Abstract
Accumulating evidence indicates that the adolescent hippocampus is highly susceptible to alcohol-induced structural damage and behavioral deficits. Microglia are vitally important brain constituents needed to support and maintain proper neural function; however, alcohol’s effects on microglia have only recently gained attention. The microglial response to alcohol during adolescence has yet to be studied; therefore, we examined hippocampal microglial activation in an adolescence binge alcohol exposure model. Adolescent male Sprague-Dawley rats were administered ethanol 3 times/day for 4 days and were sacrificed 2, 7, and 30 days later. Bromo-deoxy-Uridine was injected 2 days after ethanol exposure to label dividing cells. Microglia morphology was scored using the microglia marker Iba-1, while the extent of microglial activation was examined with ED-1, major histocompatability complex-II (MHC-II), and tumor necrosis factor (TNF)-α expression. Ethanol induced significant morphological change in hippocampal microglia, consistent with activation. In addition, ethanol increased the number of BrdU+ cells throughout all regions of the hippocampus 2 days after the last dose. Confocal microscopy showed that the proliferating BrdU+ cells in each region were Iba-1+ microglia. Importantly, newly born microglia survived and retained their morphological characteristics 30 days after ethanol exposure. Ethanol did not alter hippocampal ED-1, MHC-II, or TNF-α expression, suggesting that a single period of binge ethanol exposure does not induce a full microglial-driven neuroinflammatory response. These results establish that ethanol triggers partial microglial activation in the adolescent hippocampus that persists through early adulthood, suggesting that alcohol exposure during this unique developmental time period has long-lasting consequences.
Keywords: Adolescence, Alcoholism, Ethanol, Hippocampus, Microglial Activation, Neurodegeneration, Neuroinflammation
INTRODUCTION
The relationship between excessive alcohol consumption during adolescence and the increased tendency to develop an alcohol use disorder as an adult has led to intense focus on understanding how alcohol interacts with the brain during this unique developmental period (Grant and Dawson, 1997). Evidence suggests that adolescents are highly sensitive to alcohol’s rewarding and memory impairing effects (Doremus-Fitzwater et al., 2010), while they display reduced sensitivity to its motor impairing and sedative effects (Little et al., 1996), a combination hypothesized to drive excessive alcohol consumption (Nixon and McClain, 2010; Spear and Varlinskaya, 2005). Several lines of evidence suggest that the adolescent hippocampus is particularly vulnerable to alcohol-induced impairments and damage (Crews et al., 2000; White and Swartzwelder, 2005). Reports consistently show that excessive alcohol consumption during adolescence reduces hippocampal volume and interferes with hippocampal-dependent learning and memory function (De Bellis et al., 2000; Nagel et al., 2005; Schweinsburg et al., 2010; Sircar et al., 2009; Weitemier and Ryabinin, 2003). In addition, hippocampal neurogenesis, a process that continues throughout life and contributes significantly to structural and functional aspects of the hippocampus, is inhibited by alcohol intoxication (Crews et al., 2006c; Morris et al., 2010; Nixon and Crews, 2002).
Recent hypotheses contend that alcohol-induced brain damage may result from a neuroinflammatory response within the hippocampus involving proinflammatory cytokines, cyclooxygenase-2 (Cox-2), and inducible nitric oxide synthase (iNOS) upregulation (Crews et al., 2006a; Knapp and Crews, 1999; Pascual et al., 2009; Qin et al., 2008; Zou and Crews, 2010). Microglia, the brain’s resident immune cells, have been identified as significant sources of cytokines and other immune modulators that participate in neuroinflammatory reactions involved in brain injury and neurodegenerative diseases (Block et al., 2007). Under normal conditions, microglia have a highly ramified morphology and display a high degree of plasticity that allows them to continuously sample their surrounding environment and quickly react to even the smallest homeostatic perturbations (Nimmerjahn et al., 2005). Once microglia detect injury, they undergo an activation response that involves morphological transformation, cytokine production, and upregulation of cell surface receptors (Streit and Xue, 2009). Microglial activation may occur in a graded process, such that different activation states exist depending on the injury type, severity, and location (Kreutzberg, 1996; Streit and Xue, 2009). The level of activation achieved and the cytokines and other mediators produced likely influence whether microglia exacerbate injury or promote recovery.
The effects of alcohol on microglia are poorly understood. Postmortem studies show that a microglial reaction occurs in the brain of chronic alcoholics as characterized by increased expression Iba-1 and GluT5, two microglia-specific markers (He and Crews, 2007). Although some hallmarks of activation such as microglial proliferation, increased CD11b and observable major histocompatibility complex (MHC)-II expression have been described in rodent models of alcohol abuse, full phagocytic microglial activation has not been reported in vivo (Crews et al., 2006b; Fernandez-Lizarbe et al., 2009; Nixon et al., 2008; Ward et al., 2009). Furthermore, these observations were made in young adults and to date, no reports have shown how binge alcohol exposure affects microglia in an adolescent model. Therefore, we examined the microglial response in a 4-day binge model of an alcohol use disorder known to produce neurodegeneration in adolescent rats.
METHODS
Animals
Fifty-three adolescent male Sprague-Dawley (Charles River Laboratories, Portage, MI) rats were used in this study. Upon arrival (postnatal day 30), rats were individually housed, maintained on a 12h light/dark cycle, and provided food and water ad libitum. Rats were assigned to one of three time points: T2, T7, or T30 (T2, e.g. sacrificed 2 days after the last dose of alcohol). Time points were chosen based off of previous alcohol-induced microglia responses observed in adult rats (Nixon et al., 2008). All experiments began on postnatal day 35 following a 5-day acclimation period and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (NRC, 1996) and were approved by the University of Kentucky’s Institutional Animal Use and Care Committee.
Adolescent Binge Alcohol Model
At postnatal day 35, adolescent rats were exposed to ethanol in a 4-day binge model as described previously (Majchrowicz, 1975; Morris et al., 2010). Briefly, a nutritionally complete liquid diet (Vanilla Ensure Plus®) containing either ethanol (25% w/v) or isocaloric amounts of dextrose was administered via intragastric gavage every 8h for 4 days. Ethanol groups were given an initial 5g/kg dose with subsequent doses titrated based on the following 6 point intoxication behavior scale: 0-normal rat (5g/kg), 1-hypoactive (4g/kg), 2-ataxic (3g/kg), 3-delayed righting reflex (2g/kg), 4-loss of righting reflex (1g/kg), 5-loss of corneal eye blink reflex (0g/kg). Control rats received dextrose-containing diet equal to the average volume of ethanol diet administered to the ethanol rats. Food pellets were not available during the 4 day binge, but were returned to cages following the last dose.
