) | 1.61 (0.74–3.52) | 16/15c | 0.77 (0.34–1.72) | 0.83 (0.36–1.89) | 29/26 | 1.23 (0.68–2.20) | 1.31 (0.73–2.37) |
| ≥45 | 10/13 | 1.15 (0.47–2.81) | 1.15 (0.46–2.86) | – | – | – | 13/18 | 0.82 (0.38–1.75) | 0.80 (0.37–1.72) |
| P-trend | 0.98 | 0.83 | 0.86 | 0.97 | 0.76 | 0.85 | |||
| ORcontd | 1.03 (0.87–1.22) | 1.03 (0.87–1.23) | 0.93 (0.73–1.18) | 0.95 (0.74–1.21) | 0.99 (0.86–1.14) | 0.99 (0.86–1.14) | |||
| Alcohol from liquor (g/day) | |||||||||
| 0e | 89/113 | 1.00 | 1.00 | 212/230 | 1.00 | 1.00 | 301/343 | 1.00 | 1.00 |
| >0 to <5 | 224/223 | 1.29 (0.91–1.82) | 1.31 (0.93–1.86) | 183/192 | 1.02 (0.77–1.35) | 0.99 (0.74–1.31) | 407/415 | 1.11 (0.90–1.38) | 1.10 (0.89–1.37) |
| 5 to <10 | 75/67 | 1.45 (0.93–2.26) | 1.43 (0.92–2.24) | 27/33 | 0.87 (0.50–1.50) | 0.80 (0.46–1.39) | 102/100 | 1.16 (0.84–1.62) | 1.13 (0.81–1.57) |
| 10 to <15 | 28/39 | 0.88 (0.49–1.57) | 0.87 (0.48–1.57) | 24/19 | 1.44 (0.76–2.73) | 1.44 (0.75–2.76) | 52/58 | 1.02 (0.67–1.55) | 1.00 (0.65–1.52) |
| 15 to <30 | 61/42 | 1.80 (1.09–2.98) | 1.67 (1.00–2.79) | 10/13 | 0.83 (0.36–1.95) | 0.74 (0.31–1.76) | 71/55 | 1.46 (0.97–2.18) | 1.33 (0.88–2.01) |
| 30 to <45 | 15/18 | 1.05 (0.50–2.23) | 1.03 (0.48–2.23) | 19/13c | 1.55 (0.74–3.25) | 1.35 (0.63–2.87) | 29/28 | 1.17 (0.67–2.03) | 1.04 (0.59–1.81) |
| ≥45 | 21/13 | 2.16 (1.01–4.61) | 2.23 (1.02–4.87) | – | – | – | 26/16 | 1.93 (1.00–3.71) | 1.86 (0.95–3.64) |
| P-trend | 0.06 | 0.12 | 0.22 | 0.46 | 0.03 | 0.11 | |||
| ORcontd | 1.12 (0.98–1.28) | 1.10 (0.95–1.27) | 1.14 (0.90–1.43) | 1.09 (0.86–1.37) | 1.12 (1.00–1.26) | 1.10 (0.97–1.23) | |||
The P values for interaction of continuous alcohol (type) by sex are 0.67 (total alcohol), 0.20 (beer), 0.73 (wine), and 0.86 (liquor)
Adjusted for age (continuous), cohort, and sex (in sex combined models). The specific types of alcohol were mutually adjusted for the other types of alcohol. The PHS cohort and EPIC-Umea center were removed from the specific alcohol beverage analyses as alcohol type was not available
Adjusted for age (continuous), cohort, sex (in sex combined models), race (Caucasian, Asian, other), smoking status (never, former smokers quit ≥10 years, former smokers quit <10 years, current smokers <20 cigarettes/day, current smokers = 20 cigarettes/day, current smokers >20 cigarettes/day, unknown), diabetes (yes, no, missing), and BMI (continuous). The specific types of alcohol were mutually adjusted for the other types of alcohol
The category is collapsed including beginning number and up
Continuous ORs are expressed as per 15 g increase
This zero category includes only those who did not drink that particular type of alcohol; nondrinkers of any type of alcohol were included as a separate category but data are not shown
Among men, a statistically significant increase in risk was observed for the top category of alcohol intake from liquor (OR = 2.23, 95% CI = 1.02–4.87, 45 or more g/day vs. none, Table 3). Among women, liquor was not frequently consumed at the high levels (i.e., 45 or more g/day) so there was insufficient data to examine this association; no association was observed for the category of 30 or more grams of alcohol per day (vs. none). The association for alcohol from liquor was not statistically significant when men and women were combined (OR = 1.86, 95% CI = 0.95–3.64, 45 or more g/day vs. none). No associations were observed for grams of alcohol consumed from wine and beer (Table 3). P values for heterogeneity between cohort studies were not statistically significant for the different types of alcoholic beverages.
