Abstract
Background
The neurophysiological mechanisms underlying improved upper-extremity motor skills have been partially investigated in patients with good motor recovery but are poorly understood in more impaired individuals, the majority of stroke survivors.
Objective
The authors studied changes in primary motor cortex (M1) excitability (motor evoked potentials [MEPs], contralateral and ipsilateral silent periods [CSPs and ISPs] using transcranial magnetic stimulation [TMS]) associated with training-induced reaching improvement in stroke patients with severe arm paresis (n = 11; Upper-Extremity Fugl-Meyer score (F-M) = 27 ± 6).
Methods
All patients underwent a single session of reaching training focused on moving the affected hand from a resting site to a target placed at 80% of maximum forward reaching amplitude in response to a visual “GO” cue. Triceps contribute primarily as agonist and biceps primarily as antagonist to the trained forward reaching movement. Response times were recorded for each reaching movement.
Results
Preceding training (baseline), greater interhemispheric inhibition (measured by ISP) in the affected triceps muscle, reflecting inhibition from the nonlesioned to the lesioned M1, was observed in patients with lower F-M scores (more severe motor impairment). Training-induced improvements in reaching were greater in patients with slower response times at baseline. Increased MEP amplitudes and decreased ISPs and CSPs were observed in the affected triceps but not in the biceps muscle after training.
Conclusion
These results indicate that along with training-induced motor improvements, training-specific modulation of intrahemispheric and interhemispheric mechanisms occurs after reaching practice in chronic stroke patients with substantial arm impairment.
Keywords: stroke, arm movements, transcallosal inhibition, stroke rehabilitation, transcranial magnetic stimulation
Introduction
Reaching represents a fundamentally important human behavior and a key building block for functional independence in activities of daily living. Single-neuron and neural population activity studies have documented the role of the primary motor cortex (M1) in the planning and execution of reaching movements,1,2 including the transformation from a relatively abstract representation of the motor plan to the “mechanical details of their implementation.”3 This involvement includes feed-forward control of dynamics as well as online control of reaching direction and speed.4-6 In addition, M1 also contributes to learning of novel reaching movements.2,7,8
After a relatively severe stroke, abnormal M1 function is often accompanied by dysfunctional reaching and consequently poststroke motor disability.9 The motor impairments that contribute to dysfunctional reaching after stroke include difficulty activating elbow extensors (triceps) and shoulder flexors together in the implementation of a functional forward reaching movement10 and abnormal co-contraction of the biceps during such movements.11,12 Motor training in the setting of rehabilitative treatments can partially ameliorate reaching deficits even in patients with severe impairment.13,14 During the past 20 years, extensive research has been conducted on the ability of motor training to improve upper-extremity motor function after stroke.15,16 Noninvasive neuroimaging and neurophysiological techniques have allowed improved understanding of the mechanisms underlying these behavioral improvements, most often related to distal hand function and in patients with predominantly mild to moderate motor deficits. The cortical mechanisms of training-induced reaching improvements in patients with more severe hemiparesis have been less commonly studied. Yet this group of patients represents a substantial proportion of stroke patients17,18 and the one with the greatest need for effective treatments.
Evaluation of training-induced modulation of cortical physiology in severely involved stroke patients is particularly challenging and, consequently, underinvestigated. Contributing reasons include the inability of these patients to perform distal hand movements as required for many functional magnetic resonance imaging (fMRI) and transcranial magnetic stimulation (TMS) investigations, the difficulty of carrying out fMRI studies of proximal arm movements because of motion artifacts in the scanner, and high motor thresholds and frequently absent motor evoked potentials (MEPs) to TMS. In these situations, it is also not expedient to evaluate intracortical physiology using paired-pulse TMS techniques, such as short interval intracortical inhibition19 and paired-pulse measurements of, for example, interhemispheric inhibition between primary motor areas,20,21 which require the presence of relatively stable and reliable test MEPs.
Here, we studied the cortical mechanisms of training-induced improvements in forward reaching in patients with relatively severe hemiparesis, using a combination of TMS measurements recorded during low-level isometric motor activation. We hypothesized that training-induced improvements in reaching would be accompanied by modulation of intracortical functions that is specific to the muscle being actively trained. When patients with significant arm paresis attempt forward reaching movements, the biceps are excessively activated,10,22-24 and the triceps are underactivated.25 In addition, greater triceps activation is correlated with better reaching performance,25 and practice-induced improvements in reaching are accompanied by improved triceps activation and larger elbow extension amplitude during reaching.13,14,25,26 We therefore consider the triceps to be a prime mover in the forward reaching task we studied27 and a key muscle targeted by the reaching practice. Given these assumptions, we predicted increased corticospinal excitability and decreased interhemispheric and intracortical inhibition targeting the affected triceps muscle (partial agonist to the trained task) and to a lesser extent the affected biceps muscle (partial antagonist to the trained task).
