Abstract
Objective
Clinical and experimental studies demonstrate the important roles of vascular smooth muscle cells (VSMC) in the pathogenesis of atherosclerosis. We have previously determined that osteogenic transcription factor, Runx2, is essential for VSMC calcification. The present studies characterized Runx2-regulated signals and their potential roles in vascular calcification.
Methods and Results
In vivo studies with atherogenic ApoE−/− mice demonstrated that increased oxidative stress was associated with upregualtion of Runx2 and receptor activator of nuclear factor κB ligand (RANKL), which colocalized in the calcified atherosclerotic lesions and were juxtaposed to infiltrated macrophages and osteoclast-like cells that are positively stained for an osteoclast marker, tartrate-resistant acid phosphatase (TRAP). Mechanistic studies using RNA interfering, a luciferase reporter system, chromatin immunoprecipitation and electrophoretic mobility shift assays identified that Runx2 regulated the expression of RANKL via a direct binding to the 5'-flanking region of the RANKL. Functional characterization revealed that RANKL did not induce VSMC calcification, nor RANKL was required for oxidative stress-induced VSMC calcification. Using a co-culture system, we demonstrated VSMC-expressed RANKL induced migration as well as differentiation of bone marrow-derived macrophages into multinucleated, TRAP-positive osteoclast-like cells. These effects were inhibited by the RANKL antagonist, osteoprotegerin, and with VSMC deficient in Runx2 or RANKL.
Conclusions
We demonstrate that Runx2 directly binds to the promoter and controls the expression of RANKL, which mediates the crosstalk between calcifying VSMC and migration and differentiation of macrophages into osteoclast-like cells in the atherosclerotic lesions. Our studies provide novel mechanistic insights into the regulation and function of VSMC-derived RANKL in the pathogenesis of atherosclerosis and vascular calcification.
Keywords: RANKL, Runx2, calcification, osteoclastogenesis, migration
INTRODUCTION
Atherosclerosis is characterized by the formation of raised, often calcified, lesions in the arterial intima leading to narrowing of the vessel lumen. Vascular calcification reduces arterial compliance1; and therefore represents a key factor in the hemodynamic consequences of atherosclerosis. Accumulating evidence has demonstrated that vascular calcification is a cell-regulated process with many similarities to the mechanisms of embryonic osteogenesis, not simply a passive precipitation of crystals2–4. Vascular calcification reflects an osteochondrogenic transformation of vascular smooth muscle cells (VSMC), which is associated with increased expression of growth factors, matrix proteins, and other bone-related markers5–7.
High levels of oxidative stress have been linked with increased prevalence of arterial calcification in hypercholesterolemia, hypertension, diabetes mellitus, and end stage renal disease8. Therefore, the generation of oxidative stress is considered to be a common final pathway by which these factors predispose to vascular calcification. Oxidative stress in atherosclerosis may lead to generation of reactive oxygen species (ROS) by vascular cells, endothelial dysfunction, and plaque disruption9. We have previously reported that H2O2, a cell-permeable ROS, induces VSMC calcification in vitro10. Receptor activator of NF-kappaB ligand (RANKL) is a member of the tumor necrosis factor superfamily, which is the key regulator for osteoclast formation. RANKL is highly expressed in lymphoid tissues and trabecular bone, particularly in areas associated with active bone remodeling or inflammatory osteolysis11. In normal vessels and non-calcified arteries or valves, RANKL is frequently undetectable. By contrast, RANKL has been reported to be up-regulated and expressed in calcified atherosclerotic lesions12. Consistently, mice lack of osteoprotegerin (OPG), the decoy receptor for RANKL, exhibit increased vascular calcification13. Inhibition of RANKL by human monoclonal antibody was recently found to attenuate vascular calcium deposition in human RANKL knock-in mice14. These emerging studies suggest a role of RANKL in the atherosclerotic calcification. However, the molecular mechanisms underlying RANKL upregulation and its role in atherosclerotic lesions remain largely unknown. In a human osteoblast-like cell line and primary mouse bone marrow stromal cells, H2O2- or xanthine/xanthine oxidase-generated ROS promotes the expression of RANKL15. Therefore, oxidative stress may contribute to the increased expression of RANKL found in calcified atherosclerotic tissues.
The present studies determined the effect of oxidative stress on the expression of RANKL in VSMC and the underlying molecular mechanisms. We have previously demonstrated that the master osteogenic transcription factor, Runx2, is an essential regulator for oxidative stress-induced VSMC calcification10. In the present study, we have found that Runx2 directly binds to the promoter and induces the expression of RANKL in VSMC. Functional characterization demonstrated that RANKL does not directly induce VSMC calcification; and that VSMC-expressed RANKL is not required for oxidative stress-induced VSMC calcification, though it promotes osteoclastogenesis and migration of macrophages. These findings support an important role of VSMC-expressed RANKL in regulating infiltration and differentiation of macrophages during VSMC calcification, which may shed light on the origin and formation of the osteoclast-like cells observed in calcified atherosclerotic lesions. Thus, oxidative stress may promote vascular calcification directly via Runx2, and indirectly via Runx2-upregulated RANKL, which promotes recruitment and differentiation of osteoclast-like cells in calcifying atherosclerotic lesions.
METHODS
Primary mouse aortic smooth muscle cells (VSMC, passages 3–5) and atherogenic ApoE−/− mice were used for in vitro and in vivo studies. In vitro co-culture was performed with VSMC and bone marrow macrophages from wild type C57BL6 mice. Details of materials and experimental procedures are in the Methods section in the Online Data Supplement.