We examined alcohol intake by smoking status because smoking may modify the association between alcohol and pancreatic cancer (Table 4). Tests for interaction were not statistically significant, and there were no indications that the associations were modified by smoking status. A statistically significant association was observed for the second category of wine intake among current smokers; however, given the lack of a dose–response trend across categories, this finding is most likely to be due to chance.
Table 4.
Pooled odds ratios (ORs) and 95% confidence intervals (CIs) for alcohol consumption in relation to pancreatic cancer risk, by smoking status: PanScan
| Never smokers |
Former smokers |
Current smokers |
||||
|---|---|---|---|---|---|---|
| Cases/controls, n | OR (95% CI)a | Cases/controls, n | OR (95% CI)b | Cases/controls, n | OR (95% CI)b | |
| 584/631 | 304/337 | 376/319 | ||||
| Total alcohol (g/day) | ||||||
| 0 | 250/241 | 1.26 (0.92–1.72) | 58/55 | 1.03 (0.61–1.72) | 55/47 | 0.91 (0.54–1.55) |
| >0 to <5 | 158/198 | 1.00 | 83/91 | 1.00 | 95/74 | 1.00 |
| 5 to <10 | 71/78 | 1.08 (0.72–1.63) | 45/66 | 0.75 (0.45–1.26) | 52/47 | 0.97 (0.58–1.64) |
| 10 to <15 | 40/40 | 1.23 (0.75–2.02) | 22/21 | 1.30 (0.65–2.61) | 33/33 | 0.90 (0.50–1.62) |
| 15 to <30 | 38/49 | 0.96 (0.59–1.56) | 46/40 | 1.42 (0.82–2.46) | 56/59 | 0.76 (0.46–1.25) |
| ≥30 | 27/25 | 1.21 (0.66–2.22) | 50/64 | 0.94 (0.57–1.57) | 85/59 | 1.12 (0.69–1.81) |
| P-trend | 0.62 | 0.55 | 0.82 | |||
| ORcontd | 0.96 (0.81–1.13) | 1.04 (0.92–1.17) | 1.01 (0.92–1.11) | |||
| Alcohol from beer (g/day) | ||||||
| 0e | 150/161 | 1.00 | 88/95 | 1.00 | 76/62 | 1.00 |
| > 0 to <5 | 101/138 | 0.76 (0.53–1.10) | 83/88 | 1.11 (0.69–1.78) | 133/121 | 0.87 (0.56–1.36) |
| 5 to <10 | 20/35d | 0.55 (0.30–1.02) | 21/21 | 1.49 (0.70–3.13) | 41/33 | 1.08 (0.59–1.97) |
| 10 to <15 | – | – | 9/13 | 1.08 (0.41–2.89) | 19/19 | 0.82 (0.38–1.75) |
| ≥15 | 14/9 | 1.40 (0.56–3.50) | 20/27 | 0.96 (0.46–2.04) | 48/30 | 1.30 (0.70–2.40) |
| P-trend | 1.00 | 0.86 | 0.71 | |||
| ORcontc | 1.00 (0.70–1.43) | 0.98 (0.75–1.28) | 1.02 (0.91–1.16) | |||
| Alcohol from wine (g/day) | ||||||
| 0e | 36/38 | 1.00 | 26/31 | 1.00 | 146/150 | 1.00 |
| > 0 to <5 | 155/205 | 0.82 (0.49–1.39) | 111/122 | 1.11 (0.59–2.06) | 117/71 | 1.78 (1.16–2.73) |
| 5 to <10 | 49/50 | 1.14 (0.61–2.13) | 38/33 | 1.13 (0.53–2.38) | 21/16 | 1.32 (0.63–2.75) |
| 10 to <15 | 23/27 | 0.99 (0.47–2.09) | 23/22 | 1.15 (0.49–2.66) | 6/14 | 0.52 (0.18–1.52) |
| ≥15 | 22/23 | 1.11 (0.51–2.38) | 23/36 | 0.71 (0.32–1.56) | 27/14 | 1.77 (0.82–3.83) |
| P-trend | 0.60 | 0.22 | 0.95 | |||
| ORcontc | 1.09 (0.78–1.53) | 0.85 (0.65–1.10) | 0.99 (0.78–1.26) | |||
| Alcohol from liquor (g/day) | ||||||
| 0e | 132/160 | 1.00 | 66/75 | 1.00 | 47/46 | 1.00 |
| > 0 to <5 | 114/146 | 0.93 (0.66–1.32) | 105/123 | 0.98 (0.63–1.54) | 136/89 | 1.57 (0.94–2.61) |
| 5 to <10 | 18/13 | 1.64 (0.76–3.51) | 19/21 | 1.15 (0.55–2.39) | 43/47 | 1.05 (0.56–1.94) |
| 10 to <15 | 9/10 | 1.06 (0.41–2.76) | 9/9 | 1.94 (0.66–5.69) | 22/27 | 0.97 (0.46–2.03) |