Methods
Participants
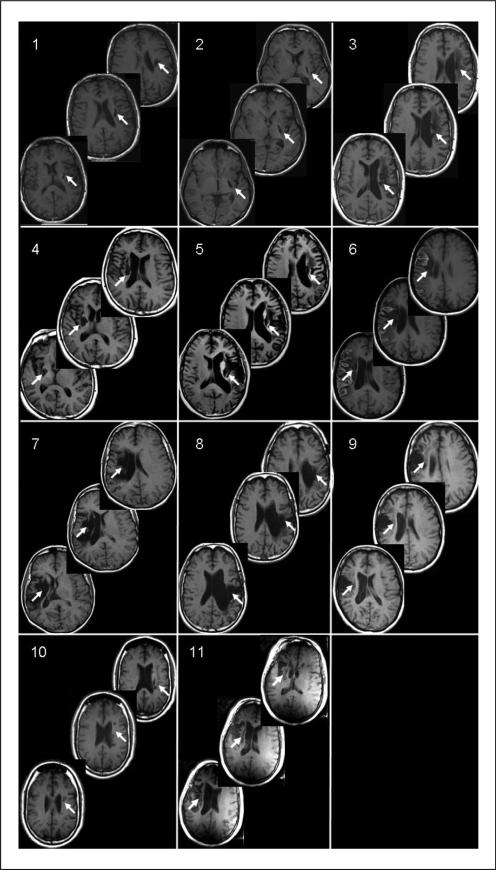
Participants provided written informed consent according to the Declaration of Helsinki under a study protocol approved by the NIH/NINDS Institutional Review Board, and all study procedures were conducted in accordance with institutional guidelines. A total of 11 individuals with chronic hemispheric stroke participated in the study (5 female; 54 ± 6 years old; 7 ± 1 years poststroke; 6 with left hemisphere lesions; all with sparing of M1, Figure 1).
Figure 1.
Lesion location: structural magnetic resonance images (MRI; T1-weighted) from 11 participants with chronic stroke. All participants had a single stroke that occurred more than 1 year prior to participating in the study. Images for participant 6 are postcontrast. Note that the lesion extends through at least 3 slices in all patients
Table 1 displays the clinical characteristics of each participant. Participants had little or no active finger or wrist movements and scored 26.7 ± 6.0 (40.5% ± 9.1% of the maximum score) on the Upper-Extremity Fugl-Meyer Assessment (F-M).
Table 1.
Patient Characteristics
| Patient | Age (years) | Gender | Time Since Stroke (years) | Lesioned Hemisphere | Stroke Etiology | UE Fugl-Meyer Score | UE Fugl-Meyer (Percentage of Maximum Score) | Hand Dominance Prior to Stroke | MRC Score (Fingers and Wrist) | Sensory Impairment | Hyper-Reflexia |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | M | 3.6 | Left | Hemorrhagic | 22 | 33 | Right | 0-1 | + | + |
| 2 | 52 | M | 4.3 | Left | Hemorrhagic | 21 | 32 | Right | 1-2 | + | + |
| 3 | 58 | M | 9.0 | Left | Ischemic | 29 | 44 | Right | 0 | + | + |
| 4 | 62 | F | 3.4 | Right | Ischemic | 33 | 50 | Right | 0-1 | + | + |
| 5 | 56 | M | 9.4 | Left | Ischemic | 22 | 33 | Right | 0-1 | + | + |
| 6 | 44 | F | 3.0 | Right | Ischemic | 27 | 41 | Right | 0-1 | + | + |
| 7 | 50 | F | 11.8 | Right | Ischemic | 23 | 35 | Right | 0-1 | + | + |
| 8 | 60 | M | 12.1 | Left | Hemorrhagic | 20 | 30 | Right | 0 | + | + |
| 9 | 50 | F | 5.5 | Right | Ischemic | 37 | 56 | Right | 1-2 | + | + |
| 10 | 60 | M | 1.8 | Left | Ischemic | 33 | 50 | Right | 1-3 | - | + |
| 11 | 53 | F | 8.2 | Right | Ischemic | N/A | N/A | Right | 2-4 | + | + |
| Mean | 54.9 | 6.55 | 26.7 | 40.5% | |||||||
| SD | 3.7 | 3.67 | 6.0 | 9.1% |
Abbreviations: UE, upper extremity; MRC, Medical Research Council; N/A, not available; SD, standard deviation.