To verify intoxication, blood ethanol concentrations (BECs) were determined from tail blood collected 90 minutes after the first dose on the third day (Table 2). Blood was centrifuged at 1800 × g for 5 min, then stored at −20°C. BECs were determined from plasma using a GM7 Alcohol Analyzer calibrated to a 300mg/dl external standard (Analox, Lunenberg, MA).
Table 2.
Ethanol Binge Data
| Group | N | Intoxication Behavior |
Ethanol Dose (g/kg/d) |
BEC (mg/dL) | Peak Withdrawal |
|---|---|---|---|---|---|
| T2 | 5 | 0.8 ± 0.13 | 12.7 ± 0.40 | 410 ± 20.9 | 3.3 ± 0.14 |
| T2 (ELISA) | 7 | 1.3 ± 0.11 | 11.0 ± 0.34 | 346 ± 31.1 | 2.6 ± 0.20 |
| T7 | 7 | 0.7 ± 0.09 | 13.0 ± 0.27 | 363 ± 21.7 | 3.5 ± 0.12 |
| T30 | 6 | 1.1 ± 0.07 | 11.8 ± 0.20 | 304 ± 15.3 | 2.0 ± 0.44 |
Overt withdrawal behavior was scored for 16h beginning 10h after the last ethanol dose following the scale reported by Penland and colleagues (Majchrowicz, 1975; Penland et al., 2001). Rats were observed and scored for 30 min of each hour. The highest withdrawal behavior achieved for each hour across withdrawal (peak) were analyzed and reported.
Bromo-deoxy-Uridine (BrdU) Incorporation
In the T2 and T30 groups, dividing cells were labeled by injection of the S-phase marker, BrdU (300mg/kg, i.p.; Sigma-Aldrich, St.Louis, MO). The T2 group was euthanized 2h after BrdU injection in order to examine cell proliferation, while the T30 group was euthanized 28 days later to analyze long-term survival of cells born at the T2 time point.
Immunohistochemistry
Tissue preparation
For immunohistochemistry, rats were sacrificed 2, 7, or 30 days following binge ethanol exposure via sodium pentobarbital overdose (Nembutal®, MWI Veterinary Supply, Nampa, ID) followed by transcardial perfusion of 0.1M phosphate buffered saline (PBS; pH 7.4) then 4% paraformaldehyde. Brains were dissected out, postfixed in 4% paraformaldehyde for 24h, washed, and stored in PBS at 4°C until sectioning. Brains were cut at 40µm on a vibrating microtome (Leica Microsystems, Wetzlar, Germany) in a 1:12 series and stored in cryoprotectant in 24-well plates at −20°C.
DAB labeling
Immunohistochemistry was conducted similar to previous reports (Morris et al., 2010; Nixon and Crews, 2002). Briefly, free-floating tissue sections were washed in tris-buffered saline (TBS) then incubated in 0.6–1% H2O2 for 30 min. For BrdU and Ki67, specific antigen retrieval steps were included as previously reported (Morris et al., 2010; Nixon and Crews, 2002). Following washes in TBS, sections were incubated in blocking buffer (3% normal serum/0.1% Triton-X/TBS) for 30 min. After overnight incubation in primary antibody (Table 1), sections were washed 3 × 10 min in blocking buffer, then incubated in species appropriate biotinylated secondary antibody (Vector Laboratories, Burlingame, CA). Colorization of antibody binding was achieved with an avidin-biotin-complex (ABC Elite Kit, Vector Laboratories) and nickel-enhanced diaminobenzidine (DAB; Polysciences, Waltham, MA). Sections were mounted to glass slides and when necessary, tissue was lightly counterstained and coverslipped in Cytoseal (Richard Allen Scientific, Kalamazoo, MI).
Table 1.
Antibodies
| Anti- (Source) | Dilution | Secondary Antibody |
|---|---|---|
| BrdU (Chemicon) | 1:5000 | Biotinylated hs anti-ms |
| Ki67 (Vector) | 1:200 | Biotinylated hs anti-ms |
| MHC-II (Serotec, Ox-6) | 1:500 | Biotinylated hs anti-ms |
| ED-1 (Serotec, CD68) | 1:500 | Biotinylated hs anti-ms |
| Iba-1 (Wako) | 1:1000 (DAB) | Biotinylated gt anti-rb |
| 1:400 (Fluor) | AlexaFluor gt anti-rb 546 | |
| BrdU (Accurate) | 1:400 | AlexaFluor gt anti-rat 488 |
Fluorescent labeling
Similar to previous reports, tissue to be labeled with fluorescent secondaries was washed in TBS (3 × 5min), then incubated in blocking buffer for 30 min. Tissue was incubated for 40 hours in primary antibody (Table 1) followed by 3 × 10 min washes in blocking buffer. Under dark, opaque boxes, sections were then incubated for 1h in fluorescent-coupled secondary antibodies (Table 1; Invitrogen, Carlsbad, CA) and washed in TBS (3 × 10 min) before mounting to glass slides, drying and coverslipping in ProLong mounting media (Invitrogen).
TNF-α Enzyme-linked Immunosorbant Assay (ELISA)
Tissue preparation
Rats were rapidly decapitated 2 days following binge ethanol exposure. Brains were immediately extracted, then hippocampus was dissected and snap-frozen on dry ice before storage at −80°C.
ELISA
Hippocampal tissue samples were weighed and then homogenized in ice-cold lysis buffer (25mM HEPES pH 7.4, 0.1% 3-[(3-cholamidopropyl)dimethyl-ammonio]1-propanesulfonate, 5mM MgCl2, 1.3mM EDTA, 1mM EGTA, 10µg/ml pepstatin, aprotinin, and leupeptin, and 1 mM PMSF; Rabuffetti et al., 2000). Homogenates were centrifuged at 20,000 × g for 15 min at 4°C and supernatant was stored at −80°C. Total protein content was determined using a Pierce BCA Protein Assay Kit (Thermo Scientific, Waltham, MA). TNF-α was measured using an ELISA kit according to the manufacturer’s instructions (Invitrogen; Cat # KRC3011). The detection limit for TNF- α in this kit is 4 pg/ml. Absorbance at 450nm was measured on a DXT880 Multimode Detector plate reader (Beckman Coulter, Brea, CA). All samples, standards, and positive controls were run in duplicate so that all tissue could be run on a single plate, reducing a potential source of variability. Cytokine protein concentration was divided by the total protein concentration obtained in the BCA assay to correct for differences in tissue volume.