| ≥15 | 12/14 | 1.01 (0.44–2.31) | 22/16 | 1.63 (0.75–3.50) | 69/56 | 1.23 (0.69–2.21) |
| P-trend | 0.43 | 0.17 | 0.96 | |||
| ORcontc | 0.88 (0.65–1.20) | 1.18 (0.93–1.49) | 1.01 (0.82–1.24) | |||
The P values for interaction of continuous alcohol (type) by smoking status are 0.98 (total alcohol), 0.84 (beer), 0.59 (wine), and 0.52 (liquor)
Adjusted for age (continuous), cohort, sex, race (Caucasian, Asian, other), diabetes (yes, no, missing), and BMI (continuous). The specific types of alcohol were mutually adjusted for the other types of alcohol. The PHS cohort and EPIC-Umea center were removed from the specific alcohol beverage analyses as alcohol type was not available
Adjusted for age (continuous), cohort, gender, race (Caucasian, Asian, other), smoking (dose continuous, duration continuous), diabetes (yes, no, missing), and BMI (continuous). The specific types of alcohol were mutually adjusted for the other types of alcohol
Continuous ORs are expressed as per 15 g increase
The category is collapsed including beginning number and up to 15
Nondrinkers of any type of alcohol were included in all analyses, but data are not shown
We observed no interactions for alcohol and BMI (P value = 0.87) or alcohol and folate intake (P value = 0.65). Furthermore, the overall pooled estimate for total alcohol intake was similar to the main findings (Table 3) after removing the first 2 and 5 years of follow-up (multivariable OR = 1.47, 95% CI = 0.90–2.41, and OR = 1.51, 95% CI = 0.88–2.60, respectively, for 60 or more g/day of alcohol vs. none).
Discussion
In this large pooled nested case–control study, we observed no significant overall increase in risk of pancreatic cancer with moderate to high levels of alcohol intake. The associations were similar when stratified by sex or smoking status. We observed a greater than twofold increase in risk among men drinking 45 or more grams of alcohol from liquor per day compared to none, but the same association was not statistically significant for men and women combined. Other alcoholic beverages were not associated with risk of pancreatic cancer and results from stratified analyses by smoking status, BMI, and folate intake and were similar to the overall findings.
Our findings agree with most observational studies examining alcohol intake and pancreatic cancer [7]; only 4 of the 12 cohorts included in this pooled analysis have previously reported individual results on alcohol and pancreatic cancer [10–12]. To date, four prospective cohort studies (out of 16) observed an increase in risk with high alcohol intake [16–18, 22]; however, no adjustment for smoking was made in one study [18], and the small number of cases (n = 66) in another study [17] led to unstable estimates. In four cohort studies among alcoholics, the incidence rates of pancreatic cancer were higher than those in the general population, with standardized incidence ratios (SIRs) ranging between 1.3 and 2.6 [23–26]. Those latter studies, however, could not adjust for potential confounding factors, including smoking, which could account for the excess risk observed.