The key inclusion criterion was the ability, while seated at a table, to move the affected hand forward at least 8 cm without leaning forward with the trunk. We also required that participants had lesions with sparing of M1. The key exclusion criterion was voluntary affected hand wrist and finger extension of >10°, a criterion that would make the patient eligible for rehabilitation interventions aimed at patients with more mild motor impairments, such as constraint-induced movement therapy, which have previously been studied.28 We also excluded patients with any disability that would interfere with performance of the reaching task and any contraindications to TMS.
Study Design
The study was performed over the course of 4 sessions (Figure 2C). On the first day (pretest 1), participants performed the reaching response time test described below. In addition, physiological measurements were made to familiarize the participant with the TMS procedures. On the second day, reaching training was immediately preceded (pretest 2) and followed (posttest) by measurements of cortical function and reaching response time. To measure any behavioral retention effects, we performed follow-up tests of reaching response time 24 hours (24-hour retention) and 30 days (30-day retention) after the single training session.
Figure 2.
Study design: tests of affected-arm reaching response time and primary motor cortex (M1) physiology were performed before and after a period of repetitive reaching practice (200 repetitions). (A) Reaching response time was measured with the participant seated in a high-backed chair with the trunk restrained. A 6-cm diameter button placed on the table in front of the participant served as the reaching target. Verbal instruction: “When you see the Go signal, quickly reach out with your (left or right) hand to press the button.” (B) M1 physiology was measured with constant, low-level background activation of the biceps or triceps in the affected arm. Visual feedback of current muscle activation and a target level was displayed on a monitor in front of the participant. Transcranial magnetic stimulation (TMS) was applied to the lesioned and nonlesioned hemispheres. Stereotactic coregistration of the TMS coil with the patient's MRI was used to verify that the hotspot was located on the precentral gyrus in the area of the hand knob. (C) The precise sequence of testing and training procedures across the testing days is depicted, including measurement of behavioral retention 24 hours and 30 days after training
Reaching Response Time
Participants were seated in a high-backed chair at an adjustable-height table that was positioned just above the participant's thighs (Figure 2A). Crossed nonelastic nylon straps were used to prevent forward leaning of the trunk. Both hands were placed at the front edge of the table on targets marking the starting position. A response pad with an adjustable 6-cm diameter circular button was placed at 80% of the maximum forward distance that could be voluntarily reached by the affected arm. A monitor in front of the participant displayed a randomly timed “Go!” command. Superlab (Cedrus Corporation, San Pedro, CA) was used to deliver the “Go” signals and to calculate the reaching response time from the “Go” signal to button press (sample rate = 100 Hz). Participants were given the following instructions: “When you see the Go signal, quickly reach out and press the button with your (left or right) hand.” All testing was performed on the affected arm. The primary behavioral outcome measure was affected-arm response time measured in 2 blocks of 10 trials. We considered triceps and biceps as contributing agonist and antagonist, respectively, to the elbow extension required for this forward reaching task. Although both muscles also cross the shoulder joint, their primary function is at the elbow, and their long heads are generally agreed to have only a stabilizing role at the shoulder.29,30 Patients with severe motor impairments often have electromyograph (EMG) activity at rest and virtually always show less brisk changes in EMG when voluntarily starting movements, making determination of EMG onset and termination required for reaction and movement time measurements unreliable. To avoid these pitfalls, we chose overall response time (from the “Go” signal to button press) as our primary end-point measure.
EMG Recordings
The skin over the biceps and triceps muscles of both arms was prepared with mild abrasion and cleansed with alcohol. Disposable self-adhesive electrodes with conductive gel were applied 2 cm apart to the short and long head of the biceps and triceps, respectively, over the area of greatest muscle bulk in a tendon-muscle montage. Amplified and filtered EMG signals were recorded at 2 kHz in 5-s epochs beginning 1 s before the delivery of the TMS pulse using Signal software (CED, Cambridge). All tests of M1 excitability were conducted on the affected arm's biceps or triceps muscle separately. Because no MEP could be elicited in these muscles at rest even at the highest output of the stimulator, all physiological testing procedures were conducted with low-level background activation of the target muscle (~10% maximum voluntary isometric contraction [MVIC]). To keep background activation levels stable, participants viewed a monitor that provided online visual feedback of current background EMG activity as well as a fixed target level of activity (Figure 2B). Rest periods were given whenever the EMG began to either substantially drop from the target level or become otherwise unstable, as determined by the investigator, and whenever requested by the patient. Measurements of M1 excitability were obtained immediately before and following a session of affected-arm reaching training.