RESULTS
Runx2 and RANKL upregulation as well as macrophage infiltration and TRAP-positive cells are associated with vascular calcification in ApoE−/− mice
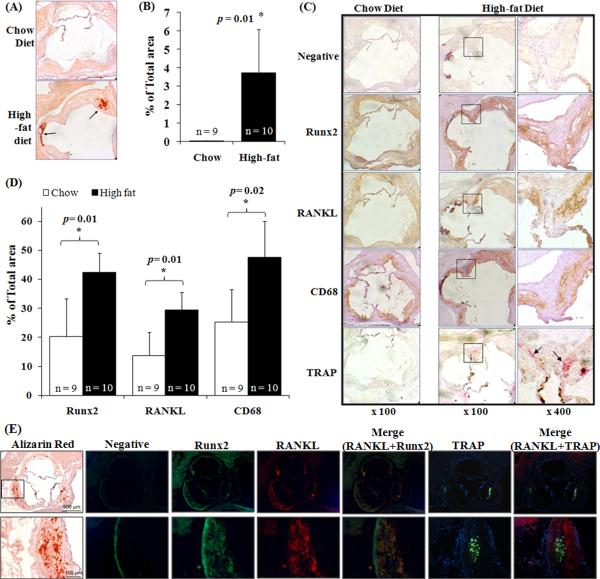
We have previously demonstrated that oxidative stress induces the expression of Runx2, which is essential for VSMC calcification in vitro. Using an atherosclerosis model, ApoE−/− mice16, we characterized the expression of Runx2 in vascular calcification in vivo. A significant increase in calcification was found in the aortic roots obtained from ApoE−/− mice fed a high-fat diet compared with those from chow -fed control animals (p=0.01, Fig. 1A and 1B), which was associated with high-fat diet-induced increased oxidative stress (Supplemental Fig. 1). Increases in Runx2- and RANKL-immunopositive areas were found concurrently with enhanced vascular calcification in the high-fat diet-fed animals when compared with those from chow-fed mice (Fig. 1C). Quantitative analysis confirmed significant up-regulation of Runx2 and RANKL expression in the high-fat diet mice (p=0.01 for each, Fig. 1D).
Figure 1. Vascular calcification is associated with increased expression of Runx2 and RANKL as well as macrophage infiltration and TRAP-positive cells.
(A) Frozen aortic root sections from chow diet- or high-fat diet-fed ApoE-deficient mice were obtained at the level of the aortic valve leaflets and stained with Alizarin Red. Arrows indicate calcium mineral deposition. (B) Calcification at the aortic root sections was measured using ImageJ software (NIH Bethesda, MD). The percentage of tissue area with calcification was calculated by dividing the calcified area by the total area of the aortic root. Bar values are expressed as the mean ± SD with number of the mice shown above each bar. (C) Frozen aortic root sections were stained with antibodies for Runx2, RANKL, CD68 and secondary antibody alone (negative control) and histochemically stained for TRAP. Immunopositive areas shown in boxes in low magnification (× 100, left panels) are shown at higher magnification (× 400, right panels). Arrows indicate TRAP-positive cells. (D) Runx2-, RANKL-, and CD68-immunopositive areas in the aortic root sections. The immunopositive areas were quantified as a percent of total aortic root area in each section. Bar values are expressed as mean ± SD with number of the mice shown above each bar. (E) Immunofluorescent staining of Runx2, RANKL, and TRAP in frozen aortic root sections from high-fat diet-fed ApoE-deficient mice. Negative control shows autofluorescence (green). Boxes in the low magnification image (upper panels) are shown in higher magnification in the lower panels. Alizarin red staining showing on left panel is to indicate the calcified areas.
In addition, increased macrophage infiltration was demonstrated in the aortic root of ApoE−/− mice fed the high-fat diet (p=0.02, Fig. 1D), as determined by staining with a macrophage marker, CD6817. The area stained positive for macrophages in the high-fat diet-fed mice was closely associated with RANKL-immunopositivity (Fig. 1C). Tartrate-resistant acid phosphatase (TRAP)-positive cells were observed in the aortic roots of high-fat diet-fed animals (Fig. 1C), which were mainly found in the areas of highest expression of RANKL and macrophage infiltration (Fig. 1C). The greatest expression of Runx2/RANKL, and of CD68/TRAP, was in the aortic sinuses at base of valve leaflets, a common site of atherosclerotic lesions. With immunofluorescent staining, we further confirmed the co-localization of Runx2 and RANKL in the calcified atherosclerotic lesions from high-fat diet fed ApoE−/− mice (Fig. 1E). Importantly, TRAP-positive cells were found in close apposition to the RANKL-positive areas (Fig. 1E), supporting a link between RANKL induction and formation of osteoclast-like cells.
Oxidative stress induces the expression of RANKL in VSMC during calcification
To determine the effects of oxidative stress on the expression of RANKL in calcifying VSMC, we characterized the effects of a series of concentrations of H2O2 (0.05–0.4 mM) on RANKL expression by primary mouse VSMC. Based on quantitative real-time PCR, H2O2 dose-dependently induced expression of RANKL in parallel with VSMC calcification (Fig. 2A and 2B). Similarly, oxidative stress induced the expression of RANKL in rat and human VSMC (Supplemental Fig 3). Increased expression of RANKL protein by oxidative stress was identified predominantly in the cell lysates (Fig. 2C), indicating that oxidative stress-induced RANKL is expressed primarily within VSMC. Furthermore, H2O2 increased RANKL mRNA in a time-dependent manner, concurrently with H2O2-induced Runx2 expression (Fig. 2D).
Figure 2. Oxidative stress induces the expression of RANKL in VSMC during calcification.