Microglia Morphological Evaluation
Microglia morphology was analyzed on a 1:12 series of Iba-1 stained tissue sections from the T2 group. Dorsal hippocampal sections (3–4/brain) were examined with an Olympus BX-51 microscope equipped with a Proscan II motorized stage, DP70 digital camera, and VIS image analysis software (version 3.6.4.0, Visiopharm, Hoersholm, Denmark). The CA fields and dentate gyrus were traced separately at 100×. A 200µm × 200µm frame was randomly placed over the regions of interest using the newCAST stereology platform. The frame was moved randomly over the tissue using a 600µm step length for the dentate gyrus and an 800µm step length for the CA fields. Only microglia with cell bodies falling entirely within the borders of the frame were analyzed. This sampling method resulted in the morphological scoring of 100–200 Iba-1+ microglia per region. Since Iba-1 labels microglia regardless of activation state, we used morphological criteria to distinguish resting microglia from activated and phagocytic microglia (Kreutzberg, 1996; Raivich et al., 1999). Microglia scored as resting or not activated had characteristic long, highly ramified processes with comparatively small cell bodies; activated microglia had large cell bodies and shortened, swollen processes that were less ramified than those present on resting microglia; and phagocytic microglia had large, ameoboid cell bodies with no or few short, swollen processes (Kreutzberg, 1996; Raivich et al., 1999). ED-1 and MHC-II expression were examined qualitatively at T2, T7, and T30 to further characterize the microglia activation profile.
BrdU Quantification
BrdU immunoreactivity at T2 and T30 was analyzed. Multipanel images containing dorsal hippocampus (Bregma −2.52 through −4.56; Paxinos and Watson, 2009) were collected at 100× using the same microscope set up described above. CA1, CA2/3, dentate molecular layer, granule cell layer, and hilus were outlined to delineate regions of interest, and BrdU+ profile counts for each region were obtained using Image ProPlus software (version 6.3.1.542; MediaCybernetics, Bethesda, MD).
Confocal Microscopy
Colocalization of BrdU and Iba-1 was assessed qualitatively at T2 within the hippocampus using an inverted laser scanning confocal microscope (Leica TCS SP5; Wetzlar, Germany). The phenotype of 20 BrdU cells per region was quantified at T30, similar to previous reports (Nixon et al., 2008). Images were obtained using a 63X oil immersion lens with Z-plane stacks collected at 0.8µm thickeness. BrdU colabeling with Iba-1 was assessed by reconstructing Z-stacks and rotating in 3D similar to previous work (Morris et al., 2010; Nixon et al., 2008).
Statistics
Statistical analysis was performed with JMP software (version 8.0.1; SAS Institute, Cary, NC). Microglia morphology at T2 and BrdU phenotype data was analyzed with Pearson’s chi square test. BrdU profile counts and TNF-α ELISA were compared with two-tailed, unpaired t-tests. Data are reported as mean ± SEM and differences were considered significant at p < 0.05.
RESULTS
Adolescent binge ethanol exposure alters microglia morphology
Details of the alcohol intoxication parameters for all groups, including BEC, intoxication score, daily ethanol dose, and peak withdrawal score are summarized in table 2. Four day binge ethanol exposure resulted in peak BECs on day 3 of 353 ± 67 mg/dL.
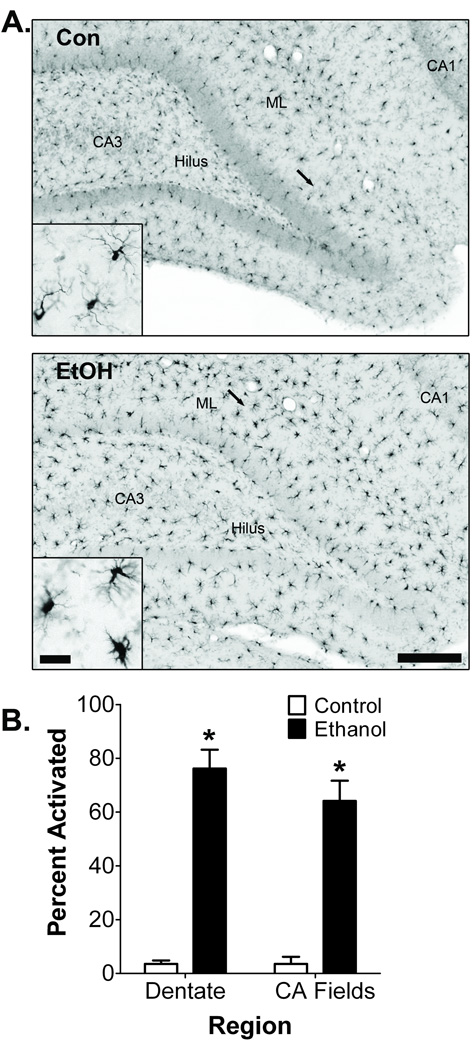
Microglia morphology, which is an indicator of microglia activation, was examined 2 days after binge ethanol exposure using Iba-1 immunohistochemistry. Iba-1 is a calcium binding protein that labels all microglia regardless of activation state (Ito et al., 1998). Iba-1+ cells were found throughout all hippocampal regions; however, distinct morphological differences in Iba-1 expression between adolescent control and ethanol rats were evident (Fig. 1A). Iba-1+ cells in control rats had small cell bodies with thin, highly ramified processes, consistent with the morphology of resting microglia. In contrast, Iba-1+ cells in ethanol rats contained large cell bodies and thick processes characteristic of activated microglia morphology. Amoeboid-shaped Iba-1+ cells characteristic of fully activated, phagocytic microglia were not observed in either control or ethanol rats. Semi-quantitative morphological analysis confirmed that binge ethanol exposure shifts a significant proportion of Iba-1+ microglia to an activated morphology within the dentate gyrus (p < 0.001) and CA fields (p < 0.001; Fig. 1B).
Figure 1. Effect of binge ethanol exposure on adolescent hippocampal microglia morphology.