Motor Cortical Function
MEP amplitude
A figure-8-shaped TMS coil (each wing 9 cm in diameter) attached to a Magstim 200 stimulator (The Magstim Company Ltd, Wales, UK) was used to deliver TMS pulses. Because affected-arm MEPs could not be elicited at rest, the optimal coil position for each muscle was determined by identifying the location where an MEP or silent period was elicited during background activation of the target muscle. Additionally, we required that the lesioned hemisphere coil position could not vary more than 1 cm from the mirror location of the nonlesioned hemisphere M1 hot-spot, consistent with previous investigations31 and confirmed that the hotspot was located on the precentral gyrus (Figure 2B) using a stereotactic neuronavigation system and the participant's own MRI (Nexstim Inc, Helsinki, Finland). TMS stimuli were delivered at 80%, 90%, and 100% of maximum stimulator output (in fixed order, 10 trials per intensity) to the lesioned hemisphere M1 hotspot during voluntary activation of the target muscle, as described above. To monitor cortico-spinal excitability of the nonlesioned hemisphere, recruitment curves from this hemisphere were collected with both arms at rest. Stimuli were delivered from 90% to 150% of resting motor threshold of the unaffected arm's target muscle (biceps and triceps, 10 trials at each intensity) and were collected before and after training.
Contralateral silent period (CSP)
The CSP is measured in the context of background activation of the target muscle and is a period of reduced EMG activity, which follows any poststimulus facilitation. The CSP was calculated from the same trials as those used to calculated MEP amplitude (details of data analysis below).
Ipsilateral silent period (ISP)
Once again the inability to reliably elicit MEPs in the affected arm at rest or even in some cases with background activation prevented the use of common paired-pulse measurements of interhemispheric inhibition.20,21 Instead, we measured the ISP, a period of inhibition of ongoing EMG activity that has been shown to be transcallosally mediated and convey information in this case on inhibition from the nonlesioned to the lesioned M1.20,21,32,33 To obtain this measure, a TMS stimulus was applied to the nonlesioned hemisphere M1 hotspot during contraction of the affected arm. Stimuli were applied at 5 intensities from 110% to 150% of resting motor threshold of the unaffected arm's target muscle (biceps or triceps). A total of 20 trials were collected at each of the 5 intensities in randomized order.
Training Procedures
Reaching training with trunk restraint, similar to that used by others,13,14 was performed with the participant positioned in the same manner as for the reaching response time testing described above. In all, 200 repetitions of the reaching task with the affected arm were performed in 20 blocks of 10 trials. Patients were instructed to quickly reach out and press the button after the “Go” signal appeared. Participants were allowed to initiate each new block of 10 trials at will by pressing a “Ready” button with their unaffected hand. In this way, rest periods were allowed between blocks of trials as needed. On average, training sessions lasted 35 to 50 minutes.
Data Analysis
Response time was calculated as the time elapsing between the “Go” signal and the button press. For each testing session, 20 trials were recorded and the median value used for subsequent analysis. Accuracy was quantified as the percentage of successful button presses out of the 20 attempted trials.
For analysis of EMG data, 5-s windows, which included 1 s of prestimulus data, were averaged together and the resulting waveform used for subsequent analysis. MEP amplitude was measured as the peak-to-peak amplitude of the EMG activity occurring between 15 and 55 ms after the delivery of the TMS pulse in the averaged, nonrectified trials. Affected-arm biceps MEP amplitudes from 2 participants were not included because the EMG signal during the MEP time window was obscured by prolonged stimulus artifact.
To quantify CSP, the point where the averaged, rectified EMG level dropped and stayed below prestimulus levels (defined as the average activity recorded from a 100 ms period [150 to 50 ms] prior to stimulus onset) for at least 10 ms was considered to be the CSP onset, and the time point when it returned to and stayed at or above prestimulus levels for at least 10 ms was considered the offset. CSP duration was defined as the time between stimulus delivery and the CSP offset and is the most widely accepted method of quantifying the CSP.34-42 We also measured the percentage inhibition during the CSP for descriptive purposes. Affected-arm biceps CSP from 2 participants were not included in the analysis because the EMG during the expected CSP time window was obscured by prolonged stimulus artifact.