(A) VSMC were exposed to control or 0.05 to 0.4 mM H2O2 in osteogenic media for 3 weeks with media changes every 2–3 days. In vitro VSMC calcification was determined by Alizarin Red staining. Representative images of stained dishes (upper) and microscopic views (× 40, lower) from 4 independent experiments are shown. (B) Expression of RANKL in VSMC exposed to 0.05 to 0.4 mM H2O2 in osteogenic media for 3 weeks was determined by quantitative real-time PCR (n=4, *p <0.05, **p <0.01 and ***p<0.005 compared with control conditions). (C) RANKL protein was measured in media and cell lysates from cells under oxidative stress for 2 weeks. Results from 2 independent ELISA assays performed in triplicate are shown (*p<0.05 compared with control condition in media or cell lysates). D) Expression of Runx2 and RANKL during VSMC calcification was determined by RT-PCR. VSMC were exposed to 0.4 mM H2O2 in osteogenic media for up to 21 days. Representative RT-PCR results of 3 independent experiments are shown. (E). RANKL promoter activity in VSMC under oxidative stress was determined using the Dual-Luciferase Reporter Assay System with six deletion mutants of RANKL promoter-reporters RL(FL), RL(−700), RL(−550), RL(−400), RL(−200), RL(−150), RL(−50) and a plasmid expressing Renilla Luciferase as an internal control reporter. Results shown are relative luciferase activities in each of the conditions compared with that of the RL(FL) under control condition, which was assigned a value of 1.0. Results from 3 independent experiments performed in duplicate are shown (* p < 0.05 vs. RL(FL) control).
H2O2-responsive element in the RANKL promoter
To elucidate the molecular mechanism of oxidative stress-induced expression of RANKL, we examined the H2O2-responsive region on the RANKL gene using a series of luciferase reporter constructs containing deletion mutants of the RANKL 5'-flanking region: RL(FL; −950), RL(−700), RL(−550), RL(−400), RL(−200), RL(−150), and RL(−50)18. H2O2 induced higher promoter activities in VSMC transfected with RL(FL), RL(−700), RL(−550), and RL(−400) compared with controls (Fig. 2E), but not in VSMC transfected with RL(−200). Therefore, the −400 to −200bp region is essential for the H2O2-induced transcription of the RANKL gene in VSMC.
Runx2 regulates RANKL transcription
Sequence analyses of the sequence of the −400 to −200bp region of the RANKL promoter identified multiple binding sites for the key osteogenic transcription factor, Runx2 (Runx2-1, Runx2-2, Runx2-3, and Runx2-4, Fig. 3A). We previously reported that Runx2 is essential for H2O2-induced VSMC calcification10. To characterize Runx2 binding domains in the RANKL promoter, ChIP assay was performed using an anti-Runx2 antibody. Segments of the RANKL promoter containing the putative Runx2-binding sites were amplified by PCR using the appropriate primer sets (whole:−438/−85, R1&2:−438/−240, and R3&4:−290/−85) and DNA templates extracted from protein/DNA cross-linked samples. Runx2 was found to bind specifically to the R3&4 region (−290/−85) as well as to the entire (−438/−85) region of the RANKL promoter (Fig. 3B). With primer sets spanning each of the putative Runx2-binding regions of the RANKL promoter (R1–R4), the Runx2 binding sites were located preferentially to the R3 region, and to a lesser extent, the R4 region (Fig. 3C).
Figure 3. Runx2 regulates RANKL transcription.
(A) Putative transcription factors which bind to 5'-flanking and promoter regions of murine RANKL (AF332141) were identified through the TFSEARCH engine (http://www.cbrc.jp/research/db/TFSEARCH.html). Three Runx2-binding sites were identified within the −400 to −200 bp region and one additional binding site was found near −200 bp. The putative Runx2 binding sites (Runx2-1, Runx2-2, Runx2-3, and Runx2-4) and primers for ChIP assay are indicated. (B) ChIP assay with Runx2 immunoprecipitates (anti-Runx2; Santa Cruz) and PCR using primer sets for whole, R1&2, and R3&4. Representative pictures of 3 independent experiments are shown. (C) ChIP assay with PCR primer sets R1, R2, R3, and R4. Representative pictures of 3 independent experiments are shown. (D) EMSA was performed using four probes (P1, P2, P3, and P4) containing the putative Runx2 binding elements. Probes carrying mutations of the Runx2-binding sites (P1m, P2m, P3m, and P4m) were used as negative controls. Representative EMSA pictures of 3 independent experiments are shown. (E) Competitive EMSA was performed using 100-fold molar excess amounts of unlabeled probes. Probes carrying mutation of the Runx2-binding sites were used as negative controls. Representative EMSA pictures of three independent experiments are shown. (F) Specific antibody for Runx2 was used to detect supershift in Runx2 binding complex. A representative EMSA picture of 2 independent experiments are shown. (G) VSMC with stable Runx2 knockdown by shRNA against Runx2 (Lenti-shRunx2) or control lentiviruses encoding GFP (Lenti-GFP) were exposed to 0.4 mM H2O2 for 2 weeks. Expression of Runx2 protein was determined by Western blot analysis. A representative blot of 2 independent experiments is shown. (H) Expression of RANKL transcript in control or Runx2 knockdown VSMC in G was determined by RT-PCR. Representative results from two independent experiments are shown. (I) VSMC were transduced with Ad-Runx2 or control virus (Ad-GFP) and cultured in osteogenic media for 2 weeks. Expression of RANKL transcript was determined by RT-PCR. Representative RT-PCR results of 2 independent experiments are shown.
Furthermore, direct binding of Runx2 to the RANKL promoter was confirmed by EMSA with oligonucleotides derived from putative Runx2-binding sites in the RANKL promoter (P1:−378/−354, P2:−337/−313, P3:−216/−192, and P4:−190/−166). The previously-reported Runx2 consensus binding probe (Pcon) was used as a positive control19. As with the results of ChIP assay, binding complexes were detected predominantly with the P3 probe, and with other probes to a much lesser extent. Mutations of the Runx2 binding sites on all probes (P1m, P2m, P3m, and P4m) abolished their binding to Runx2, indicating binding specificity (Fig. 3D). The P3 putative Runx2 binding site was found to be conserved among the mouse, rat and human RANKL gene, but the P1, 2 and 4 sites were not (Supplemental Fig 4). Runx2 binding to the P3 probe was blocked by excess amounts of cold P3 probe and Runx2 consensus probe (Pcon), but not by P3 probe with mutation in Runx2 binding site (P3m) (Fig. 3E). Moreover, antibody specific for Runx2 produced a supershift of the Runx2 binding complex, confirming the specificity of Runx2 in the DNA-protein complex (Fig. 3F). In addition, mutation of P3 inhibited H2O2- and Runx2-induced luciferase activity driven by RANKL promoter (Supplemental Fig 4). Taken together, these results indicate a specific and predominant binding of Runx2 to the P3 region of RANKL promoter.
