Abstract
Neurons exist within a microenvironment that significantly influences their function and survival. While there are many environmental factors that can potentially impact neuronal function, activation of the innate immune system (microglia) is an important element common to many neurological and pathological conditions associated with memory loss. Learning and memory processes rely on the ability of neurons to alter their transcriptional programs in response to synaptic input. Recent advances in cell-based imaging of plasticity-related immediate-early gene (IEG) expression have provided a tool to investigate plasticity-related changes across multiple brain regions. The activity-regulated, cytoskeleton-associated IEG Arc is a regulator of protein synthesis–dependent forms of synaptic plasticity, which are essential for memory formation. Visualisation of Arc provides cellular level resolution for the mapping of neuronal networks. Chronic activation of the innate immune system alters Arc activity patterns, and this may be a mechanism by which it induces the cognitive dysfunction frequently associated with neuroinflammatory conditions. This review discusses the use of Arc expression during activation of the innate immune system as a valid marker of altered plasticity and a predictor of cognitive dysfunction.
Keywords: synaptic plasticity, leaning and memory, immediate early gene, Arc, neuroinflammation, innate immune system, cytokines, neuronal networks, fractalkine, hippocampal circuits
Introduction
Who we are is determined by our perceptions and actions, which are largely guided by our memory; therefore, our ability to remember determines who we are as an individual. The ability of our brain to form and store new memories involves modulation of the strength and efficacy of synaptic signalling and is mediated by de novo synthesis of genes and proteins. Dysfunction of hippocampal synaptic plasticity results in loss of memory functions and is characteristic of many neurodegenerative diseases, such as Alzheimer’s disease, HIV-associated dementia, autism, Down syndrome and multiple sclerosis (Akiyama et al., 2000; Akiyama et al., 2001; Banati et al., 2000; McGeer and McGeer, 1998; Mhatre et al., 2004; Morganti-Kossmann et al., 2001; Vargas et al., 2005). Additionally, dysfunction of hippocampal synaptic plasticity may be a long term consequence of traumatic brain injury (TBI) (McAllister, 1992), therapeutic brain irradiation (Meyers et al., 2000) or normal aging. Although neuronal dysfunction is the ultimate consequence of these disorders, alterations in the neuronal microenvironment, by activation of the innate immune system, seems to be the key factor for the progression of these pathologies (Akiyama et al., 2000; Akiyama et al., 2001; Banati et al., 2000; McGeer and McGeer, 1998; Mhatre et al., 2004; Morganti-Kossmann et al., 2001; Vargas et al., 2005).
Microglial cells are bone-marrow/mesenchymal-derived monocytes and constitute the resident innate immune system of the brain as well as the key cellular mediators of neuroinflammatory processes (Barger and Basile, 2001). Once activated, microglial cells release potentially harmful molecules, such as proinflammatory cytokines, chemokines, reactive oxygen species and complement proteins, leading to a self-propagating cycle and resulting in chronic neuroinflammation, which may compromise synaptic plasticity.
Synaptic plasticity requires de novo gene expression and protein synthesis for the development of enduring synaptic modifications and long-term changes in behaviour (Deisseroth et al., 2003; Lee et al., 2005). The immediate early gene (IEG) Arc (activity-regulated cytoskeleton-associated protein) is expressed in response to synaptic activity in the principal neurons of different brain structures (cortex, hippocampus, amygdala and striatum). Arc expression is required for the formation of durable plasticity processes that underlie memory consolidation and correlates both temporally and spatially with the stimulus that induced its transcription (Guzowski et al., 2000; Lyford et al., 1995). The correspondence in circuit dynamics between electrophysiology and Arc expression has led to the suggestion that expression of Arc may serve as a reliable monitor of cellular activity, reflecting spatial and contextual information processing (Guzowski et al., 1999). As a result, Arc could be used to study altered hippocampal circuits (Rosi et al., 2009). It has been recently reported that dysregulation of Arc expression parallels cognitive dysfunctions observed in several different inflammatory-related conditions (Frank et al., 2010; Hein et al., 2010; Rosi et al., 2006). Arc dysregulation is also involved in the comorbidity of anxiety and alcohol drinking and the neuro-adaptation of drug addiction (Hearing et al., 2010; Lucas et al., 2008; Pandey et al., 2008). Understanding how neuroinflammation may affect synaptic plasticity is one of the most important goals for basic and clinical neuroscience. This review discusses the use of the plasticity-related behaviourally-induced Arc expression during neuroinflammatory conditions as a reliable marker for studying altered hippocampal circuits.
Brain Inflammation
Neuroinflammation begins as a host defence mechanism associated with neutralisation of an insult and restoration of normal structure and function, similar to inflammation in peripheral organs. However, if neuroinflammation is not regulated, it can result in a self-propagating and deleterious process. Microglial cells are considered to be the resident immune system of the brain and react to various insults, such as viruses, bacteria, circulating pathogens, physical injury, chemical insults, signalling molecules released by neurons and aggregation of modified proteins though activation of Toll-like receptors (TLRs). Microglia, respond to TLRs ligands and produce proinflammatory mediators, such as proinflammatory cytokines, chemokines, reactive oxygen species and complement proteins, which lead to chronic neuroinflammation (Akiyama et al., 2000; Rivest, 2009). The released molecules can target the deleterious agents (bacteria, pathogens, etc.) and remove the degenerating stimulus; however, the proinflammatory mediators may also be deleterious and lead to astrocyte dysfunction and excessive extracellular glutamate levels, resulting in excitotoxicity. If this process does not resolve spontaneously, sustained neuroinflammation will result in altered microglial cell function and impair the normal neuroprotective role of these cells (Vilhardt, 2005). Chronically activated microglia and their products are key mediators of the neuroinflammatory cascade, which may lead to neuronal damage (Barger and Basile, 2001). Therefore neuroinflammation (or activation of the brain’s innate immune system) can have beneficial or harmful outcomes, and this is critically dependent on the duration of the inflammatory response and the type of stimulus inducing it (Michelucci et al., 2009). The classical activation of microglia, described above, is characterised by a phenotype called M1 (Michelucci et al., 2009); in contrast, alternative activation of microglia can lead to an anti-inflammatory phenotype called M2 (Michelucci et al., 2009). These two phenotypes can be distinguished based on their gene expression profile (Michelucci et al., 2009). For the purpose of the present review, only the detrimental aspects of the classical activation will be considered in the context of altered synaptic functions.
To study neuroinflammation and its consequences on neuronal functioning in vivo, microglia can be activated by several compounds, including lipopolysaccharide (LPS), β-amyloid (Aβ), interferon-γ and other proinflammatory cytokines. LPS is known to preferentially activate microglial cells (Lehnardt et al., 2003). The innate immune response induced by LPS is mainly mediated by microglia (Rivest, 2009) that are activated through stimulation of TLR4/CD14 receptors (Lehnardt et al., 2003). Chronic LPS infusion leads to a cascade of self-propagating cellular events including blockade of glutamate uptake by astrocytes (Rothwell, 1997), increased levels of prostaglandins (Katsuura et al., 1989), nitric oxide production (Morimoto et al., 2002; Perez-Capote et al., 2005), cytokine production (Bernardino et al., 2005) and enhanced release of glutamate from astrocytes (Bezzi et al., 2001; Emerit et al., 2004). Slow, chronic LPS infusion directly into the ventricular system results in increased activation of microglia selectively within the dentate gyrus (DG) and CA3 areas of the hippocampus and the piriform and entorhinal cortices (Hauss-Wegrzyniak et al., 1998a; Rosi et al., 2005b). Chronic LPS infusion results in astrogliosis (Rosi et al., 2005a), increases in tissue levels of interleukin-1beta (IL-1β) and tumour necrosis factor-α (TNFα), elevated β-APP induction (Hauss-Wegrzyniak et al., 1998a) and increases in the prominent component of the proinflammatory cytokine signalling cascade, nuclear factor κ binding protein (Rosi et al., 2005a). The autocrine and paracrine effects of the proinflammatory cytokine TNF-α up-regulate TLRs expression, spreading the inflammatory process though the parenchyma (Rivest, 2009).