The ISP was quantified by measuring ISP inhibition or the degree to which the background EMG was suppressed during the silent period, the most commonly used and best validated approach for this measure.20,21,32,33 ISP inhibition is the area below the prestimulus average taken between the silent period onset and offset, expressed as a percentage of prestimulus EMG area calculated over an equal duration. ISP inhibition is therefore the percentage suppression of the background EMG, calculated as [1 – (ISP Area/Prestimulus Area)] × 100. For descriptive purposes, we also measured ISP latency and the duration from ISP onset to offset.
Statistical Analysis
A 1-way repeated measures analysis of variance (ANOVA) was performed to test reaching response time across test sessions. A 2-way repeated measures ANOVA with the factors Stimulation Intensity and Test Session was performed to test for differences in affected-arm MEP amplitude, CSP duration, and ISP inhibition as well as possible differences in the level of background EMG activity during testing. Finally, we tested for baseline differences between biceps and triceps MEP amplitude, CSP duration, and ISP inhibition using a Student's paired t test. For between-muscle comparisons, MEP amplitudes were normalized to the level of prestimulus EMG (defined as the average rectified activity recorded over the 100 ms before stimulus onset). We calculated Pearson product moment coefficients to describe any baseline relationships between these physiological measures and arm impairment severity and between practice-induced changes in behavior and changes in the physiological measurements. Significance levels were set at .05. All statistical analyses were run using SPSS 14.0 (SPSS Inc, Chicago, IL). Data are reported below as mean ± standard error.
Results
Motor Cortical Function and Arm Impairment at Baseline
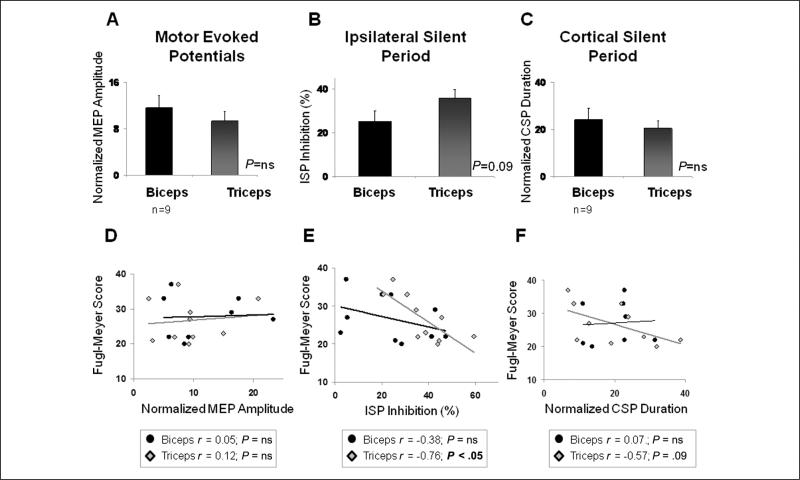
Figure 3 displays the physiological measurements in the affected-arm biceps compared with triceps (averaged across stimulation intensities) at baseline, before any training occurred, and shows their respective relationships to the severity of arm impairment observed at baseline. Inspection of this figure reveals a tendency toward greater interhemispheric inhibition (ISP) in triceps than biceps (P = .09; Figure 3B) and that patients with greater interhemispheric inhibition in the triceps muscle were those with more severe arm impairment (P < .05; Figure 3E). Patients with longer CSP durations in the triceps muscle also tended to be more severely impaired (P = .09; Figure 3F).
Figure 3.