Runx2 is essential for oxidative stress-induced RANKL expression and sufficient to induce RANKL
To determine whether Runx2 is required for oxidative stress-induced expression of RANKL, we employed lentivirus-mediated shRNA to knockdown the expression of Runx2 in VSMC10. As expected, H2O2-induced Runx2 expression in VSMC was not affected by control virus (lenti-GFP), however, Runx2 expression was inhibited in VSMC infected with shRNA against Runx2 (Fig. 3G). The inhibitory effect of lentiviral shRNA on Runx2 expression resulted in blockage of H2O2-induced RANKL expression in VSMC (Fig. 3H), confirming that Runx2 is essential for H2O2-induced expression of RANKL in VSMC. In addition, adenovirus-mediated over-expression of Runx2 alone was sufficient to induce the expression of RANKL in VSMC (Fig. 3I), which is consistent with our previous finding that overexpression of Runx2 induces VSMC calcification10. Therefore, increased expression of Runx2, either induced by H2O2 or mediated by viral infection, up-regulates the expression of RANKL in calcifying VSMC.
RANKL does not induce VSMC calcification and is not required for oxidative stress-induced VSMC calcification
To assess whether RANKL directly induces VSMC calcification, recombinant RANKL protein (100 ng/ml) was added to VSMC in osteogenic media with or without 0.4 mM H2O2. As expected, H2O2 induced calcification of VSMC (Fig. 4A), whereas RANKL did not (Fig. 4A and 4B). OPG (50 ng/ml), a soluble decoy receptor of RANKL, did not block calcification induced by oxidative stress (Fig. 4A). In addition, H2O2-induced calcification was not inhibited in VSMC from RANKL-deficient mice compared with WT VSMC, as determined by von Kossa cytochemical staining as well as by quantification of calcium levels in cell lysates (Fig. 4C and 4D), indicating that RANKL signaling is not essential for oxidative stress-induced VSMC calcification.
Figure 4. RANKL does not induce VSMC calcification and is not required for oxidative stress-induced VSMC calcification.
(A) RANKL (100 ng/ml) and/or OPG (50 ng/ml) were added to VSMC with or without the addition of 0.4 mM H2O2 in osteogenic media for 3 weeks with media changes every 2–3 days. In vitro VSMC calcification was determined by von Kossa staining. Representative images of stained dishes (upper) and microscopic views (× 100, lower) from 3 independent experiments are shown. (B) VSMC were cultured in DMEM with 10% FBS and 10 mM beta-glycerophosphate with or without the addition of RANKL (100 ng/ml) for 10 days. Calcification was quantified in cell lysates by the o-cresolphthalein complexone method. Results shown are from 3 independent experiments (NS, no statistically significant difference). (C) VSMC from WT or RANKL-deficient mice were treated with 0.4 mM H2O2 in osteogenic media for 3 weeks. Representative images of von Kossa stained dishes (upper) and microscopic views (× 100, lower) from 2 independent experiments are shown. (D) In separate experiments, VSMC from WT or RANKL-deficient mice were treated with 0.4 mM H2O2 in osteogenic media for 1 or 2 weeks; and calcium mineral was quantified by the o-cresolphthalein complexone method. Results from 2 independent experiments performed in triplicates are shown (*p <0.05 compared with control conditions, no statistically significant differences were found between WT and RANKL−/− VSMC).
Oxidative stress-stimulated VSMC promote migration of bone marrow macrophages in a Runx2/RANKL-dependent manner
Soluble RANKL protein has been shown to exhibit chemotactic properties toward human monocytes (24). To test whether RANKL expression by VSMC may affect BMM migration, freshly isolated mouse BMM were indirectly exposed to VSMC using the Transwell migration assay. Soluble RANKL protein at 100 ng/ml was used as a positive control. As shown in Fig. 5A, oxidative stress-stimulated VSMC increased the migration of BMM by 5.2-fold compared with serum free control, while unstimulated VSMC had no effect on BMM migration. The effects of oxidative stress-stimulated VSMC on BMM migration was blocked in VSMC from RANKL-deficient mice or in VSMC with Runx2 knockdown, demonstrating the requirement of Runx2-regulated RANKL production by VSMC. Furthermore, addition of OPG abolished the effect of oxidative stress-stimulated VSMC on BMM migration (Fig 5B), supporting a role of RANKL expressed by VSMC in regulating BMM migration.
Figure 5. Oxidative stress-stimulated VSMC promote BMM migration in a Runx2/RANKL-dependent manner.
A) After VSMC were cultured in osteogenic media for 2 weeks with or without 0.4 mM H2O2, cells were coated on the lower side of Transwell filter, and DiI-labeled BMMs were added to the upper chambers. After 24 hours, migrated BMMs were measured using a microplate fluorescence reader. Results from 3 independent experiments performed in duplicate are shown (p = NS for unstimulated WT VSMC vs. serum-free, p = 0.01 for oxidative stress-stimulated WT VSMC vs. serum-free). B) WT VSMC were pre-incubated in osteogenic media for 2 weeks with or without 0.4 mM H2O2, then coated on the lower side of Transwell. Effects of conditioned media from the pre-incubation or addition of OPG (50ng/ml) to the upper side of the Transwell on macrophage migration was determined. Results from 3 independent experiments performed in duplicate are shown (*p = 0.005 for oxidative stress-stimulated VSMC vs. serum-free control). Addition of OPG abolished the effects.