Similar patterns of activated microglia and brain inflammation have also been observed during normal aging (Gavilan et al., 2007) and as a consequence of traumatic brain injury (TBI), both in humans and in animal models (Holmin et al., 1995; Oehmichen et al., 2009). Clinically relevant radiation therapy is also associated with stimulation of the innate immune system (Schaue and McBride); similar to LPS-induced inflammation, ionising irradiation induces an increase in TLR4/CD14 and expression of TNF-α (reviewed by: Schaue and McBride, 2010).
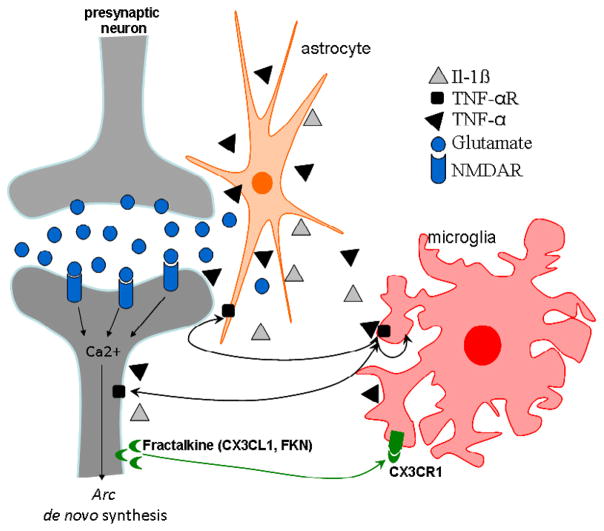
The mechanisms by which activated microglia contribute to neuronal dysfunction are not well understood and are still under study; however, altered communication between neurons and glia have been suggested as a possible mechanism (Neumann, 2001). Recent findings indicate that neurons are not simply passive targets of microglia; they are instead thought to control microglia activity (Biber et al., 2007), which suggests that dialogue between neurons and microglia is essential to the maintenance of physiologically normal neuronal functioning. The chemokine fractalkine (FKN; CX3CL1; neurotactin) is a neuronally derived signal, which has been shown to regulate the neurotoxic effects of microglia. Importantly, FKN binds and activates a single receptor, CX3CR1. In vivo, the FKN ligand is principally expressed on neurons, whereas CX3CR1 is found on microglia (Cardona et al., 2006). Disruption of FKN-CX3CR1 signalling has been shown to exacerbate neurotoxicity in animal models of many neuropathological disorders, including AD (Cardona et al., 2006). The FKN/CX3CR1 signalling pathway may mediate altered coupling of neuronal activity with macromolecular synthesis, which has been implicated in plasticity and memory during neuroinflammation (Fig. 1).
Figure 1.
Proposed mechanism for the regulation of communication among microglia, neurons and astrocytes during chronic neuroinflammation. In response to different CNS injuries, microglial cells are activated, and they release proinflammatory factors, such as TNF-α and IL-1β; this characteristic phenotype of classically activated microglia is called M1 (Michelucci et al., 2009). Consequently, these proinflammatory compounds influence the activity of surrounding astrocytes and neurons. This neuroinflammatory environment results in altered glutamate transmission and indirectly promotes neuronal damage by increasing glutamate. The immediate early gene Arc is expressed in response to synaptic activity in cortical and hippocampal glutamatergic neurons, and the elevated levels of glutamate found during inflammatory conditions may alter de novo synthesis of Arc. The chemokine fractalkine (FKN; CX3CL1; neurotactin) is a neuronally-derived ligand principally expressed on neurons, while the CX3CR1 receptor is found on microglia.
Arc, Learning and Memory
Memory formation is a temporally graded process, which requires transcription and translation in the first hours after acquisition (learning). The hippocampus is a brain region critical for the acquisition, consolidation and retrieval of declarative memories (for review see: (Eichenbaum, 2001; Squire, 1994). This process involves modulation of the strength and efficacy of synaptic signalling (i.e., synaptic plasticity), which in turn involves de novo gene expression (Deisseroth et al., 2003). Gene expression induced during learning produces proteins that alter the composition of networks and provides a mechanism for translating synaptic plasticity into changes in synaptic strength (memory). A number of activity-regulated genes have been identified for this function (Nedivi et al., 1993). While IEGs, such as c-fos and zif268, are also involved in mechanisms associated with the maintenance of memory (Guzowski et al., 2001b), Arc is the only known activity-induced gene that correlates both temporally and spatially with the stimulus that induced its transcription (Guzowski et al., 2001a) and that is essential for consolidation of synaptic plasticity and memory (Plath et al., 2006). Arc is expressed at very low levels in the brains of caged control animals. Following exploration of a novel environment, Arc is selectively expressed in glutamatergic neurons in the forebrain (Vazdarjanova et al., 2006), where its expression peaks around 30 minutes after behavioural induction (Ramirez-Amaya et al., 2005). Arc protein is found in the postsynaptic density within the NMDA receptor complex, and its induction requires NMDA receptor activation (Lyford et al., 1995; Steward and Worley, 2001). Shortly after its transcription, Arc mRNA is detected in the distal dendrites of recently activated synapses (Steward et al., 1998; Steward and Worley, 2001). Once translated, Arc mRNA and protein are usually rapidly degraded (for review see: Bramham et al., 2010). This process is dependent on time, place and amount of protein expressed and represents a specialised system able to mediate adaptive changes. Additionally, it requires a variety of post-transcriptional mechanisms that are not yet totally understood (Bramham et al., 2010).
Arc shows many unique and important characteristics, which make it a particularly attractive IEG gene to assess in the context of altered synaptic plasticity. First, Arc is rapidly activated by robust, patterned synaptic activity related to learning and memory behaviour (reviewed in: Guzowski, 2002). Arc KO mice are only impaired in hippocampus-dependent functions (Plath et al., 2006). The temporal dynamics of Arc expression in principal neurons are well established. Arc mRNA is induced in the nucleus of neurons within 5 minutes of novel environment exploration and can be visualised by fluorescence in situ hybridisation (FISH; Fig. 2A). It disappears from the nucleus within 10–15 minutes and rapidly moves to the cytoplasm and to the dendrites of activated synapses (Fig. 2B), where it is locally translated (Fig. 2D) (Guzowski et al., 1999). Arc protein plays a fundamental role in the maintenance of long-term potentiation (LTP) and spatial learning and memory processes (Guzowski, 2002; Guzowski et al., 2000; Steward et al., 1998). Given the established role of Arc in the maintenance of LTP, it has been used as a reliable marker for functional integration of newborn neurons into hippocampal networks (Kee et al., 2007; Ramirez-Amaya et al., 2006). Importantly, it has been reported that Arc is repeatedly induced in the same pyramidal neurons in the hippocampus during exploration of the same environment, a process than can be quantified using cellular-compartmental analysis of temporal activity with FISH (catFISH; Fig. 2C; Fig. 5) (Guzowski et al., 2001a; Vazdarjanova and Guzowski, 2004; Rosi et al., 2009). These latter findings are compatible with parallel cell recording experiments in the hippocampus of animals exposed to similar behavioural conditions (Wilson and McNaughton, 1993). The temporal and spatial characteristics of Arc expression corroborate neuronal activity profiles obtained using well-accepted electrophysiological recordings in different hippocampal subfields (Guzowski et al., 1999; Ramirez-Amaya et al., 2005; Rosi et al., 2005b; Rosi et al., 2006).