A recent pooled analysis reported a significant 22% increase in risk of pancreatic cancer among those consuming ≥30 g/day vs. none [27]. In this analysis, 5 of the cohort studies were also included in our analysis but 9 others were different. Unlike this study, the increase in risk was stronger in women than in men (women, RR = 1.41, 95% CI = 1.07–1.85; men, RR = 1.12, 95% CI = 0.89–1.39), and no association was observed for the specific types of alcoholic beverages (wine, beer or spirits). However, overall the relative risk was almost identical to ours (RR = 1.23, 95% CI = 0.97–1.57, for >30 g/day vs. >0 to <5 g/day, Fig. 1). In addition, the NIH-AARP cohort study recently published findings on alcohol with 1,149 pancreatic cancer cases and observed a 45% increased risk among those consuming >3 drinks (95% CI = 1.17–1.80) and a 55% increase in risk among those consuming 6 or more drinks of alcohol a day compared to >0 to <1 drinks/day (RR = 1.55, 95% CI = 1.13–2.13, P-trend = 0.004) [22]. In that study, liquor consumption was significantly associated with risk (RR = 1.62, 95% CI = 1.24–2.10, ≥3 drinks/day vs. 0), whereas beer and wine were not. A large cohort study of women with 1,325 pancreatic cancer cases did not observe an increase in risk (RR = 1.07, 95% CI = 0.85–1.35, for 15 or more drinks/week vs. non-drinkers) [15], but the cutpoint for the highest category of alcohol intake may not have been sufficiently high to detect an increase in risk.
The two-fold increase in risk observed for high liquor intake among men is noteworthy, especially given similar findings in the NIH-AARP cohort study [22]. Although chance or confounding may explain these findings, alternative explanations are possible. For example, men reporting consuming 45 or more grams of alcohol from liquor per day may be more likely to be alcoholics than those consuming other alcoholic beverages. The observation, in this analysis, that women did not have a higher risk of pancreatic cancer with increasing liquor intake may support this explanation, as women are less likely to be alcoholics [43]. As alcoholism can lead to acute and chronic pancreatitis, the elevated risk observed may be a consequence of this condition (whether clinical or sub-clinical) as it is a well-established risk factor for pancreatic cancer [6]. Information on pancreatitis diagnosis was not available for the majority of cohorts included in this study and thus could not be included in the analysis. As alcohol intake is positively associated with a number of lifestyle factors that are linked to socioeconomic status, it is also plausible that the observed increase risk is due to confounding by an unknown risk factor that is associated with alcohol intake.
In addition to chronic pancreatitis, alternative mechanisms through which alcohol may act to increase pancreatic carcinogenesis are equally plausible. These include increased risk of diabetes type II among heavy alcohol drinkers, and metabolic effects on the inflammatory response [44]. Alcohol can influence the inflammatory response through a number of pathways, including activation of nuclear transcription factors, increased production of reactive oxidation species, activation of pancreatic stellate cells, which leads to fibrosis, and dysregulation of proliferation and apoptosis [44]. Alcohol may also act in synergy with cigarette smoke compounds to increase the inflammatory response. In this study, however, we did not observe a synergistic effect of alcohol and cigarette smoke. Based on our findings, and the literature to date, it appears unlikely that the metabolic effects of alcohol are sufficient to increase the risk of pancreatic cancer.
By combining a number of large cohort studies, we were able to pool a large number of pancreatic cancer cases to examine a broad range of alcoholic exposure and conduct analyses stratified by potential effect modifiers. All exposure data were collected prior to cancer diagnosis, avoiding any possibility of recall bias. In addition, the cohort studies in this analysis all have high follow-up rates and detailed endpoint ascertainment. The major limitation to the study is the possibility of measurement error in the exposure assessment, given that only one measurement of alcohol use was available in this analysis and that changes in alcoholic consumption might have occurred before or after the measurement. Measurement error could have led to attenuation of the true risk estimate. In addition, alcohol intake was assessed with varying questionnaires across the cohorts, each with potentially different measurement error.
In this pooled analysis, we observed no significant association for moderate to high levels of alcohol intake in relation to risk of pancreatic cancer. However, a 23% increase in risk for ≥30 g/day observed in this study was comparable to a significant 22% increase in risk reported by a recent pooled cohort study [27]. Folate intake, BMI, cigarette smoking history, and sex did not modify the overall association between alcohol intake and pancreatic cancer. Examination of different types of alcoholic beverages provided some suggestion that heavy liquor use may be associated with a higher risk of pancreatic cancer among men.
Footnotes
The authors Dominique S. Michaud, Alina Vrieling, Li Jiao, Julie B. Mendelsohn, Emily Steplowski, Shannon M. Lynch, Jean Wactawski-Wende, and Rachael Z. Stolzenberg-Solomon are writing committee members.
Contributor Information
Dominique S. Michaud, Division of Epidemiology, Public Health and Primary Care, Imperial College London, London, UK Department of Epidemiology, Harvard School of Public Health, Boston, MA.