Oxidative stress-stimulated VSMC promote osteoclastic differentiation of macrophages in a Runx2/RANKL-dependent manner
RANKL is a central regulator of osteoclast formation, and RANKL produced by osteoblasts induces osteoclastic differentiation of osteoclast precursors11. The presence of TRAP-positive osteoclast-like cells in areas of high RANKL expression promoted us to test the hypothesis that oxidative stress-induced RANKL in VSMC during calcification may induce osteoclast formation. With the use of a co-culture system of VSMC and BMM as a source of osteoclast precursors, we determined that H2O2-stimulated VSMC induced the formation of multinucleated TRAP-positive cells from BMM, compared with unstimulated VSMC (Fig. 6A). However, in VSMC from RANKL-deficient mice, H2O2 treatment did not induce any multinucleated TRAP-positive cells in co-culture with BMM (Fig. 6A), indicating a direct effect of VSMC-derived RANKL. Furthermore, osteoclastic differentiation was restored by addition of soluble RANKL (100 ng/ml) to the co-culture of RANKL−/− VSMC with BMMs (Fig. 6A). Addition of OPG, however, completely blocked the stimulatory effects obtained with H2O2 (Fig. 6B).
Figure 6. Oxidative stress-stimulated VSMC promote osteoclastic differentiation of BMM in a Runx2/RANKL-dependent manner.
BMMs were plated on top of VSMC that were pre-incubated in osteogenic media for 2 weeks with or without 0.4 mM H2O2; cultured in α-MEM containing 10% Hi-FBS/10 ng/ml M-CSF with or without RANKL (100 ng/ml); and stained for TRAP 1 week later. (A) Representative images of stained dishes (left) and microscopic views (× 100, right) from 4 independent experiments performed in duplicate. (B) Effect of OPG (500 ng/ml) on the co-culture. Representative images of TRAP stained dishes (upper) and microscopic views (× 100, lower) from 2 independent experiments performed in duplicate are shown. (C) Runx2 KO VSMC were co-cultured with BMM for 1 week. Representative images of TRAP stained dishes (left) and microscopic views (× 100, right) from 3 independent experiments performed in duplicate are shown.
Furthermore, VSMC with Runx2 knockdown by lentiviral shRNA failed to promote the formation of multinucleated TRAP-positive cells in a co-culture system when compared with wild type VSMC (Fig. 6C), confirming that Runx2 is essential for oxidative stress-induced RANKL expression in VSMC and that it functions in regulating osteoclastic differentiation.
DISCUSSION
Using the atherogenic ApoE−/− mice, we determined that increased Runx2 and RANKL were colocalized in the calcified atherosclerotic lesions, in close apposition to TRAP-positive osteoclast-like cells. We demonstrated for the first time that oxidative stress-enhanced expression of RANKL in primary murine VSMC, which was mediated by the osteogenic transcription factor Runx2 via a direct binding to the RANKL promoter. VSMC-derived RANKL was not essential for oxidative stress to induce VSMC calcification; and RANKL did not induce VSMC calcification when added in the culture media. Instead, VSMC-derived RANKL was found to promote migration and osteoclastic differentiation BMM. These observations provide novel evidence for the molecular mechanism underlying RANKL upregulation in atherosclerotic calcification and the function of Runx2/RANKL in regulating the crosstalk between calcifying VSMC and macrophages in the genesis of TRAP-positive osteoclast-like cells in atherosclerotic lesions.
Increased expression of RANKL was found to colocalize with Runx2 in the calcified atherosclerotic lesions (Fig. 1). Such an observation is consistent with previous reports that RANKL expression is increased in calcified atherosclerotic lesions but not in normal vessels12, and mice deficient in OPG develop arterial calcifications13. RANKL is a transmembrane protein expressed in the bone marrow microenvironment and stimulates osteoclast progenitor cells to differentiate into osteoclasts20. Under normal steady-state conditions in adults, mineral deposition by osteoblasts and mineral resorption by osteoclasts are delicately balanced to maintain bone homeostasis21. Similar to the observation in human atherosclerotic arteries22, we identified TRAP-positive multinucleated osteoclast-like cells in close apposition to the calcified areas. The high levels of RANKL and the presence of TRAP-positive osteoclast-like cells in calcified atherosclerotic lesions supports the notion that molecular mechanisms similar to bone remodeling processes are manifested within mineralized atherosclerotic artery walls21.
Increased oxidative stress in the atherosclerotic lesions may contribute to the increase in RANKL expression. Oxidative stress induced expression of RANKL in mouse, rat and human VSMC (Fig. 2 and Supplemental Fig 3), suggesting a common regulatory mechanism. These results are consistent with a previous report that ROS induces RANKL expression in bone marrow stromal cells in vitro15. Similarly, RANKL expression is increased during BMP-2- and calcitriol-induced vascular calcification23–25. The molecular mechanism underlying H2O2-induced expression of RANKL in VSMC is mediated by Runx2 via a direct binding to the RANKL promoter (Fig. 2&3). Runx2 is a transcription factor that belongs to the runt-domain gene family and is essential for osteoblast differentiation and gene expression of bone matrix proteins26. We previously demonstrated that oxidative stress induces VSMC calcification in vitro via Runx2-dependent signals10. Runx2 was found to be essential for the expression of RANKL (Fig. 3H) and sufficient to induce RANKL in VSMC (Fig. 3I); findings consistent with the inhibition of RANKL expression in Runx2-deficient osteoblast cells27. By contrast, calcitriol-induced RANKL expression was not affected in Runx2-deficient calvarial cells28. In the ST2 mouse bone marrow stromal cells, decreased Runx2 expression by PKA activation was associated with increased RANKL expression29. Accordingly, the effects of Runx2 on RANKL expression appear to differ among cell types and their response to different stimuli.