Figure 2.
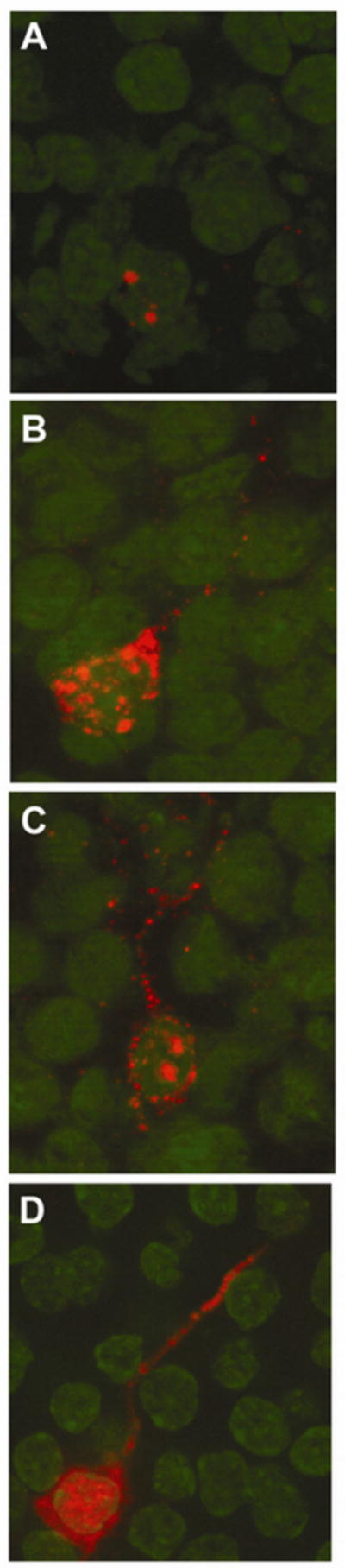
Qualitative characterisation of Arc mRNA and Arc protein expression in granule cell neurons of the DG, following exploration of a novel environment. Arc was induced by two 5 min behavioural explorations of a novel environment, which were separated by 25 min. Intranuclear foci of Arc mRNA induced by the second exploration (A), 5 min before tissue collection, and cytoplasmic Arc mRNA (B) induced by the first exploration, 30 min before tissue collection, were detected using fluorescent in situ hybridisation. Both nuclear foci (second exploration) and cytoplasmic Arc mRNA (first exploration) were seen in 90% of cells immunoreactive for Arc (C). Arc protein, induced by the first 5 min exploration, was detected 30 min later in the cytoplasm and dendrites. Digoxigenine-labelled Arc antisense probe was detected with CY3 (red, A, B, and C), and immunofluorescence was used to detect Arc protein (red, D). Cell nuclei were counterstained with FITC (green); magnification for all images, ×63.
Figure 5.
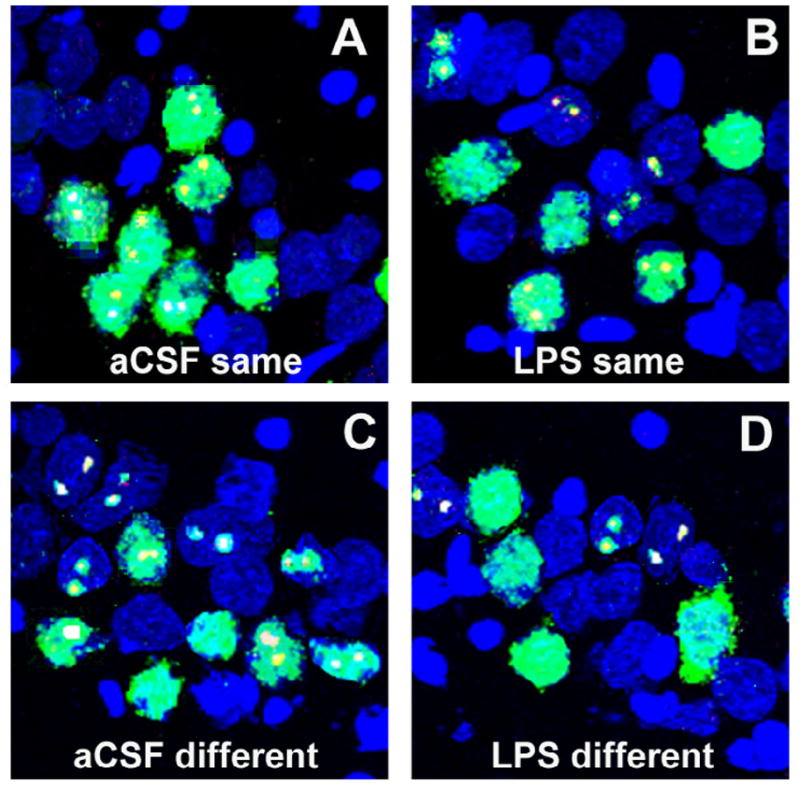
Samples of Arc catFISH images in area CA3. Confocal projection images showing Arc expression following exploration of either the same (A, C) or different (B, D) environment in control animals chronically infused with artificial cerebrospinal fluid (aCSF; A, B) or in an animal infused with lipopolysaccharide (LPS; C, D). Digoxigenin-labelled Arc-intronic antisense probe was detected with Cy3 (red). Fluorescein-labeled Arc antisense full probe was detected with FITC (green) and cell nuclei were counterstained with DAPI (blue). The nuclear Arc appears yellow as a result of the overlap of red digoxigenin and green FITC-labelled Arc. Control animals exposed to exploration of the same environment primarily had neurons stained for both nuclei (Arc-nuclei, from the second exploration) and cytoplasm (Arc-cyto, from the first exploration), with only a small number of neurons containing only Arc-cyto or Arc-foci (A). When, the aCSF-treated animals explored two different environments showed similar numbers of neurons stained for Arc-cyto only (from the first exploration), Arc-nuclei only (from the second exploration) or both (B). In contrast, animals from the LPS-treated group showed similar population of neurons stained with Arc-nuclei, Arc-cyto or both when exploring the same (C) or different environment (D). Scale bar = 50 mm.
The hippocampus is formed by several anatomically distinct subregions (DG, CA1 and CA3), and the functional relationships among them are essential for spatial learning and memory encoding. Importantly, there is considerable evidence for functional heterogeneity among the three subfields. The DG receives information from the entorhinal cortex and projects to the CA3 area, which then transmits the information to the CA1 region. In addition to the inputs from CA3, CA1 also receives direct input from the entorhinal cortex independent of the DG-CA3 circuit (for review see: Martin and Clark, 2007). Therefore, to better understand the mechanisms of altered cognition during neuroinflammatory conditions, it is important to separately analyse CA1, CA3 and DG regions. With the use of catFISH and immunohistochemistry to detect the cellular expression of Arc (Fig. 2), it is possible to independently study activation of the three different subregions at a cellular level. Therefore, Arc expression has been utilised extensively to map neuronal networks that underlie information processing and plasticity (Guzowski et al., 2001a; Ramirez-Amaya et al., 2005; Rosi et al., 2005b; Rosi et al., 2009; Vazdarjanova and Guzowski, 2004).