A) Representative Iba-1 images in the adolescent hippocampus from control (n = 5) and ethanol (n = 5) groups at T2. Microglia in control animals had morphological characteristics consistent with resting microglia, while binge ethanol exposed rats had swollen cell bodies with thicker, less ramified processes, consistent with activation. Arrows denote area represented in inset. Scale bar = 250µm; 30µm for inset. Con = control, EtOH = ethanol, ML = dentate gyrus molecular layer. B) Iba-1 microglia morphological analysis demonstrated that binge ethanol exposure transforms microglia into an activated state both within the dentate gyrus and CA fields. *p < 0.05.
Adolescent binge ethanol exposure induces microglia proliferation
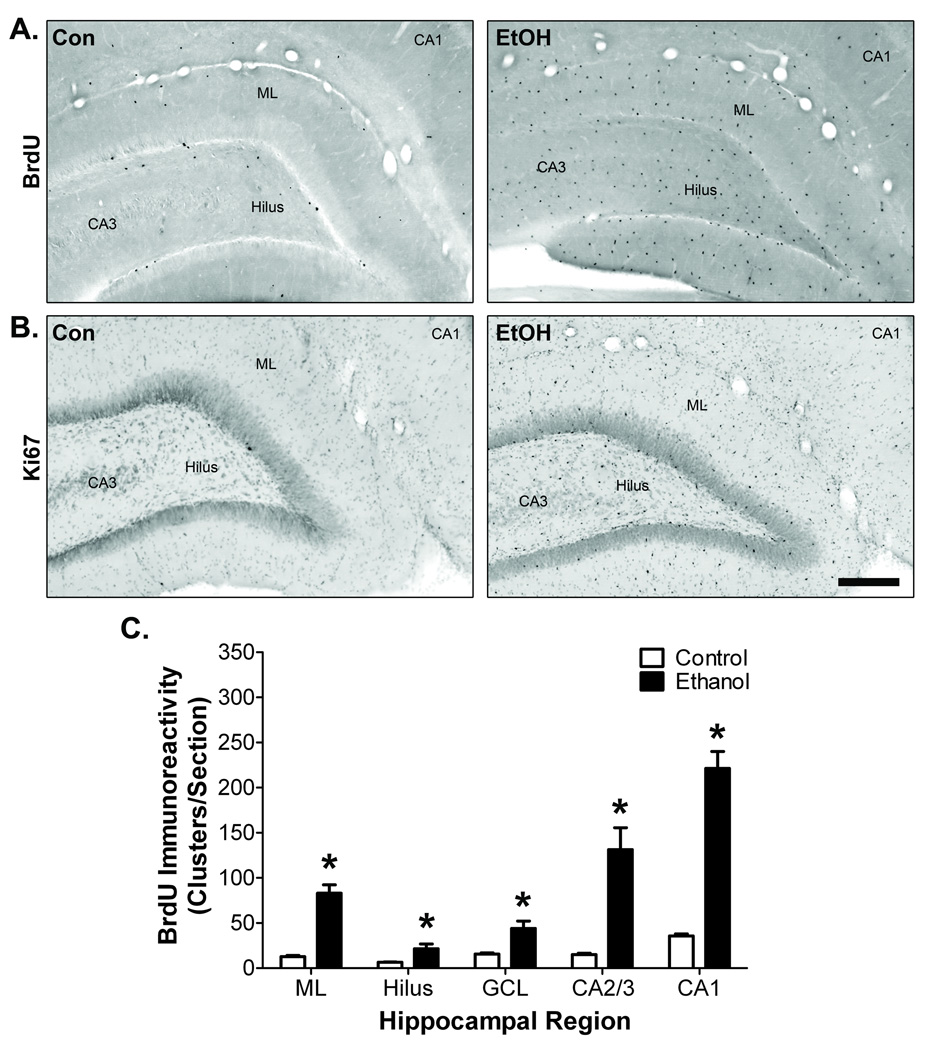
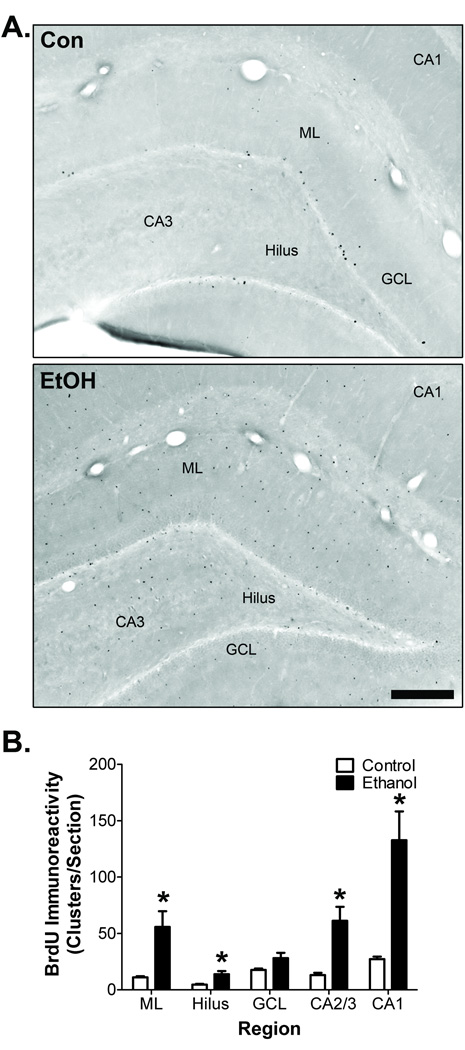
Cell proliferation is an important component of many microglial reactions (Ladeby et al., 2005). To determine if microglia proliferation accompanies ethanol-induced morphological transformation in adolescent rats, hippocampal BrdU incorporation was examined 2 days after binge treatment. In control rats, BrdU+ cells were mostly confined to the subgranular zone of dentate gyrus, although sparse BrdU+ immunoreactivity was present in the dentate molecular layer, hilus, and CA fields (Fig. 2A). In ethanol-exposed rats, numerous BrdU+ cells were located in all regions of the hippocampus. This pattern of cell proliferation in control and ethanol rats was confirmed with Ki-67, an endogenous cell proliferation marker (Fig. 2B). Image analysis demonstrated that binge ethanol exposure significantly increased the number of BrdU+ cell clusters found in the dentate molecular layer by 6.5-fold (t = 7.47, p = 0.015), hilus by 3.3-fold (t = 2.81, p = 0.048), granule cell layer by 2.8-fold (t = 3.43, p = 0.025), CA2/3 by 8.7-fold (t = 4.78, p = 0.0086), and CA1 by 6.2-fold (t = 9.79, p < 0.001; Fig. 2C).