The Runx2 binding domains were identified within −400bp to −200bp in the RANKL promoter in VSMC, with a predominant binding of Runx2 to the −206bp to −201bp region (P3, Fig. 3), which is the most conserved sequence among human, rat and mouse RANKL gene (Supplemental Fig 4). Mutation of P3 was found to inhibit Runx2 binding (Fig 3, E, F), as well as H2O2–induced and Runx2-induced luciferase activity of the RANKL promoter (Supplemental Fig. 4). Similar to our finding, deletion or mutation of Runx2 binding sites within the RANKL promoter or over-expression of a dominant negative Runx2 abolished BMP2- and Smad1-mediated activation of RANKL promoter activity in chondrocytes30. Despite structural similarity to the core Runx2-binding sequences (5'-ACCPuCPu-3'), the other putative Runx2 binding site showed significantly less binding activity, suggesting the importance of overall genomic context containing the core sequences for Runx2 activity. In the ST2 bone marrow stromal cells, mutation of each of the three putative Runx2 binding sites on the RANKL promoter (corresponding to the P1, P3 and P4 in the present study), especially the P3 and P4 sites, decreased the basal promoter activity of RANKL; and inhibited PKA-induced RANKL expression29. Therefore, our study is consistent with the study in ST2 cells with respect to supporting the requirement for direct binding of Runx2 on the RANKL promoter, especially the P3 region, in regulating RANKL expression.
Unlike previous reports that RANKL promotes an osteogenic phenotype in aortic myofibroblast31 and rat aortic VSMC calcification32, we did not find a direct effect of RANKL on mouse VSMC calcification (Fig. 4A), excluding the possibility of autocrine or paracrine effects of VSMC-derived RANKL on calcification. Similar results were also found in calcifying vascular cells; treatment of RANKL (100 ng/ml) in osteogenic media did not induce calcification of these cells (Supplemental Fig. 2). Differences in model cell systems may explain the differences between the present results and prior reports31,32. The difference in expression of RANK, the receptor for RANKL, in mouse and rat VSMC was not statistically significant, which may not contribute to the different effects of RANKL on the calcification of mouse and rat VSMC (Supplemental Fig 5). In addition, patterns of mineral deposition seen by von Kossa staining are more diffuse in rat cells and more nodular in mouse cells32, suggesting that RANKL induction of calcium positive stains in rat aortic VSMC may occur via a distinct mechanism of biomineralization or via increased intracellular calcium. In any case, oxidative stress promoted calcification in RANKL-deficient VSMC, supporting the concept that VSMC-derived RANKL is not essential for direct regulation of VSMC calcification in vitro (Fig. 4B).
We found that, in VSMC, the majority of RANKL protein induced by oxidative stress was cell-associated (Fig. 2C), unlike in activated murine T lymphocytes which secrete an active soluble form of RANKL into the culture medium33. In MC3T3-E1 cells, mechanical strain has been shown to increase membrane-bound RANKL at the expense of soluble RANKL, via a reduction in ectodomain shedding34. The dominance of VSMC-associated RANKL over soluble RANKL in the context of oxidative stress may explain why RANKL protein is concentrated in areas of atherosclerosis, whereas serum levels of soluble RANKL are inversely correlated with presence of coronary artery disease35.
VSMC exposed to oxidative stress induced the migration of BMM in a Runx2/RANKL-dependent manner, supporting the role of oxidative stress-induced RANKL from VSMC in regulating macrophage infiltration (Fig. 5). Macrophage infiltration plays significant role in the formation of atherosclerotic lesions36. Aikawa et al. found that macrophage infiltration and inflammation precede the osteogenic conversion of VSMC37. Deficiency of OPG in ApoE−/− mice accelerates atherosclerotic lesion progression, and increases atherosclerotic calcification and macrophage infiltration, suggesting a role of RANKL in up-regulating calcification and macrophage infiltration in vivo20. Consistently, we demonstrated increased macrophage infiltration in close association with Runx2/RANKL expression in calcified vessel walls (Fig. 1C & E). RANKL has been reported to induce migration of MonoMac-6 monocytic cells as well as peripheral blood mononuclear cells in a dose-dependent manner with an potency similar to that of monocyte chemoattractant protein-1 (MCP-1)38, a chemotactic factor that contributes to atherogenesis39. The effect of RANKL on macrophage recruitment may be attributed to its induction of several cytokines including CCL22 (macrophage-derived chemokine), MCP-1, and interleukin-840–42. The chemokines, in turn, increase RANKL expression43, amplifying the inflammatory process44,45. In addition, RANKL has been found to up-regulate RANK expression on monocytes, to promote cell survival and activate their capacity for antigen presentation through induction of co-stimulatory molecules46,47.
In bone, RANKL expression by osteoblastic and/stromal cells is essential for the complete development of multinucleated bone-resorptive osteoclasts from monocytic precursors11, and for the resorptive activity and survival of mature osteoclasts as well48. Consistently, we found that VSMC-derived RANKL induced the differentiation of BMM into multinucleated TRAP-positive cells, an effect that was antagonized by exogenous OPG (Fig. 6). These results are consistent with the observation by Collin-Osdoby et al. that TNF-α-activated human microvascular endothelial cells, which express RANKL on the cell surface, promote osteoclastogenesis in co-cultures with human monocyte precursors via a RANKL-mediated mechanism49. In contrast, CVC and conditioned media from CVC were previously shown to inhibit osteoclastic differentiation in a co-culture system50; however, CVC expression of RANKL expression during osteoblastic differentiation and mineralization was not determined50. The role of osteoclasts in atherosclerotic calcification is still not clear. In keeping with the hypothesis that the process of vascular calcification resembles that observed in bone tissue, net calcium deposition in vessel walls might result from focal perturbation of the balance between osteoblast-like cells and osteoclast-like cells21. Thus, our observations support the notion that osteoclast-like cells in the vascular wall may represent important cellular mediators of mineral resorption in arteries. Nevertheless, our observation that oxidative stress promotes VSMC induction of osteoclastogenesis may shed lights on the origin and potential role of osteoclast-like cells in atherosclerotic plaque. Further studies are warranted to characterize the pathological function of osteoclast-like cells in the regulation of vascular calcification.