Inflammation and Arc
Investigating how the hippocampus processes episodic memory information during neuropathological conditions is important for understanding their influence on cognition. Given the specificity and the well-characterised dynamics of behaviourally-induced Arc expression and its critical role in synaptic plasticity and memory, Arc represents a unique marker for assessing how pathological conditions affect specific neuronal functions associated with cognitive performance.
Normally, Arc protein functions in a transient manner, and it has been proposed that sustained Arc expression may generate synaptic noise and thereby inhibit long-term memory formation (Guzowski et al., 2000). Supporting this prediction, observations on normal animals have indicated that abnormally elevated Arc is linked to slow learning (Kelly and Deadwyler, 2003). Subsequently, several studies have led to the conclusion that optimal and transient levels of Arc are necessary for appropriate hippocampal functioning. Abnormal increases (Lacor et al., 2004; Rosi et al., 2009; Rosi et al., 2005b; Rosi et al., 2006) or reductions (Frank et al., 2010; Hein et al., 2010; Rosi et al., 2008; Rosi et al., 2010) of behaviourally-induced Arc are associated with brain inflammation and result in altered hippocampus-dependent cognitive functions.
Increased Arc and inflammation
To define the effects of microglia activation on cognition, we previously investigated whether LPS-induced chronic neuroinflammation affects the overall hippocampal pattern of rapid de novo gene expression associated with learning and memory. Using a well-characterised animal model of chronic brain inflammation (Hauss-Wegrzyniak et al., 1998a; Hauss-Wegrzyniak et al., 1998b; Rosi et al., 2005a; Rosi et al., 2004; Rosi et al., 2005b; Rosi et al., 2006) produced by a long-lasting, slow infusion of LPS into the 4th ventricle of rats, we investigated the cellular changes within specific neuronal populations known to be vulnerable in brains of AD patients. Chronic LPS infusion resulted in hippocampus-dependent spatial memory deficits, detected by water maze testing (Rosi et al., 2006), along with a decrease in the number of NMDA receptors, which was not accompanied by neuronal loss (Rosi et al., 2004). By chronically infusing very small amounts of LPS (0.25 μg/h) into the 4th ventricle for 28 days, we observed that activated microglial cells, expressing major histocompatibility complex II (MHCII), alter the coupling of neural activity with de novo synthesis of Arc (Rosi et al., 2005b) (Fig. 3). When animals were twice allowed to explore a novel environment for 5 minutes (learning paradigm), separated by 30, 45 or 90 minute intervals, both transcription and translation of behaviourally-induced Arc were significantly altered by the presence of neuroinflammation (Rosi et al., 2005b; Rosi et al., 2006). The increases in behaviourally-induced Arc occurred selectively in hippocampal CA3 pyramidal and DG granule neurons, which are cell fields containing the highest number of activated microglia induced by LPS infusion (Rosi et al., 2005b) (Fig. 3A). This alteration was activity-dependent and occurred only in animals engaged in a learning paradigm; LPS-induced inflammation did not affect basal levels of Arc expression.
Figure 3.
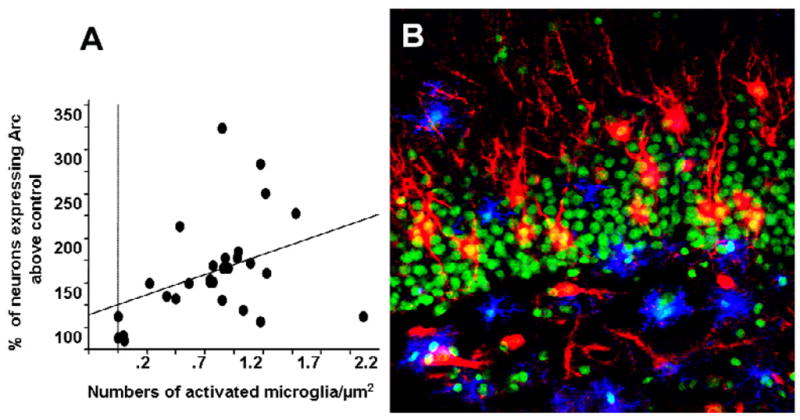
A. Significant correlation between the number of activated microglia cells and the percentage increase in the expression of behaviourally-induced Arc in LPS-treated rats above control animals. [Adapted from (Rosi et al., 2005b)]. B. Double immunohistochemical staining for Arc protein (red) and activated microglia (MHCII-positive cells, blue) within the DG of LPS-infused rats. Nuclei were counterstained with Sytox-Green. Scale bar = 100 μm.
In line with these reports of altered Arc, in vitro studies have shown that exposure of dissociated hippocampal neurons to neurologically active Aβ oligomers induces an increase in Arc expression (Lacor et al., 2004). This finding suggests that dysregulation of Arc expression may contribute to cognitive impairment and memory loss in AD.
Decreased Arc and inflammation
Activated MHCII-positive microglial cells are also found in the DG of old animals (Gavilan et al., 2007). Old animals show reduced numbers of neurons expressing Arc in the DG (Small et al., 2004). When challenged with a peripheral immune stimulus that further stimulates the innate immune system, old animals show dramatic reductions in the levels of Arc together with significantly impaired cognition (Frank et al., 2010). These data suggest that stimulation of the innate immune system plays, through central proinflammatory cytokines, a salient role in the changes of Arc and impairment of cognitive functions.
MHCII antigens are upregulated in microglia both in experimental models of TBI and in human TBI (Holmin et al., 1995; Oehmichen et al., 2009). Traumatic brain injury results in both acute and chronic disruption of cognitive processes, which may be mediated through a disruption of hippocampal circuitry and activation of the innate immune system (Dixon et al., 1999; Pierce et al., 1998; Rosi et al., 2010). Long-term effects TBI result in a significant reduction in the number of neurons expressing behaviourally-induced Arc in the DG in hemispheres both ipsilateral and contralateral to the trauma (Rosi et al., 2010). The reduction of Arc expression after trauma could explain the inability of animals to properly recall the location of the platform during the probe trial of the water maze test (impaired consolidation of long term memory) (Rosi et al., 2010).
Therapeutic cranial irradiation, commonly used to treat brain tumours, induces long-lasting cognitive impairments that affect the quality of life (Butler et al., 2006; Meyers and Brown, 2006). Animal models of therapeutic irradiation show elevated hippocampal neuroinflammation (Rosi et al., 2008), altered hippocampus-dependent cognitive functions (Rola et al., 2004) and a significant reduction in the number of neurons expressing behaviourally-induced Arc in the DG (Rosi et al., 2008). The changes in Arc were only observed in animals engaged in a leaning paradigm, but not in animals resting in the home cage, and developed only a long time after radiation exposure (Rosi et al., 2008). These studies show that there is a time-dependent effect on the changes in Arc expression and the development of neuroinflammation. One week after radiation exposure, only changes in the translation of Arc were observed, but no changes in the translation machinery were observed. This effect was accompanied by slight changes in the number of activated microglial cells. In contrast to what has been seen one week after irradiation, two months after radiation exposure, both transcription and translation of behaviourally-induced Arc in the DG were altered when the animals were exposed to a learning experience. At this time, elevated numbers of microglial cells were observed in the DG (Rosi et al., 2008).
Overexpression of the proinflammatory cytokine IL-1β in a transgenic mouse model has been associated with chronic, elevated microglia activation and high levels of MHCII in the hippocampus (Hein et al., 2010). These animals show impaired contextual and spatial memory, together with significant reduction of behaviourally-induced Arc levels. These data further suggest that changes in the expression of behaviourally-induced Arc are a good cellular monitor of cognition. The changes detected in IL-1β transgenic mice, however, reflect the overall hippocampal levels of Arc and do not isolate possible regional differences. Immunohistochemical analysis of behaviourally-induced Arc has the advantage of mapping recently activated neurons and networks of neurons at the cellular level and allows for the delineation of differences across different hippocampal subfields (Rosi et al., 2009; Rosi et al., 2005b; Small et al., 2004).