Figure 2. Hippocampal cell proliferation following binge ethanol exposure during adolescence.
A) Representative images at T2 showing BrdU immunoreactivity throughout the hippocampus. B) Representative images at T2 showing Ki67 immunoreactivity throughout the hippocampus. Scale bar = 250µm. Con = control, EtOH = ethanol, ML = dentate gyrus molecular layer. C) Image analysis demonstrated that binge ethanol exposure increased the number of BrdU immunoreactive clusters in every hippocampal region examined. *p < 0.05 for group comparisons within each region (n = 5/group).
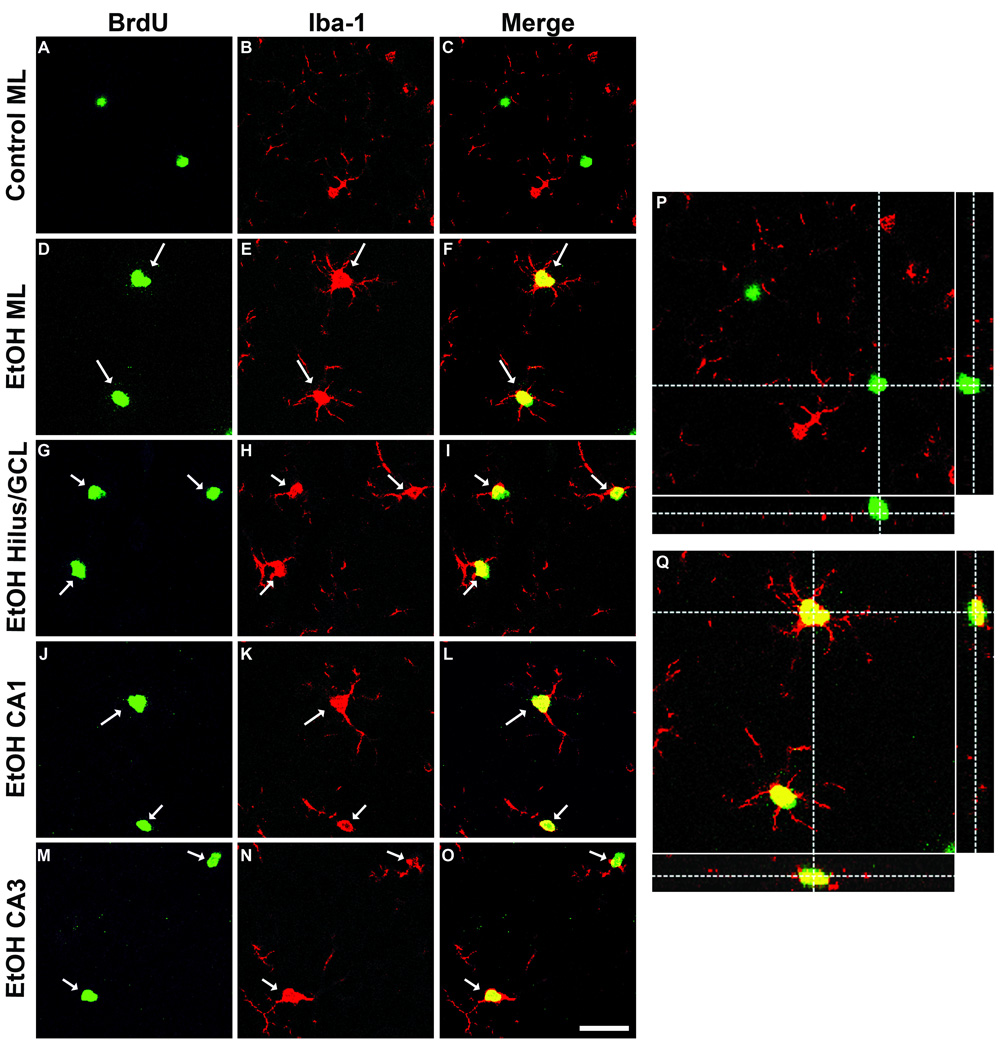
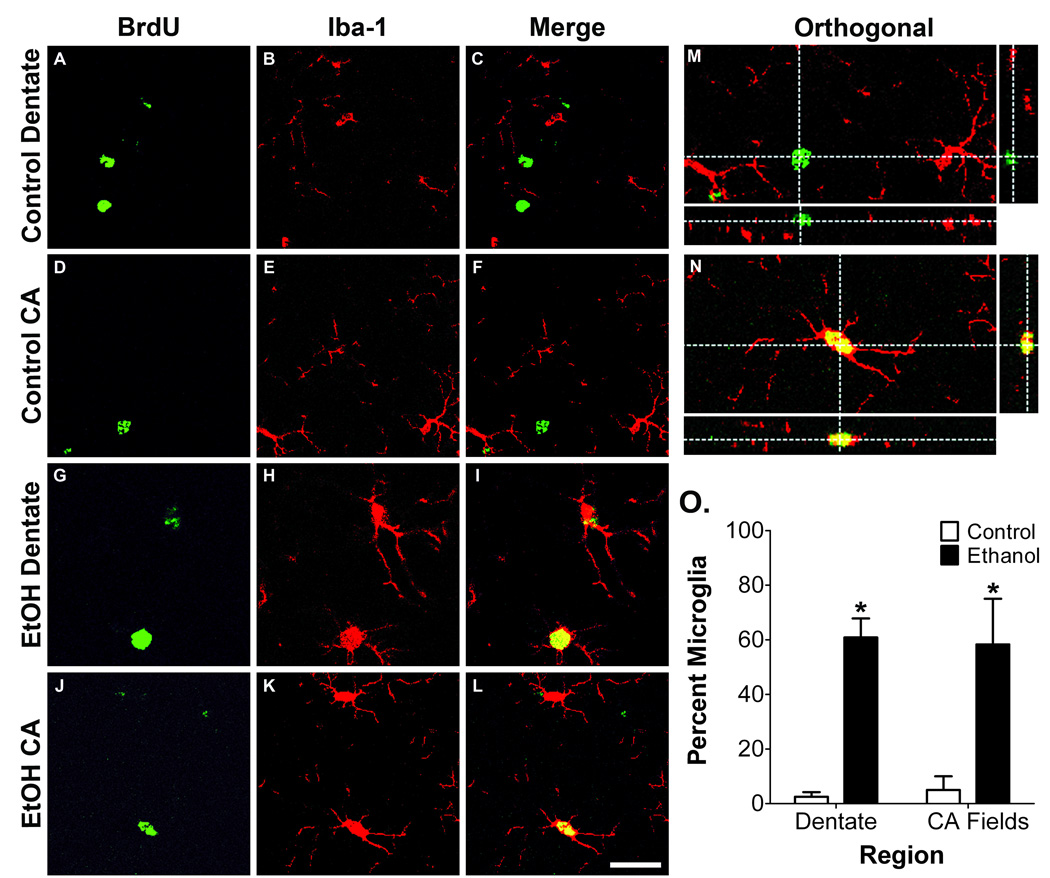
To determine if BrdU+ cells found within the hippocampus 2 days following binge ethanol exposure were microglia, co-localization of BrdU with Iba-1 was examined with confocal microscopy. 3-D reconstruction of Z-stack images revealed BrdU/Iba-1 co-labeling in the dentate molecular layer, granule cell layer, hilus, CA2/3, and CA1 (Fig. 3), indicating that the microglial reaction initiated by binge ethanol exposure results in widespread proliferation of hippocampal microglia.