Taken together, our studies demonstrate the molecular mechanisms underlying Runx2-regulated expression of RANKL and its function during oxidative stress-induced VSMC calcification. The results indicate that expression of Runx2, RANKL, and vascular calcification are associated with macrophage infiltration and TRAP-positive cells in atherosclerotic lesions of ApoE−/− mice; that VSMC-derived RANKL, induced by Runx2-dependent signaling, increases migration and osteoclastic differentiation of macrophages; that RANKL does not directly regulate osteogenic differentiation of VSMC; and that osteoclast-like cells arise in the proximity to mineralized areas of atherosclerotic lesions, making it likely that osteoclastic cells indirectly regulate vascular calcification, as seen in the interplay of osteoblasts and osteoclasts in regulating bone homeostasis.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Dr. Jay M McDonald (University of Alabama at Birmingham) for helpful discussion and critical review. We thank Dr. Xu Feng (University of Alabama at Birmingham) for providing the RANKL-reporter constructs.
SOURCES OF FUNDING This work was supported by grants from National Institutes of Health HL092215 and AR055339 (YC), HL081202 (LD), DK081346 (YT), a VA Merit Review Award BX000369 (YC) and an American Heart Association Award 0865081E (YC). CHB was supported by a NIH T32 Pre-doctoral Training Grant.
Footnotes
DISCLOSURES None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Demer LL. Effect of calcification on in vivo mechanical response of rabbit arteries to balloon dilation. Circulation. 1991;83:2083–2093. doi: 10.1161/01.cir.83.6.2083. [DOI] [PubMed] [Google Scholar]
- 2.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 3.Tintut Y, Parhami F, Bostrom K, Jackson SM, Demer LL. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–7553. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- 4.Canfield AE, Doherty MJ, Wood AC, Farrington C, Ashton B, Begum N, Harvey B, Poole A, Grant ME, Boot-Handford RP. Role of pericytes in vascular calcification: a review. Z Kardiol. 2000;89(Suppl 2):20–27. doi: 10.1007/s003920070096. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi K, Nakamura S, Nishida W, Sobue K. Bone morphogenetic protein-induced MSX1 and MSX2 inhibit myocardin-dependent smooth muscle gene transcription. Mol Cell Biol. 2006;26:9456–9470. doi: 10.1128/MCB.00759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao JS, Aly ZA, Lai CF, Cheng SL, Cai J, Huang E, Behrmann A, Towler DA. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann N Y Acad Sci. 2007;1117:40–50. doi: 10.1196/annals.1402.075. [DOI] [PubMed] [Google Scholar]
- 7.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radical Biology and Medicine. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 8.Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–E696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- 9.Stocker R, Keaney JF., Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 10.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh MC, Choi Y. Biology of the TRANCE axis. Cytokine Growth Factor Rev. 2003;14:251–263. doi: 10.1016/s1359-6101(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 12.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 13.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, Kostenuik PJ, Erben RG, Hofbauer LC. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009;175:473–478. doi: 10.2353/ajpath.2009.080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai XC, Lu D, Liu AL, Zhang ZM, Li XM, Zou ZP, Zeng WS, Cheng BL, Luo SQ. Reactive oxygen species stimulates receptor activator of NF-kappaB ligand expression in osteoblast. J Biol Chem. 2005;280:17497–17506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld ME, Carson KG, Johnson JL, Williams H, Jackson CL, Schwartz SM. Animal models of spontaneous plaque rupture: the holy grail of experimental atherosclerosis research. Curr Atheroscler Rep. 2002;4:238–242. doi: 10.1007/s11883-002-0025-3. [DOI] [PubMed] [Google Scholar]
- 17.Gautier EL, Huby T, Witztum JL, Ouzilleau B, Miller ER, Saint-Charles F, Aucouturier P, Chapman MJ, Lesnik P. Macrophage apoptosis exerts divergent effects on atherogenesis as a function of lesion stage. Circulation. 2009;119:1795–1804. doi: 10.1161/CIRCULATIONAHA.108.806158. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Yang H, Liu W, Cao X, Feng X. Sp1 and Sp3 regulate the basal transcription of receptor activator of nuclear factor kappa B ligand gene in osteoblasts and bone marrow stromal cells. J Cell Biochem. 2005;96:716–727. doi: 10.1002/jcb.20569. [DOI] [PubMed] [Google Scholar]
- 19.Mehrotra M, Saegusa M, Voznesensky O, Pilbeam C. Role of Cbfa1/Runx2 in the fluid shear stress induction of COX-2 in osteoblasts. Biochem Biophys Res Commun. 2006;341:1225–1230. doi: 10.1016/j.bbrc.2006.01.084. [DOI] [PubMed] [Google Scholar]
- 20.Bennett BJ, Scatena M, Kirk EA, Rattazzi M, Varon RM, Averill M, Schwartz SM, Giachelli CM, Rosenfeld ME. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE−/− mice. Arterioscler Thromb Vasc Biol. 2006;26:2117–2124. doi: 10.1161/01.ATV.0000236428.91125.e6. [DOI] [PubMed] [Google Scholar]
- 21.Doherty TM, Uzui H, Fitzpatrick LA, Tripathi PV, Dunstan CR, Asotra K, Rajavashisth TB. Rationale for the role of osteoclast-like cells in arterial calcification. FASEB J. 2002;16:577–582. doi: 10.1096/fj.01-0898hyp. [DOI] [PubMed] [Google Scholar]
- 22.Jeziorska M, McCollum C, Wooley DE. Observations on bone formation and remodelling in advanced atherosclerotic lesions of human carotid arteries. Virchows Arch. 1998;433:559–565. doi: 10.1007/s004280050289. [DOI] [PubMed] [Google Scholar]