Interestingly, reduced expression of the plasticity-related Arc, as a consequence of chronically activated microglia, has also been observed outside the hippocampus (Centonze et al., 2009). This observation further suggests that the effect of activated microglia upon synaptic plasticity is not region-specific (i.e., the hippocampus), and the mechanisms involved in alteration of synaptic plasticity during neuroinflammation may be common to different brain regions.
Reconciling increases and decreases of Arc in the DG
Although the percentage of Arc-expressing neurons in response to a behavioural paradigm in the DG is low (<5%) (Ramirez-Amaya et al., 2005; Rosi et al., 2005a), this percentage represent a relatively high number of neurons given the total number of granule cells. The maintenance of a small fraction of active Arc during a behavioural experience is critical for proper hippocampal functioning and is consistent with electrophysiological recordings showing sparse activity in the DG during behaviour (Jung and McNaughton, 1993) and with the principle of sparse distributed coding (McNaughton, 1987). This principle suggests that the maximal efficient storage/function requires only a fraction of the total number of neurons in the DG (McNaughton et al., 1996). Therefore, a modest change in the number of cells expressing Arc in the DG may be sufficient to disrupt the finely regulated sparse coding and thereby decrease the memory ability of the system. Together these data suggest that an optimal level of Arc needs to be expressed in response to a behavioural experience, and excessive increases or reductions would alter the principle of the sparse coding needed for the storage of non-overlapping information.
Neurogenesis, Arc and inflammation
The mechanisms underlying the cognitive impairments associated with inflammatory conditions are likely to be multifactorial, but one important possibility involves the process of hippocampal neurogenesis. Evidence supports the role of adult neurogenesis in neural plasticity underlying animal cognition. Neurogenesis in the DG has been shown to be required for hippocampus-dependent memory functions (Dupret et al., 2008; Imayoshi et al., 2008), and the number of newborn granule cells has been shown to correlate with hippocampus-dependent memory (Shors et al., 2001). Moreover, newborn neurons develop synaptic responsiveness and electrophysiological properties similar to those of existing granule cells (Song et al., 2002; van Praag et al., 2002).
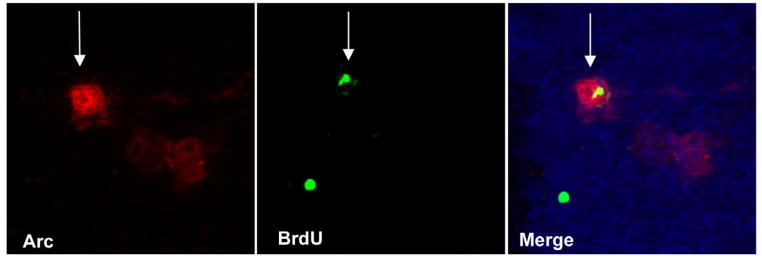
Inflammation and microglia activation in the DG have been shown to be detrimental to the survival of new and mature granule cells during the early stage of their formation (Monje and Palmer, 2003). The increased excitatory drive in new and mature granule cells is attributable to an increase in network activity in hippocampal neural circuits caused by inflammation (Jakubs et al., 2008). Despite the constant formation of newborn neurons, only a small percentage of these survive and functionally integrate into hippocampal networks (Zhao et al., 2006). However, the mere number of newborn neurons is not an appropriate predictor for functioning; increased neurogenesis has been reported in animal models of epilepsy, stroke, trauma, Alzheimer’s disease, Parkinson’s disease and Huntington’s disease (Parent, 2003). Given the established role of Arc in synaptic plasticity, Arc expression can be used as a reliable marker for integration of newborn neurons in relevant hippocampal networks (Fig. 4). Using this approach, it has been demonstrated that during a learning experience, Arc is preferentially induced in newborn neurons (Ramirez-Amaya et al., 2006). Inflammation regulates functional integration of these newborn neurons (Jakubs et al., 2008). Although there is an elevated number of newborn neurons after traumatic brain injury, there is no evidence for integration of these neurons into behaviourally relevant networks (Rosi et al., 2010). Consistent with the hypothesis that integration of newborn neurons is necessary for hippocampus-dependent memory formation, these animals show reduced Arc expression and impaired cognition (Rosi et al., 2010). LPS-induced chronic neuroinflammation significantly affects the functional integration of the newborn neurons into relevant hippocampal networks (Belarbi K, Rosi S, unpublished observations).
Figure 4.
Representative image of a newborn (BrdU+, FITC, green) neuron (NeuN+, CY5, blue) that is functionally integrated (Arc +, CY3, red) into the granule cell layer of the dentate gyrus. Arc expression was induced by exploration of a novel environment.
Hippocampal circuits and activated microglia
Memory is a network phenomenon encoded by ensembles of neurons within specific brain areas, among which the hippocampus plays a crucial role. Hippocampal network function is essential for discrimination and retrieval of information and enables effective navigational behaviour. Given the correspondence in circuit dynamics between electrophysiological recordings and measurements of Arc, Arc expression can be used as a reliable monitor of cellular activity, reflecting spatial and contextual information processing (Guzowski et al., 1999). Using the immediate early gene-based brain-imaging method called catFISH, it is possible to detect primary transcripts at genomic alleles (Fig. 2A,C). This technique provides exceptional temporal and cellular resolution to map the activity history of an individual neuron (Fig. 2), the spatial distribution of thousands of activated neurons and the visualisation of neuronal ensembles activated by distinct behavioural experiences (Fig. 5)(Guzowski et al., 1999; Rosi et al., 2009; Vazdarjanova and Guzowski, 2004). When rodents are exposed to the same spatial learning environment twice, Arc transcription from each experience occurs predominantly in a single population of neurons (Fig. 5A). By contrast, when animals are exposed to two different environments, Arc transcription occurs in statistically-independent neural populations (Fig. 5C) (Guzowski et al., 1999; Rosi et al., 2009). catFISH provides cellular and temporal resolution for monitoring not only the proportion of neurons activated by an experience but also the degree of overlap of ensembles for similar or different experiences (Guzowski et al., 1999; Rosi et al., 2009; Vazdarjanova and Guzowski, 2004). Both electrophysiological and Arc catFISH studies have shown overlapping CA1 and CA3 neuronal populations after two consecutive explorations of the same environment (pattern completion) (Fig. 5A). After exploration of two distinct environments, the neuronal populations engaged in CA1 and CA3 have a low degree of overlap (pattern separation) (Fig. 5C). After a given behavioural experience, the CA3 hippocampal neurons are able to form stable and independent neural representations, also called spatial maps (Leutgeb et al., 2004). LPS-induced chronic neuroinflammation disrupts the ability of the CA3 area to form these stable spatial maps. Using the catFISH imaging technique, we demonstrated that during chronic neuroinflammation, the CA3 networks show disrupted ability to encode spatial information (Fig. 5B, D) and CA1 neurons can work independently of CA3 (Rosi et al., 2009). Furthermore, the size of the neuronal populations activated by a learning experience in the CA3 area is significantly higher than the size of neuronal populations activated in control animals. The reduced sparsity of CA3 networks, which refers to an abnormal number of neurons that are inappropriately activated in response to an experience, may explain the cognitive impairment induced by chronic treatment with LPS (Rosi et al., 2006). By studying hippocampal network activity, we have demonstrated that neuroinflammation not only affects the sparsity of the CA3 networks necessary to recognise similar environments, but also leads to the inability of neurons to discriminate between different environments (pattern separation) (Fig. 5B, D) (Rosi et al., 2009). The altered pattern separation induced by LPS in the CA3 area may impair the normal ability of the system to discriminate between two different environments and to ignore differences in an environment, enabling the recollection of the environment as familiar. Therefore LPS-induced chronic neuroinflammation reduces the reliability of information processing in hippocampal networks; this justifies the inability of the animals to properly solve the Morris water maze task (Rosi et al., 2006). These results are similar in some respects to changes observed in old animals (Barnes et al., 1997) that are unable to accomplish pattern completion and separation (Burke and Barnes, 2006). Alterations in the levels of Arc expression have been correlated with cognitive impairment during aging (Blalock et al., 2003) and with amyloid deposition in AD transgenic mice (Dickey et al., 2004). Interestingly, activation of microglia cells has been associated with aging (Gavilan et al., 2007) in a similar pattern to that observed during chronic LPS infusion (Rosi et al., 2005b). Increases in MHCII gene expression (activated microglia) have been reported during the early stages of AD and in the hippocampus of subjects with mild dementia (Parachikova et al., 2007). Importantly, in these subjects, the expression of MHCII has been found to inversely correlate with cognitive functions (Parachikova et al., 2007). While the changes in normal aging and the early stages of AD are distinct from those observed during LPS-induced chronic neuroinflammation, they illustrate how, in the presence of activated microglia, changes in network functions can contribute to changes in information processing, resulting in cognitive deficits.