Alina Vrieling, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Li Jiao, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Julie B. Mendelsohn, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Emily Steplowski, Information Management Services, Silver Spring, MD, USA.
Shannon M. Lynch, Division of Cancer Control and Population Science, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Jean Wactawski-Wende, Department of Social and Preventive Medicine, University at Buffalo, Suny, Buffalo, NY, USA.
Alan A. Arslan, Department of Obstetrics and Gynecology, New York University School of Medicine, New York, NY, USA Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA; NYU Cancer Institute, New York, NY, USA.
H. Bas Bueno-de-Mesquita, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands.
Charles S. Fuchs, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA Hankinson Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Myron Gross, Department of Laboratory Medicine/Pathology, School of Medicine, University of Minnesota, Minneapolis, MN, USA.
Kathy Helzlsouer, Prevention and Research Center, Mercy Medical Center, Baltimore, MD, USA.
Eric J. Jacobs, Department of Epidemiology, American Cancer Society, Atlanta, GA, USA
Andrea LaCroix, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Gloria Petersen, Department of Health Sciences Research, Mayo Clinic, Rochester, MN, USA.
Wei Zheng, Department of Medicine and Vanderbilt-Ingram Cancer Center, Vanderbilt University, Nashville, TN, USA.
Naomi Allen, Cancer Epidemiology Unit, University of Oxford, Oxford, UK.
Laufey Ammundadottir, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; Laboratory of Translational Genomics, Division of Cancer Epidemiology and Genetics, Department of Health and Human Services, National Cancer Institute, National Institutes of Health, Bethesda, MDUSA.
Manuela M. Bergmann, Department of Epidemiology, German Institute of Human Nutrition, Potsdam-Rehbrücke, Germany
Paolo Boffetta, International Agency for Research on Cancer, Lyon, France; The Tisch Cancer Institute, Mount Sinai School of Medicine, New York, NY, USA.
Julie E. Buring, Divisions of Preventive Medicine and Aging, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA Department of Ambulatory Care and Prevention, Harvard Medical School, Boston, MAUSA.
Federico Canzian, Division of Cancer Epidemiology, German Cancer Research Center (DKFZ), Heidelberg, Germany.
Stephen J. Chanock, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA Laboratory of Translational Genomics, Division of Cancer Epidemiology and Genetics, Department of Health and Human Services, National Cancer Institute, National Institutes of Health, Bethesda, MDUSA.
Françoise Clavel-Chapelon, Inserm (Institut National de la Santé et de la Recherche Médicale) and Institut Gustave Roussy, Villejuif, France.
Sandra Clipp, Johns Hopkins Bloomberg School of Public Health, George W. Comstock Center for Public Health Research and Prevention, Hagerstown, MD, USA.
Matthew S. Freiberg, Center for Research on Health Care, University of Pittsburgh, Pittsburgh, PA, USA
J. Michael Gaziano, Divisions of Preventive Medicine and Aging, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Massachusetts Veterans Epidemiology Research and Information Center, Veterans Affairs Boston Healthcare System, Boston, MA, USA.
Edward L. Giovannucci, Department of Epidemiology, Harvard School of Public Health, Boston, MA Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard School of Public Health, Boston, MA, USA.
Susan Hankinson, Department of Epidemiology, Harvard School of Public Health, Boston, MA; Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Patricia Hartge, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Robert N. Hoover, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
F. Allan Hubbell, School of Medicine, University of California, Irvine, CA, USA.
David J. Hunter, Department of Epidemiology, Harvard School of Public Health, Boston, MA Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Amy Hutchinson, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; Core Genotyping Facility, Advanced Technology Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD, USA.
Kevin Jacobs, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA; Core Genotyping Facility, Advanced Technology Program, SAIC-Frederick Inc., NCI-Frederick, Frederick, MD, USA; Bioinformed Consulting Services, Gaithersburg, MD, USA.
Charles Kooperberg, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Peter Kraft, Department of Epidemiology, Harvard School of Public Health, Boston, MA.
Jonas Manjer, Department of Surgery, Malmö University Hospital, Malmö, Sweden.
Carmen Navarro, Department of Epidemiology, Murcia Regional Health Council, Murcia, Spain; CIBER Epidemiología y Salud Pública (CIBERESP), Barcelona, Spain.
Petra H. M. Peeters, Division of Epidemiology, Public Health and Primary Care, Imperial College London, London, UK Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, The Netherlands.
Xiao-Ou Shu, Department of Medicine and Vanderbilt-Ingram Cancer Center, Vanderbilt University, Nashville, TN, USA.