- 23.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–1809. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen KQ, Olesen P, Ledet T, Rasmussen LM. Bone morphogenetic proteins regulate osteoprotegerin and its ligands in human vascular smooth muscle cells. Endocrine. 2007;32:52–58. doi: 10.1007/s12020-007-9007-0. [DOI] [PubMed] [Google Scholar]
- 25.Cardus A, Panizo S, Parisi E, Fernandez E, Valdivielso JM. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res. 2007;22:860–866. doi: 10.1359/jbmr.070305. [DOI] [PubMed] [Google Scholar]
- 26.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 27.Enomoto H, Shiojiri S, Hoshi K, Furuichi T, Fukuyama R, Yoshida CA, Kanatani N, Nakamura R, Mizuno A, Zanma A, Yano K, Yasuda H, Higashio K, Takada K, Komori T. Induction of osteoclast differentiation by Runx2 through receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin regulation and partial rescue of osteoclastogenesis in Runx2−/− mice by RANKL transgene. J Biol Chem. 2003;278:23971–23977. doi: 10.1074/jbc.M302457200. [DOI] [PubMed] [Google Scholar]
- 28.Notoya M, Otsuka E, Yamaguchi A, Hagiwara H. Runx-2 is not essential for the vitamin D-regulated expression of RANKL and osteoprotegerin in osteoblastic cells. Biochem Biophys Res Commun. 2004;324:655–660. doi: 10.1016/j.bbrc.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 29.Mori K, Kitazawa R, Kondo T, Maeda S, Yamaguchi A, Kitazawa S. Modulation of mouse RANKL gene expression by Runx2 and PKA pathway. J Cell Biochem. 2006;98:1629–1644. doi: 10.1002/jcb.20891. [DOI] [PubMed] [Google Scholar]
- 30.Usui M, Xing L, Drissi H, Zuscik M, O'Keefe R, Chen D, Boyce BF. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. J Bone Miner Res. 2008;23:314–325. doi: 10.1359/JBMR.071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36:57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, Coll B, Fernandez E, Valdivielso JM. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 33.Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 34.Kim DW, Lee HJ, Karmin JA, Lee SE, Chang SS, Tolchin B, Lin S, Cho SK, Kwon A, Ahn JM, Lee FY. Mechanical loading differentially regulates membrane-bound and soluble RANKL availability in MC3T3-E1 cells. Ann N Y Acad Sci. 2006;1068:568–572. doi: 10.1196/annals.1346.054. [DOI] [PubMed] [Google Scholar]
- 35.Schoppet M, Schaefer JR, Hofbauer LC. Low serum levels of soluble RANK ligand are associated with the presence of coronary artery disease in men. Circulation. 2003;107:e76. doi: 10.1161/01.cir.0000060815.25798.02. [DOI] [PubMed] [Google Scholar]
- 36.Duplaa C, Couffinhal T, Labat L, Moreau C, Petit-Jean ME, Doutre MS, Lamaziere JM, Bonnet J. Monocyte/macrophage recruitment and expression of endothelial adhesion proteins in human atherosclerotic lesions. Atherosclerosis. 1996;121:253–266. doi: 10.1016/0021-9150(95)05729-3. [DOI] [PubMed] [Google Scholar]
- 37.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 38.Breuil V, Schmid-Antomarchi H, Schmid-Alliana A, Rezzonico R, Euller-Ziegler L, Rossi B. The receptor activator of nuclear factor (NF)-kappaB ligand (RANKL) is a new chemotactic factor for human monocytes. FASEB J. 2003;17:1751–1753. doi: 10.1096/fj.02-1188fje. [DOI] [PubMed] [Google Scholar]
- 39.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura ES, Koizumi K, Kobayashi M, Saitoh Y, Arita Y, Nakayama T, Sakurai H, Yoshie O, Saiki I. RANKL-induced CCL22/macrophage-derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clin Exp Metastasis. 2006;23:9–18. doi: 10.1007/s10585-006-9006-1. [DOI] [PubMed] [Google Scholar]
- 41.Kim MS, Day CJ, Morrison NA. MCP-1 is induced by receptor activator of nuclear factor-{kappa}B ligand, promotes human osteoclast fusion, and rescues granulocyte macrophage colony-stimulating factor suppression of osteoclast formation. J Biol Chem. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- 42.Secchiero P, Corallini F, Barbarotto E, Melloni E, di Iasio MG, Tiribelli M, Zauli G. Role of the RANKL/RANK system in the induction of interleukin-8 (IL-8) in B chronic lymphocytic leukemia (B-CLL) cells. J Cell Physiol. 2006;207:158–164. doi: 10.1002/jcp.20547. [DOI] [PubMed] [Google Scholar]
- 43.Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2004;15:457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 45.Gleissner CA, von Hundelshausen P, Ley K. Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol. 2008;28:1920–1927. doi: 10.1161/ATVBAHA.108.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seshasayee D, Wang H, Lee WP, Gribling P, Ross J, Van Bruggen N, Carano R, Grewal IS. A novel in vivo role for osteoprotegerin ligand in activation of monocyte effector function and inflammatory response. J Biol Chem. 2004;279:30202–30209. doi: 10.1074/jbc.M403968200. [DOI] [PubMed] [Google Scholar]
- 47.Park HJ, Park OJ, Shin J. Receptor activator of NF-kappaB ligand enhances the activity of macrophages as antigen presenting cells. Exp Mol Med. 2005;37:524–532. doi: 10.1038/emm.2005.65. [DOI] [PubMed] [Google Scholar]
- 48.Valverde P, Tu Q, Chen J. BSP and RANKL induce osteoclastogenesis and bone resorption synergistically. J Bone Miner Res. 2005;20:1669–1679. doi: 10.1359/JBMR.050511. [DOI] [PubMed] [Google Scholar]
- 49.Collin-Osdoby P, Rothe L, Anderson F, Nelson M, Maloney W, Osdoby P. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem. 2001;276:20659–20672. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- 50.Tintut Y, Abedin M, Cho J, Choe A, Lim J, Demer LL. Regulation of RANKL-induced osteoclastic differentiation by vascular cells. J Mol Cell Cardiol. 2005;39:389–393. doi: 10.1016/j.yjmcc.2005.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.