Neuroinflammation and transcriptional regulation and translation of Arc
The changes in behaviourally-induced Arc during inflammatory conditions could result from 1) a failure in its transcriptional regulation and/or 2) failures in post-transcriptional mechanisms that control the amount of synthesis in response to synaptic input and/or 3) altered degradation of Arc after its induction.
Transcriptional regulation of Arc is activity-dependent and requires NMDAR activation, extracellular signal-regulated kinase (ERK) (Steward et al., 1998; Steward and Worley, 2001) and elevation of intracellular Ca2+ and cAMP (Waltereit et al., 2001). During inflammation, the chronic increase in proinflammatory cytokines (TNF-α and IL-1β) (Brown and Bal-Price, 2003 Bodles, 2004 #1393) leads to a cascade of self-propagating cellular events (Emerit et al., 2004; Griffin et al., 1998), including a blockade of glutamate uptake by glia (Robinson et al., 1993), enhanced release of glutamate from astrocytes (Bezzi et al., 2001) and disruption of normal physiological activity within the hippocampus (Angulo et al., 2004). Elevated levels of glutamate may act on non-NMDA type glutamate receptors to cause chronic membrane depolarisation, which would partially relieve voltage-dependent Mg2+ block at NMDA receptors. Subsequent activation of NMDA receptors by ordinary glutamatergic synaptic activity may thus permit a continuous influx of calcium ions into neurons, theoretically overwhelming the endogenous mechanisms that regulate calcium ion homeostasis. This scenario is known as the “weak excitotoxicity” model (Albin and Greenamyre, 1992). Inflammation can also relieve the Mg2+ blockade of voltage-gated NMDA channels by increasing nitric oxide levels and continued membrane depolarisation (Brown and Bal-Price, 2003; Emerit et al., 2004; Willard et al., 2000). As Arc expression is NMDA receptor-dependent (Steward and Worley, 2001), elevated intracellular levels of calcium induced by elevated cytokines (Bodles and Barger, 2004; Viviani et al., 2003) may affect the transcription of Arc and result in its induction in a larger than usual number of neurons.
In support of this hypothesis, we demonstrated that partial antagonism of NMDARs, using the low affinity uncompetitive NMDAR antagonist memantine, was able to restore behaviourally-induced Arc expression to control levels, without affecting its basal expression (Rosi et al., 2006). These results are consistent with the hypothesis that over-activation of NMDAR channels and an increase in calcium influx contributed to the alterations in inflammation-induced Arc at the transcriptional and post-transcriptional levels (Rosi et al., 2009; Rosi et al., 2006) (Fig. 1).
The dysregulation of calcium ion influx due to the presence of chronic brain inflammation may have significant consequences on neuronal-glia cross-talk and survival (Akiyama et al., 2000; LaFerla, 2002) as well as gene expression (Toescu et al., 2004). Memantine treatment was also able to indirectly reduce the activation of microglia and therefore improve neuronal-microglia communication (Rosi et al., 2009).
Arc is involved in trafficking of AMPA glutamate receptors (Chowdhury et al., 2006), and AMPA receptors regulate the transcription of Arc (Rao et al., 2006). NMDA receptors initiate signalling pathways that modulate the number of AMPA receptors at the cell surface to produce short-term changes in synaptic strength. NMDA and AMPA receptors regulate long-term structural plasticity at the level of gene expression (Rao and Finkbeiner, 2007). On the basis of these data, it was proposed that during synaptic plasticity, changes in the ratio of NMDA/AMPA receptors may enhance the negative feedback control of Arc and thus influence Arc transcription (Rao et al., 2006). A dynamic interplay between AMPA receptors and Arc is necessary to maintain optimal functionality (Rao and Finkbeiner, 2007). TNF-α regulates AMPA receptor trafficking (Stellwagen and Malenka, 2006); therefore, prolonged increases in TNF-α levels, such as those seen during chronic neuroinflammation, may perturb this dynamic interplay and affect Arc expression.
Decreased Arc expression may also interfere with intracellular trafficking and may impair Arc translation at dendritic level. At this level, Arc is regulated by synaptic signals (Dong et al., 2003; Yin et al., 2002). These effects could also be due to alterations in the turnover and/or translation of regulatory RNA-binding proteins (Pullmann et al., 2007) or faster Arc degradation caused by proinflammatory-induced effects (Rosi et al., 2008).
In vitro studies have shown that Arc regulation is translation dependent. Arc is a physiological target for the process known as nonsense-mediated RNA decay (NMD) (Giorgi et al., 2007; Peebles and Finkbeiner, 2007). NMD serves as control mechanism for the rapid elimination of aberrant RNA (Giorgi et al., 2007), and through this mechanism, NMD strictly limits Arc synthesis. The rapid degradation of Arc is a distinctive feature of its effective maintenance of LTP, and if this process were altered, uncontrolled over-expression and/or a reduced degradation of Arc could take place, as seen during the inflammatory conditions described above. How this process is mediated by proinflammatory conditions is still unclear, but it represents an important target for therapeutic intervention.
Summary and Conclusions
In summary, in vivo studies have shown that, during brain inflammation, changes in hippocampus-dependent memory functions consistently correlate with the expression of plasticity-related, behaviourally-induced, IEG Arc. The changes in expression of behaviourally-induced Arc, described during different inflammatory conditions, further illustrate the strong link between activation of the innate immune system and altered synaptic plasticity and support the use of Arc as reliable marker to study altered hippocampal circuitry in vivo. Given that abnormal changes in Arc are only seen in response to a learning paradigm, neuroinflammation seems to affect only the activity-dependent de novo expression of the gene (Fig. 1). While the mechanisms of these changes are not clear, the studies reviewed here demonstrate that overexpression or reduction of Arc impairs the synaptic plasticity required for memory formation and that optimal levels of Arc are necessary for proper memory processes (Fig. 6).
Figure 6.