Victoria Stevens, Department of Epidemiology, American Cancer Society, Atlanta, GA, USA.
Gilles Thomas, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Anne Tjønneland, Department of Diet, Cancer and Health, Institute of Cancer Epidemiology, Danish Cancer Society, Copenhagen, Denmark.
Geoffrey S. Tobias, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
Dimitrios Trichopoulos, Department of Epidemiology, Harvard School of Public Health, Boston, MA; Department of Hygiene and Epidemiology, University of Athens Medical School, Athens, Greece.
Rosario Tumino, Cancer Registry and Histopathology Department, “Civile-M.P.Arezzo” Hospital, Ragusa, Italy.
Paolo Vineis, Division of Epidemiology, Public Health and Primary Care, Imperial College London, London, UK.
Jarmo Virtamo, Department of Chronic Disease Prevention, National Institute for Health and Welfare, Helsinki, Finland.
Robert Wallace, Departments of Epidemiology and Internal Medicine, University of Iowa Colleges of Public Health and Medicine, Iowa City, IA, USA.
Brian M. Wolpin, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA Hankinson Channing Laboratory, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA.
Kai Yu, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Anne Zeleniuch-Jacquotte, Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA; NYU Cancer Institute, New York, NY, USA.
Rachael Z. Stolzenberg-Solomon, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg. 2008;393(4):535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92(11):2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132(6):2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 5.Michaud DS. Epidemiology of pancreatic cancer. Minerva Chir. 2004;59(2):99–111. [PubMed] [Google Scholar]
- 6.Lowenfels AB, Maisonneuve P, Lankisch PG. Chronic pancreatitis and other risk factors for pancreatic cancer. Gastroenterol Clin North Am. 1999;28(3):673–685. x. doi: 10.1016/s0889-8553(05)70080-7. [DOI] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund, American Institute for Cancer Research . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. American Intitute for Cancer Research; Washington, DC: 2008. Pancreas. [Google Scholar]
- 8.Lin Y, Tamakoshi A, Kawamura T, Inaba Y, Kikuchi S, Motohashi Y, Kurosawa M, Ohno Y. Risk of pancreatic cancer in relation to alcohol drinking, coffee consumption and medical history: findings from the Japan collaborative cohort study for evaluation of cancer risk. Int J Cancer. 2002;99(5):742–746. doi: 10.1002/ijc.10402. [DOI] [PubMed] [Google Scholar]
- 9.Isaksson B, Jonsson F, Pedersen NL, Larsson J, Feychting M, Permert J. Lifestyle factors and pancreatic cancer risk: a cohort study from the Swedish Twin registry. Int J Cancer. 2002;98:480–482. doi: 10.1002/ijc.10256. [DOI] [PubMed] [Google Scholar]
- 10.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Fuchs CS. Coffee and alcohol consumption and the risk of pancreatic cancer in two prospective United States cohorts. Cancer Epidemiol Biomarkers Prev. 2001;10(5):429–437. [PubMed] [Google Scholar]
- 11.Stolzenberg-Solomon RZ, Pietinen P, Barrett MJ, Taylor PR, Virtamo J, Albanes D. Dietary and other methyl-group availability factors and pancreatic cancer risk in a cohort of male smokers. Am J Epidemiol. 2001;153(7):680–687. doi: 10.1093/aje/153.7.680. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SS, Calle EE, Patel AV, Thun MJ. Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control. 2000;11(10):915–923. doi: 10.1023/a:1026580131793. [DOI] [PubMed] [Google Scholar]
- 13.Villeneuve PJ, Johnson KC, Hanley AJ, Mao Y. Alcohol, tobacco and coffee consumption and the risk of pancreatic cancer: results from the Canadian Enhanced Surveillance System case-control project. Canadian Cancer Registries Epidemiology Research Group. Eur J Cancer Prev. 2000;9(1):49–58. doi: 10.1097/00008469-200002000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Nilsen TI, Vatten LJ. A prospective study of lifestyle factors and the risk of pancreatic cancer in Nord-Trondelag, Norway. Cancer Causes Control. 2000;11(7):645–652. doi: 10.1023/a:1008916123357. [DOI] [PubMed] [Google Scholar]