Figure 3. Binge ethanol exposure induces proliferation of hippocampal microglia.
Representative confocal images from the T2 time point showing BrdU (A, D, G, J, M), the microglia marker Iba-1 (B, E, H, K, N), and merge (C, F, I, L, O). Co-labeling was seldom observed in the control group, while a majority of BrdU+ cells from the ethanol group expressed Iba-1 in the dentate molecular layer (D–F), GCL/hilus (G–I), CA1 (J–L), and CA3 (M–O). Scale bar = 30µm. 3-D reconstructed images with orthogonal views are shown for control (P) and ethanol (Q) molecular layer to demonstrate co-labeling in the ethanol group.
Binge ethanol exposure during adolescence partially activates hippocampal microglia
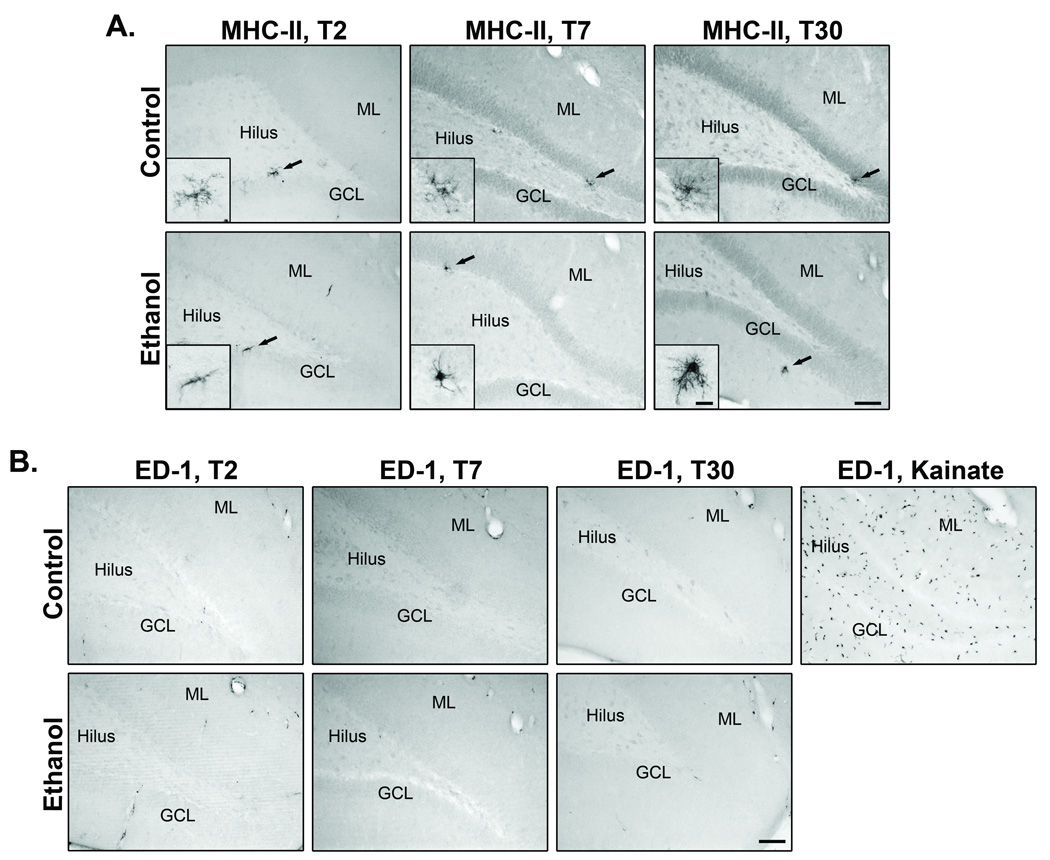
Activated microglia that have reached the phagocytic stage express ED-1 (CD68 in humans), a lysosomal antigen expressed in phagocytes, while microglia involved in immune-mediated responses upregulate MHC-II expression. MHC-II and ED-1 expression were examined at T2, T7, and T30 to determine if binge ethanol exposure induces maximal microglia activation. In control and ethanol rats, few to no MHC-II+ microglia were found along the dentate granule and molecular layers (Fig. 4A). Although there was no obvious change in the number of MHC-II expressing microglia, the few immunoreactive microglia in the ethanol group had morphological characteristics consistent with an activated morphology, whereas, those in control rats resembled the highly ramified, resting morphology (see insets). In control and ethanol rats, ED-1 expression was similarly rare. The few immunopositive cells observed appear confined to blood vessels, with essentially no ED-1+ cells in the parenchyma of the hippocampus (Fig. 4B), identical to previous reports in adult rats (Nixon et al., 2008). In addition to changes in morphology, fully activated microglia secrete various proinflammatory mediators, such as TNF-α. Therefore, we measured the expression of TNF-α at T2, the time at which microglia proliferate and show morphology characteristic of activation. Binge ethanol exposure had no effect on TNF-α expression in the hippocampus (t(12) = 0.65, p = 0.53; control = 11 ± 0.9 pg TNF-α/mg protein; ethanol = 10 ± 0.9 pg TNF-α/mg protein). Mean TNF-α content, 17µg/ml, was well within the limits of detection of the kit (<4µg/ml) and within the expected range for adolescent rat brain homogenate based on reports on neonate and adult rat (Bilboa et al., 2010; Rabuffetti et al., 2000).