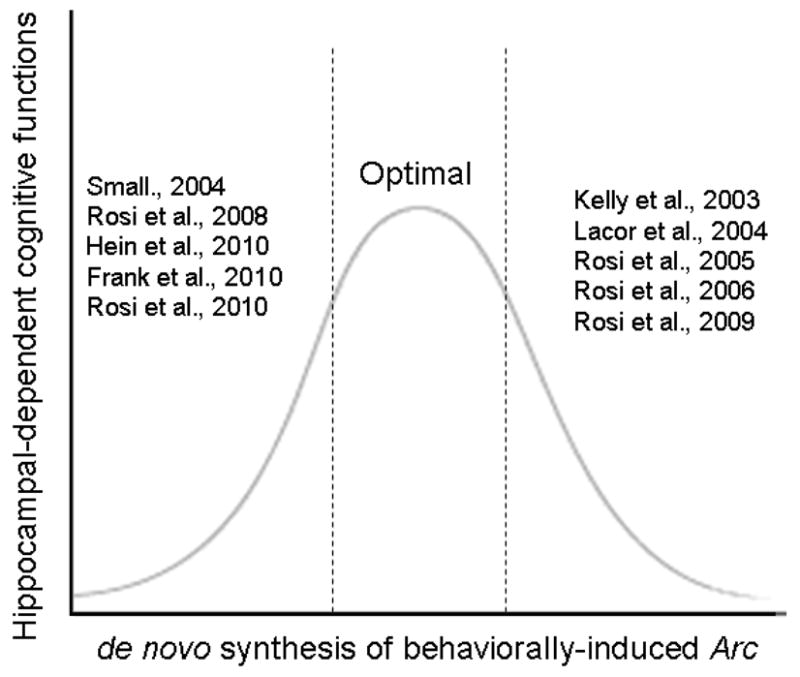
Model summarising in vivo studies reporting reduced or increased Arc expression with decreased cognitive functions. Together these studies suggest that there is an optimal level of Arc expression in response to a learning paradigm, and changes in Arc expression may affect cognitive functions.
Acknowledgments
Grant Sponsor: Alzheimer’s Association, NIH
Grant Number: NIRG 08-90589, R01 CA133216
We would like to thank Professor Giancarlo Pepeu and Doctor Karim Belarbi for their critical feedback on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Tanaka R, Sato M, Taked N. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol Med Chir (Tokyo) 2001;41:590–598. doi: 10.2176/nmc.41.590. [DOI] [PubMed] [Google Scholar]
- Albin RL, Greenamyre JT. Alternative excitotoxic hypotheses. Neurology. 1992;42(4):733–8. doi: 10.1212/wnl.42.4.733. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S, Audinat E. Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24(31):6920–7. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123 ( Pt 11):2321–37. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76(3):846–54. doi: 10.1046/j.1471-4159.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388(6639):272–5. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Bernardino L, Xapelli S, Silva AP, Jakobsen B, Poulsen FR, Oliveira CR, Vezzani A, Malva JO, Zimmer J. Modulator effects of interleukin-1beta and tumor necrosis factor-alpha on AMPA-induced excitotoxicity in mouse organotypic hippocampal slice cultures. J Neurosci. 2005;25(29):6734–44. doi: 10.1523/JNEUROSCI.1510-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4(7):702–10. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30(11):596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23(9):3807–19. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodles AM, Barger SW. Cytokines and the aging brain - what we don’t know might help us. Trends Neurosci. 2004;27(10):621–6. doi: 10.1016/j.tins.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200(2):125–40. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Bal-Price A. Inflammatory neurodegeneration mediated by nitric oxide, glutamate, and mitochondria. Mol Neurobiol. 2003;27(3):325–55. doi: 10.1385/MN:27:3:325. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7(6):517–23. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, Musella A, D’Amelio M, Cavallucci V, Martorana A, et al. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci. 2009;29(11):3442–52. doi: 10.1523/JNEUROSCI.5804-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52(3):445–59. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Mermelstein PG, Xia H, Tsien RW. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr Opin Neurobiol. 2003;13(3):354–65. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Gordon MN, Mason JE, Wilson NJ, Diamond DM, Guzowski JF, Morgan D. Amyloid suppresses induction of genes critical for memory consolidation in APP + PS1 transgenic mice. J Neurochem. 2004;88(2):434–42. doi: 10.1111/j.1471-4159.2004.02185.x. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Kochanek PM, Yan HQ, Schiding JK, Griffith RG, Baum E, Marion DW, DeKosky ST. One-year study of spatial memory performance, brain morphology, and cholinergic markers after moderate controlled cortical impact in rats. J Neurotrauma. 1999;16(2):109–22. doi: 10.1089/neu.1999.16.109. [DOI] [PubMed] [Google Scholar]
- Dong E, Caruncho H, Liu WS, Smalheiser NR, Grayson DR, Costa E, Guidotti A. A reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes. Proc Natl Acad Sci U S A. 2003;100(9):5479–84. doi: 10.1073/pnas.1031602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3(4):e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 2001;127(1–2):199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58(1):39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Hein AM, Biedenkapp JC, Watkins LR, Maier SF. IL-1RA blocks E. coli-induced suppression of Arc and long-term memory in aged F344xBN F1 rats. Brain Behav Immun. 2010;24(2):254–62. doi: 10.1016/j.bbi.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavilan MP, Revilla E, Pintado C, Castano A, Vizuete ML, Moreno-Gonzalez I, Baglietto-Vargas D, Sanchez-Varo R, Vitorica J, Gutierrez A, et al. Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J Neurochem. 2007;103(3):984–96. doi: 10.1111/j.1471-4159.2007.04787.x. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130(1):179–91. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Royston MC, Gentleman SM, McKenzie JE, Graham DI, Roberts GW, Mrak RE. Glial-neuronal interactions in Alzheimer’s disease: the potential role of a ‘cytokine cycle’ in disease progression. Brain Pathol. 1998;8(1):65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12(1):86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20(11):3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2(12):1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Imaging neural activity with temporal and cellular resolution using FISH. Curr Opin Neurobiol. 2001a;11(5):579–84. doi: 10.1016/s0959-4388(00)00252-x. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001b;21(14):5089–98. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Dobrzanski P, Stoehr JD, Wenk GL. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998a;780(2):294–303. doi: 10.1016/s0006-8993(97)01215-8. [DOI] [PubMed] [Google Scholar]
- Hauss-Wegrzyniak B, Lukovic L, Bigaud M, Stoeckel ME. Brain inflammatory response induced by intracerebroventricular infusion of lipopolysaccharide: an immunohistochemical study. Brain Res. 1998b;794(2):211–24. doi: 10.1016/s0006-8993(98)00227-3. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacol. 2010:1–12. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O’Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain Behav Immun. 2010;24(2):243–53. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmin S, Mathiesen T, Shetye J, Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta Neurochir (Wien) 1995;132(1–3):110–9. doi: 10.1007/BF01404857. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–61. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jakubs K, Bonde S, Iosif RE, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Inflammation regulates functional integration of neurons born in adult brain. J Neurosci. 2008;28(47):12477–88. doi: 10.1523/JNEUROSCI.3240-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–82. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Katsuura G, Gottschall PE, Dahl RR, Arimura A. Interleukin-1 beta increases prostaglandin E2 in rat astrocyte cultures: modulatory effect of neuropeptides. Endocrinology. 1989;124(6):3125–7. doi: 10.1210/endo-124-6-3125. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007 doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Experience-dependent regulation of the immediate-early gene arc differs across brain regions. J Neurosci. 2003;23(16):6443–51. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24(45):10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat Rev Neurosci. 2002;3(11):862–72. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- Lee PR, Cohen JE, Becker KG, Fields RD. Gene expression in the conversion of early-phase to late-phase long-term potentiation. Ann N Y Acad Sci. 2005;1048:259–71. doi: 10.1196/annals.1342.023. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100(14):8514–9. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–8. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Lucas M, Frenois F, Vouillac C, Stinus L, Cador M, Le Moine C. Reactivity and plasticity in the amygdala nuclei during opiate withdrawal conditioning: differential expression of c-fos and arc immediate early genes. Neuroscience. 2008;154(3):1021–33. doi: 10.1016/j.neuroscience.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14(2):433–45. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Clark RE. The rodent hippocampus and spatial memory: from synapses to systems. Cell Mol Life Sci. 2007;64(4):401–31. doi: 10.1007/s00018-007-6336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW. Neuropsychiatric sequelae of head injuries. Psychiatr Clin North Am. 1992;15(2):395–413. [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol. 1998;33(5):371–8. doi: 10.1016/s0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, et al. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199(Pt 1):173–85. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- McNaughton BLMR. Hippocampal synaptic enhancement and information storage within distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–9. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Geara F, Wong PF, Morrison WH. Neurocognitive effects of therapeutic irradiation for base of skull tumors. Int J Radiat Oncol Biol Phys. 2000;46(1):51–5. doi: 10.1016/s0360-3016(99)00376-4. [DOI] [PubMed] [Google Scholar]