- 15.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, Green J. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 16.Zheng W, McLaughlin JK, Gridley G, Bjelke E, Schuman LM, Silverman DT, Wacholder S, Co-Chien HT, Blot WJ, Fraumeni JF., Jr A cohort study of smoking, alcohol consumption, and dietary factors for pancreatic cancer (United States). Cancer Causes Control. 1993;4(5):477–482. doi: 10.1007/BF00050867. [DOI] [PubMed] [Google Scholar]
- 17.Harnack LJ, Anderson KE, Zheng W, Folsom AR, Sellers TA, Kushi LH. Smoking, alcohol, coffee, and tea intake and incidence of cancer of the exocrine pancreas: the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 1997;6(12):1081–1086. [PubMed] [Google Scholar]
- 18.Heuch I, Kvale G, Jacobsen BK, Bjelke E. Use of alcohol, tobacco and coffee, and risk of pancreatic cancer. Br J Cancer. 1983;48:637–643. doi: 10.1038/bjc.1983.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman DT, Brown LM, Hoover RN, Schiffman M, Lillemoe KD, Schoenberg JB, Swanson GM, Hayes RB, Greenberg RS, Benichou J, et al. Alcohol and pancreatic cancer in blacks and whites in the United States. Cancer Res. 1995;55(21):4899–4905. [PubMed] [Google Scholar]
- 20.Partanen TJ, Vainio HU, Ojajarvi IA, Kauppinen TP. Pancreas cancer, tobacco smoking and consumption of alcoholic beverages: a case-control study. Cancer Lett. 1997;116(1):27–32. doi: 10.1016/s0304-3835(97)04744-7. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Matsuo K, Sawaki A, Mizuno N, Hiraki A, Kawase T, Watanabe M, Nakamura T, Yamao K, Tajima K, Tanaka H. Alcohol drinking and one-carbon metabolism-related gene polymorphisms on pancreatic cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17(10):2742–2747. doi: 10.1158/1055-9965.EPI-08-0470. [DOI] [PubMed] [Google Scholar]
- 22.Jiao L, Silverman DT, Schairer C, Thiebaut AC, Hollenbeck AR, Leitzmann MF, Schatzkin A, Stolzenberg-Solomon RZ. Alcohol use and risk of pancreatic cancer: the NIH-AARP diet and health study. Am J Epidemiol. 2009;169(9):1043–1051. doi: 10.1093/aje/kwp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigvardsson S, Hardell L, Przybeck TR, Cloninger R. Increased cancer risk among Swedish female alcoholics. Epidemiology. 1996;7(2):140–143. doi: 10.1097/00001648-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Tonnesen H, Moller H, Andersen JR, Jensen E, Juel K. Cancer morbidity in alcohol abusers. Br J Cancer. 1994;69(2):327–332. doi: 10.1038/bjc.1994.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye W, Lagergren J, Weiderpass E, Nyren O, Adami HO, Ekbom A. Alcohol abuse and the risk of pancreatic cancer. Gut. 2002;51(2):236–239. doi: 10.1136/gut.51.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adami HO, McLaughlin JK, Hsing AW, Wolk A, Ekbom A, Holmberg L, Persson I. Alcoholism and cancer risk: a population-based cohort study. Cancer Causes Control. 1992;3(5):419–425. doi: 10.1007/BF00051354. [DOI] [PubMed] [Google Scholar]
- 27.Genkinger JM, Spiegelman D, Anderson KE, Bergkvist L, Bernstein L, van den Brandt PA, English DR, Freudenheim JL, Fuchs CS, Giles GG, Giovannucci E, Hankinson SE, Horn-Ross PL, Leitzmann M, Mannisto S, Marshall JR, McCullough ML, Miller AB, Reding DJ, Robien K, Rohan TE, Schatzkin A, Stevens VL, Stolzenberg-Solomon RZ, Verhage BA, Wolk A, Ziegler RG, Smith-Warner SA. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev. 2009;18(3):765–776. doi: 10.1158/1055-9965.EPI-08-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Zheng W, Albanes D, Bamlet W, Berg CD, Berrino F, Bingham S, Buring JE, Bracci PM, Canzian F, Clavel-Chapelon F, Clipp S, Cotterchio M, de Andrade M, Duell EJ, Fox JW, Jr, Gallinger S, Gaziano JM, Giovannucci EL, Goggins M, Gonzalez CA, Hallmans G, Hankinson SE, Hassan M, Holly EA, Hunter DJ, Hutchinson A, Jackson R, Jacobs KB, Jenab M, Kaaks R, Klein AP, Kooperberg C, Kurtz RC, Li D, Lynch SM, Mandelson M, McWilliams RR, Mendelsohn JB, Michaud DS, Olson SH, Overvad K, Patel AV, Peeters PH, Rajkovic A, Riboli E, Risch HA, Shu XO, Thomas G, Tobias GS, Trichopoulos D, Van Den Eeden SK, Virtamo J, Wactawski-Wende J, Wolpin BM, Yu H, Yu K, Zeleniuch-Jacquotte A, Chanock SJ, Hartge P, Hoover RN. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41(9):986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen GM, Amundadottir L, Fuchs CS, Kraft P, Stolzenberg-Solomon RZ, Jacobs KB, Arslan AA, Bueno-de-Mesquita HB, Gallinger S, Gross M, Helzlsouer K, Holly EA, Jacobs EJ, Klein AP, Lacroix A, Li D, Mandelson MT, Olson SH, Risch HA, Zheng W, Albanes D, Bamlet WR, Berg CD, Boutron-Ruault MC, Buring JE, Bracci PM, Canzian F, Clipp S, Cotterchio M, de Andrade M, Duell EJ, Gaziano JM, Giovannucci EL, Goggins M, Hallmans G, Hankinson SE, Hassan M, Howard B, Hunter DJ, Hutchinson A, Jenab M, Kaaks R, Kooperberg C, Krogh V, Kurtz RC, Lynch SM, McWilliams RR, Mendelsohn JB, Michaud DS, Parikh H, Patel AV, Peeters PH, Rajkovic A, Riboli E, Rodriguez L, Seminara D, Shu XO, Thomas G, Tjonneland A, Tobias GS, Trichopoulos D, Van Den Eeden SK, Virtamo J, Wactawski-Wende J, Wang Z, Wolpin BM, Yu H, Yu K, Zeleniuch-Jacquotte A, Fraumeni JF, Jr, Hoover RN, Hartge P, Chanock SJ. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42(3):224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The ATBC Cancer Prevention Study Group The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4(1):1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 31.Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, Helzlsouer KJ. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol. 2003;157(4):335–344. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 32.Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, Feigelson HS, Thun MJ. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 33.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, Tjonneland A, Clavel-Chapelon F, Thiebaut A, Wahrendorf J, Boeing H, Trichopoulos D, Trichopoulou A, Vineis P, Palli D, Bueno-De-Mesquita HB, Peeters PH, Lund E, Engeset D, Gonzalez CA, Barricarte A, Berglund G, Hallmans G, Day NE, Key TJ, Kaaks R, Saracci R. European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 34.Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: the New York University Women's Health Study. Nutr Cancer. 1997;28(3):276–281. doi: 10.1080/01635589709514588. [DOI] [PubMed] [Google Scholar]
- 35.Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The prostate, lung, colorectal and ovarian (PLCO) cancer screening trial of the national cancer institute: history, organization, and status. Control Clin Trials. 2000;21(6 Suppl):251S–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 36.Cai H, Zheng W, Xiang YB, Xu WH, Yang G, Li H, Shu XO. Dietary patterns and their correlates among middle-aged and elderly Chinese men: a report from the Shanghai men's health study. Br J Nutr. 2007;98(5):1006–1013. doi: 10.1017/S0007114507750900. [DOI] [PubMed] [Google Scholar]
- 37.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, Li HL, Wen W, Ji BT, Li Q, Shu XO, Gao YT. The Shanghai women's health study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162(11):1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 38.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 39.Belanger CF, Hennekens CH, Rosner B, Speizer FE. The nurses’ health study. Am J Nurs. 1978;78:1039–1040. [PubMed] [Google Scholar]
- 40.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 41.Buring JE. The women's health study research group. The women's health study: summary of the study design. J Myocardiol Ischemia. 1992;4:27–29. [Google Scholar]
- 42.Physician's health study: aspirin and primary prevention of coronary heart disease. N Engl J Med. 1989;321(26):1825–1828. doi: 10.1056/NEJM198912283212610. [DOI] [PubMed] [Google Scholar]
- 43.Kung HC, Hoyert DL, Xu JQ, Murphy SL. Deaths: final data for 2005. National Center for Health Statistics; Hyattsville: 2008. National vital statistics reports. [PubMed] [Google Scholar]
- 44.Go VL, Gukovskaya A, Pandol SJ. Alcohol and pancreatic cancer. Alcohol. 2005;35(3):205–211. doi: 10.1016/j.alcohol.2005.03.010. [DOI] [PubMed] [Google Scholar]