Figure 4. The effect of binge ethanol exposure on MHC-II and ED-1 expression.
A) MHC-II representative images from T2, T7, and T30 groups. Few, if any, MHC-II+ microglia were observed in control (n = 7) and ethanol (n = 7) animals, mainly within the dentate gyrus. B) ED-1 representative images from T2, T7, and T30 groups. ED-1 immunoreactivity was mostly confined to blood vessels in both control and ethanol groups. Arrows denote area represented in inset. Scale bar = 250µm; 20µm for inset. Con = control, EtOH = ethanol, GCL = granule cell layer, ML = dentate gyrus molecular layer.
Microglia response persists into young adulthood
To examine the lasting effect of binge ethanol exposure on microglia activation, microglia were analyzed at T30, 4 weeks following BrdU injection. Many of the cells labeled with BrdU at T2 were still present at T30 (Fig 5A). Similar to the T2 time point, significantly more BrdU+ cells were observed in the ethanol rats within the dentate molecular layer (t = 3.2, p = 0.02), hilus (t = 3.3, p = 0.02), CA2/3 (t = 3.9, p = 0.01), and CA1 (t = 4.1, p = 0.009; Fig 5B). Confocal microscopy was used to phenotype the surviving BrdU+ cells (Fig. 6). Within the dentate gyrus of binge ethanol exposed rats, 60.8% of the sampled BrdU+ cells co-localized with Iba-1, compared to just 2.5% in the control group (p < 0.001). Likewise in the CA regions, 58% of BrdU+ cells in the ethanol group were microglia, while just 5% co-localized in the control group (p < 0.001). Moreover, microglia sampled from the ethanol group had retained the morphological characteristics described at T2 (Fig. 6G–L), suggesting that exposure to ethanol during adolescence results in persistent microglia activation that remains through at least early adulthood (e.g. postnatal day 65) in rats.
Figure 5. Cells born following binge ethanol exposure survive into young adulthood.
A) Representative images comparing surviving BrdU immunoreactivity in control (n = 6) and ethanol (n = 6) group 28 days after BrdU injection. B) Image analysis shows that the ethanol-induced increase in BrdU detected at T2 survives to T30. Scale bar = 250µm. GCL = granule cell layer, ML = dentate molecular layer. * p < 0.05.
Figure 6. Adolescent binge ethanol exposure generates persistent microglial reaction lasting to young adulthood.
Representative confocal images from the T30 time point showing BrdU, Iba-1, and merge. A–F) As with the T2 time point, BrdU/Iba-1 co-localization occurred sparingly in the control group (n = 6). G–L) In the ethanol group (n = 6), microglia born at T2 remained present in the dentate gyrus and CA fields through T30. Scale bar = 30µm. E) Phenotype analysis demonstrated that significantly more BrdU+ cells co-localized with Iba-1 in the ethanol group compared to controls. Scale bar = 30µm. Asterisks denote p < 0.05 for group comparisons.
DISCUSSION
Until recently, the effects of alcohol on microglia have largely been overlooked, likely due to a lack of evidence indicative of full activation in alcohol models (Nixon et al., 2008; Zahr et al., 2010). We provide intriguing evidence that a single 4-day binge exposure episode during adolescence sufficiently triggers widespread hippocampal microglial proliferation accompanied by distinct morphological transformation towards an activated phenotype. Interestingly, microglia generated in response to alcohol exposure survived and retained their altered morphological characteristics into young adulthood, indicating that a single binge drinking episode can have long-lasting consequences on hippocampal microglial reactivity. However, there was no indication that alcohol’s effects on microglia involved phagocytosis, as evidenced by a lack of ED-1 expression. The absence of ED-1+ phagocytic microglia is consistent with previous results in adult rats subjected to the same experimental paradigm (Nixon et al., 2008). The effects on microglia proliferation and morphology coupled with the failure to acquire phagocytic properties suggest that alcohol drives microglia to an intermediate state of activation (Raivich et al., 1999). This is supported by functional evidence demonstrating that binge alcohol exposure had no effect on TNF-α expression. In addition, binge alcohol exposure failed to produce significant MHC-II expression, indicating that alcohol-induced partial activation of microglia does not involve an immune-mediated response.
The functional relevance of the microglial response to alcohol deserves further examination; especially considering that microglia born following binge alcohol exposure retain morphological characteristics consistent with partial activation from adolescence into early adulthood. The initial exposure to high alcohol concentrations could serve as a priming stimulus, whereby microglia become sensitized to subsequent neurological challenge. Increased MHC-II expression has been described in a model of intermittent binge alcohol exposure (Ward et al., 2009), suggesting that the cumulative effects of multiple withdrawal episodes may be sufficient to push partially activated microglia towards a more inflammatory phenotype. This provides support for the microglia priming idea with the subsequent challenge being additional exposure to high concentrations of alcohol. Evidence for alcohol-mediated microglia priming can also been seen in studies examining proinflammatory cytokine expression often attributed to microglia. For example, alcohol increased TNF-α, IL-1β, IL-6, Cox-2, and iNOS in intermittent binge alcohol exposure models; effects which were attenuated with Cox-2 inhibition or TLR4 knockout, known inhibitors of microglial activation (Alfonso-Loeches et al., 2010; Pascual et al., 2009). In contrast, we show that the microglial response to a single 4-day alcohol binge does not involve an increase in TNF-α. This is in agreement with similar models which show unaltered protein expression of proinflammatory TNF-α, IL-1β, IFN-γ, IL-4, IL-5, and IL-13 (Zahr et al., 2010). In addition, others found a single binge alcohol exposure to be insufficient to induce IL-1β and MCP-1; however, subsequent systemic lipopolysaccharide challenge revealed a synergistic effect between alcohol and lipopolysaccharide on IL-1β and MCP-1 production (Qin et al., 2008). This effect further supports the role of alcohol serving as a priming stimulus for full microglia activation.