- Mhatre M, Floyd RA, Hensley K. Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: common links and potential therapeutic targets. J Alzheimers Dis. 2004;6(2):147–57. doi: 10.3233/jad-2004-6206. [DOI] [PubMed] [Google Scholar]
- Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol. 2009;210(1–2):3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16(2):129–34. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossmann T. Role of cerebral inflammation after traumatic brain injury: a revisited concept. Shock. 2001;16(3):165–77. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Murasugi T, Oda T. Acute neuroinflammation exacerbates excitotoxicity in rat hippocampus in vivo. Exp Neurol. 2002;177(1):95–104. doi: 10.1006/exnr.2002.7991. [DOI] [PubMed] [Google Scholar]
- Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363(6431):718–22. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- Neumann H. Control of glial immune function by neurons. Glia. 2001;36(2):191–9. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- Oehmichen M, Jakob S, Mann S, Saternus KS, Pedal I, Meissner C. Macrophage subsets in mechanical brain injury (MBI)--a contribution to timing of MBI based on immunohistochemical methods: a pilot study. Leg Med (Tokyo) 2009;11(3):118–24. doi: 10.1016/j.legalmed.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008;28(10):2589–600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parachikova A, Agadjanyan MG, Cribbs DH, Blurton-Jones M, Perreau V, Rogers J, Beach TG, Cotman CW. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol Aging. 2007;28(12):1821–33. doi: 10.1016/j.neurobiolaging.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9(4):261–72. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Peebles CL, Finkbeiner S. RNA decay back in play. Nat Neurosci. 2007;10(9):1083–4. doi: 10.1038/nn0907-1083. [DOI] [PubMed] [Google Scholar]
- Perez-Capote K, Serratosa J, Sola C. Excitotoxic and apoptotic neuronal death induce different patterns of glial activation in vitro. J Neurochem. 2005;94(1):226–37. doi: 10.1111/j.1471-4159.2005.03183.x. [DOI] [PubMed] [Google Scholar]
- Pierce JE, Smith DH, Trojanowski JQ, McIntosh TK. Enduring cognitive, neurobehavioral and histopathological changes persist for up to one year following severe experimental brain injury in rats. Neuroscience. 1998;87(2):359–69. doi: 10.1016/s0306-4522(98)00142-0. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52(3):437–44. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Pullmann R, Jr, Kim HH, Abdelmohsen K, Lal A, Martindale JL, Yang X, Gorospe M. Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol Cell Biol. 2007;27(18):6265–78. doi: 10.1128/MCB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26(47):12237–41. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25(7):1761–8. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30(6):284–91. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci. 2006;9(7):887–95. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52(3):461–74. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9(6):429–39. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Djali S, Buchhalter JR. Inhibition of glutamate uptake with L-trans-pyrrolidine-2,4-dicarboxylate potentiates glutamate toxicity in primary hippocampal cultures. J Neurochem. 1993;61(6):2099–103. doi: 10.1111/j.1471-4159.1993.tb07447.x. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impariment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. Cranial irradiation alters the behaviorally induced immediate-early gene arc (activity-regulated cytoskeleton-associated protein) Cancer Res. 2008;68(23):9763–70. doi: 10.1158/0008-5472.CAN-08-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Belarbi K, Ferguson RA, Fishman K, Obenaus A, Raber J, Fike JR. Trauma-induced alterations in cognition and arc expression are reduced by previous exposure to (56)Fe irradiation. Hippocampus. 2010 doi: 10.1002/hipo.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Pert CB, Ruff MR, McGann-Gramling K, Wenk GL. Chemokine receptor 5 antagonist D-Ala-peptide T-amide reduces microglia and astrocyte activation within the hippocampus in a neuroinflammatory rat model of Alzheimer’s disease. Neuroscience. 2005a;134(2):671–6. doi: 10.1016/j.neuroscience.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Hauss-Wegrzyniak B, Wenk GL. Chronic brain inflammation leads to a decline in hippocampal NMDA-R1 receptors. J Neuroinflammation. 2004;1(1):12. doi: 10.1186/1742-2094-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, Fike JR, Wenk GL, Barnes CA. Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain. 2009;132(Pt 9):2464–77. doi: 10.1093/brain/awp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Vazdarjanova A, Worley PF, Barnes CA, Wenk GL. Neuroinflammation alters the hippocampal pattern of behaviorally induced Arc expression. J Neurosci. 2005b;25(3):723–31. doi: 10.1523/JNEUROSCI.4469-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Vazdarjanova A, Ramirez-Amaya V, Worley PF, Barnes CA, Wenk GL. Memantine protects against LPS-induced neuroinflammation, restores behaviorally-induced gene expression and spatial learning in the rat. Neuroscience. 2006;142(4):1303–15. doi: 10.1016/j.neuroscience.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Cytokines and acute neurodegeneration. Mol Psychiatry. 1997;2(2):120–1. doi: 10.1038/sj.mp.4000223. [DOI] [PubMed] [Google Scholar]
- Schaue D, McBride WH. Links between innate immunity and normal tissue radiobiology. Radiat Res. 2010;173(4):406–17. doi: 10.1667/RR1931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52(3):475–84. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–6. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101(18):7181–6. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5(5):438–45. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- Squire LRKBJ. Memory, hippocampus, and brain systmes. In: Gazzaniga M, editor. The Cognitive Neurosciences. Cambridge, MA: MIT; 1994. pp. 825–837. [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21(4):741–51. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30(1):227–40. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27(10):614–20. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–4. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24(29):6489–96. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498(3):317–29. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Vilhardt F. Microglia: phagocyte and glia cell. Int J Biochem Cell Biol. 2005;37(1):17–21. doi: 10.1016/j.biocel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23(25):8692–700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci. 2001;21(15):5484–93. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59(1):84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard LB, Hauss-Wegrzyniak B, Danysz W, Wenk GL. The cytotoxicity of chronic neuroinflammation upon basal forebrain cholinergic neurons of rats can be attenuated by glutamatergic antagonism or cyclooxygenase-2 inhibition. Exp Brain Res. 2000;134(1):58–65. doi: 10.1007/s002210000446. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261(5124):1055–8. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci U S A. 2002;99(4):2368–73. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]