Microglia reactions are hallmark features of most neurodegenerative diseases, resulting in the hypothesis that microglial activation directly contributes to neuronal damage and loss associated with these diseases (Block et al., 2007). Similar mechanisms have been proposed to participate in alcohol-mediated neurodegeneration based on alcohol’s effects on microglia and associated cytokine production, along with alcohol’s interaction with LPS-mediated neuroinflammation (Qin et al., 2008). However, in vivo evidence directly demonstrating microglial participation in alcohol-mediated neuronal injury or death has yet to be established. Therefore, other microglia-mediated effects involving neuroprotection or functional recovery should not be ignored (Graeber and Streit, 2010). This is particularly relevant to events occurring in the hippocampus where binge alcohol exposure strongly inhibits granule cell neurogenesis (Crews et al., 2006c; Morris et al., 2010; Nixon and Crews, 2002). After 1 week of abstinence in adult rats, alcohol-mediated inhibition of neurogenesis is followed by a large burst of neural progenitor cell proliferation that results in increased generation of new hippocampal granule neurons (Nixon and Crews, 2004). Importantly, microglia proliferation occurs after alcohol intoxication, but precedes the increase in neurogenesis occurring during abstinence, suggesting that microglia may be involved in promotion of neurogenesis (Nixon et al., 2008). Indeed, recent evidence suggests that microglia can stimulate or suppress neurogenesis depending on grade of microglia activation (Das and Basu, 2008; Mathieu et al., 2010; Molina-Holgado and Molina-Holgado, 2010). Phagocytic level activation and a proinflammatory state inhibits neurogenesis (Monje et al., 2003) whereas low grade, anti-inflammatory activation is associated with increased neurogenesis (Butovsky et al., 2006; Mathieu et al., 2010; Ziv et al., 2006).
The adolescent hippocampus is highly susceptible to structural and functional damage caused by alcohol (Crews et al., 2000; White and Swartzwelder, 2005). Continued alcohol consumption in a binge pattern during adolescence reduces hippocampal volume and interferes with hippocampal-dependent memory processes (De Bellis et al., 2000; Schulteis et al., 2008). The current study is the first to demonstrate that a single 4-day binge alcohol episode during adolescence promotes a widespread microglial reaction in the hippocampus. The nature of the microglial response to alcohol is not clear, and it is unknown whether microglia contribute directly to alcohol-mediated hippocampal injury or act as neuroprotective vehicles aiding in functional recovery; however, its long-lasting nature suggests that hippocampal microglia perform important functions in response to alcohol. Elucidating these mechanisms may provide important clues regarding the vulnerability of the adolescent hippocampus to alcohol and could lead to the development of new treatment options to promote recovery based on manipulation of microglial function.
Acknowledgements
This work was supported by NIAAA R21AA160307 and R01AA016959.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest
REFERENCES
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilboa SD, Wieselerb JL, Barrientosb RM, Tsanga V, Watkins LR, Maier SF. Neonatal bacterial infection alters fever to live and simulated infections in adulthood. Psychoneuroendocrinology. 2010;35:369–381. doi: 10.1016/j.psyneuen.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M. Microglia activated by IL-4 or IFN-[gamma] differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Crews F, Bechara R, Brown L, Guidot D, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol. Clin. Exp. Res. 2006a;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol. Clin. Exp. Res. 2006b;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol. Clin. Exp. Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006c;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Das S, Basu A. Inflammation: A new candidate in modulating adult neurogenesis. J. Neurosci. Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J. Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Graeber M, Streit W. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J. Subst. Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- He J, Crews F. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Molecular Brain Research. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol. Clin. Exp. Res. 1999;23:633–643. [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Ladeby R, Wirenfeldt M, Garcia-Ovejero D, Fenger C, Dissing-Olesen L, Dalmau I, Finsen B. Microglial cell population dynamics in the injured adult central nervous system. Brain Res. Rev. 2005;48:196–206. doi: 10.1016/j.brainresrev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol. Clin. Exp. Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacology. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mathieu P, Battista D, Depino A, Roca V, Graciarena M, Pitossi F. The more you have, the less you get: the functional role of inflammation on neuronal differentiation of endogenous and transplanted neural stem cells in the adult brain. J. Neurochem. 2010;112:1368–1385. doi: 10.1111/j.1471-4159.2009.06548.x. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Molina-Holgado F. Mending the broken brain: neuroimmune interactions in neurogenesis. Journal of Neurochemistry. 2010;114:1277–1290. doi: 10.1111/j.1471-4159.2010.06849.x. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: A mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J. Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J. Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol. Dis. 2008;31:218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, McClain JA. Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr. Opin. Psychiatry. 2010;23:227–232. doi: 10.1097/YCO.0b013e32833864fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC. Guide for the care and use of laboratory animals. Washington, D.C: The National Academic Press; 1996. [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J. Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Paxinos GW, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 2009. [Google Scholar]
- Penland S, Hoplight B, Obernier J, Crews FT. Effects of nicotine on ethanol dependence and brain damage. Alcohol. 2001;24:45–54. doi: 10.1016/s0741-8329(01)00142-2. [DOI] [PubMed] [Google Scholar]
- Qin L, He J, Hanes R, Pluzarev O, Hong J-S, Crews F. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J. Neuroinflammation. 2008;5:10. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabuffetti M, Sciorati C, Tarozzo G, Clementi E, Manfredi AA, Beltramo M. Inhibition of caspase-1-like activity by Ac-Tyr-Val-Ala-Asp-chloromethyl ketone Induces long-lasting neuroprotection in cerebral Ischemia through apoptosis reduction and decrease of proinflammatory cytokines. J. Neurosci. 2000;20:4398–4404. doi: 10.1523/JNEUROSCI.20-12-04398.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res. Brain Res. Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42:459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R, Basak AK, Sircar D. Repeated ethanol exposure affects the acquisition of spatial memory in adolescent female rats. Behav. Brain Res. 2009;202:225–231. doi: 10.1016/j.bbr.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence: alcohol sensitivity, tolerance, and intake. Recent Dev. Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Streit W, Xue Q-S. Life and death of microglia. J. Neuroimmune Pharmacol. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, De Witte P, Ballini C, Corte LD, Dexter D. Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. J. Neurochem. 2009;111:1119–1128. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: A hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. Recent Dev. Alcohol. 2005;17:161–176. doi: 10.1007/0-306-48626-1_8. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Luong R, Sullivan EV, Pfefferbaum A. Measurement of serum, liver, and brain cytokine Induction, thiamine levels, and hepatopathology in rats exposed to a 4-day alcohol binge protocol. Alcohol. Clin. Exp. Res. 2010;34:1858–1870. doi: 10.1111/j.1530-0277.2010.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews F. Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-κB and proinflammatory cytokines. Alcohol. Clin. Exp. Res. 2010;34:777–789. doi: 10.1111/j.1530-0277.2010.01150.x. [DOI] [PubMed] [Google Scholar]