Physiology and arm impairment at baseline: baseline (pretraining) physiological measurements (averaged across stimulation intensities) in the triceps and biceps muscles and their relationship to baseline upper-extremity motor impairment (Fugl-Meyer score) are depicted. Normalized baseline motor-evoked potential (MEP) amplitudes (A) and cortical silent period (CSP) durations (C) were comparable in the biceps and triceps. Although there was a tendency for the ipsilateral silent period (ISP, B) to be of larger magnitude in the triceps than biceps, this trend did not reach statistical significance (P = .09). Greater ISP inhibition in the triceps muscle was significantly correlated with lower baseline Fugl-Meyer scores (ie, more impairment; P < .05; E), and there was a trend toward longer CSP durations being associated with lower baseline Fugl-Meyer scores as well (P = .09; F). No significant relationship was detected between MEP amplitude and baseline arm impairment (D). n = 11 except where otherwise indicated
Training Effects
Reaching response time
Reaching training resulted in faster affected-arm reaching response times, an effect that remained present 24 hours later (day 3; Figure 4A; P < .05) but not 30 days later (day 30). We used paired t tests for post hoc comparisons of reaching response time between the average of the 2 pretests (day 1 and day 2 pretest) and (1) day 2 post-training (P = .062), (2) day 3 (P = .004), and (3) day 30 (P = .162). The comparison between response time at pretest and day 3 remains significant after a Bonferroni correction for multiple comparisons (α = .017). On average, response times were decreased by 14.3% ± 4.2% or, in absolute time, 253 ± 87 ms after training. Accuracy (the percentage of successful button presses) was high and not significantly changed after reaching training (average pretraining and posttraining values = 94.2% ± 1.5% and 95.0% ± 2.0%, respectively). Interestingly, training-induced improvement in response time, calculated as ([Post – Pre]/Pre) × 100, correlated with baseline response time, such that participants with the slowest response times at baseline showed the largest training-induced improvements (Figure 4B; r = 0.74; P < .05).
Figure 4.
Affected-arm reaching response time: affected-arm reaching response times were faster immediately after training (P = .06) and 24 hours later (P < .01) but not 30 days after training (P = .16; A). Patients with the slowest response times pretraining showed the greatest practice-induced improvements (B) *P < .05
Corticospinal excitability
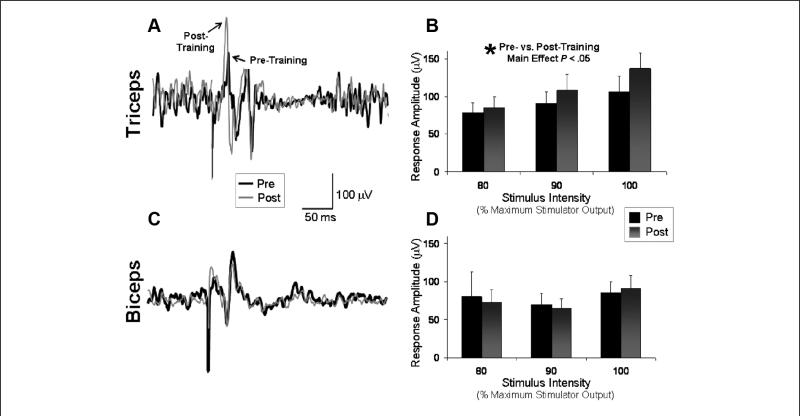
As depicted in a single participant in Figure 5A, higher peak-to-peak MEP amplitudes were observed in the affected triceps muscle after training, indicating increased corticospinal excitability in this muscle. Average triceps MEP amplitudes pretraining versus posttraining were 91.3 ± 17.0 and 109.2 ± 20.1 μV, respectively, when averaged across the 3 stimulation intensities. The results of the repeated-measures ANOVA indicated a significant main effect of Intensity, indicating higher MEP amplitudes at higher stimulation intensities (F = 9.84; P < .01) and, more important, a main effect of Time, indicating significantly higher MEP amplitudes at posttesting versus pretesting (F = 8.37; P < .05; Figure 5B). Figure 5C displays affected biceps MEP amplitudes for the same participant shown in Figure 5A. For the group, average biceps MEP amplitudes pretraining versus posttraining were 102.2 ± 33.4 and 88.7 ± 19.0 μV, respectively. Repeated measures ANOVA indicated no significant effect of Stimulation Intensity or Time and no significant Time × Intensity interaction effect (P = ns; Figure 5D).
Figure 5.
Affected-arm motor evoked potentials (MEPs): MEPs from the affected arm with stimulation of the lesioned hemisphere and background activation of the target muscle before and after a session of affected-arm reaching practice. The average of 10 trials is shown for the triceps (A) and biceps (C) for a single participant before (thick line) and after (thin line) training. Mean values (±standard error) for the group significantly increased in the triceps (B), but no significant pre–post difference was detected in the biceps (D)
On average, triceps MEP latencies were 13.3 ± 0.8 and 14.8 ± 1.1 ms, and biceps MEP latencies were 16.6 ± 1.6 and 17.2 ± 1.7 ms immediately before and after training, respectively (P = ns for each).
Ipsilateral silent period
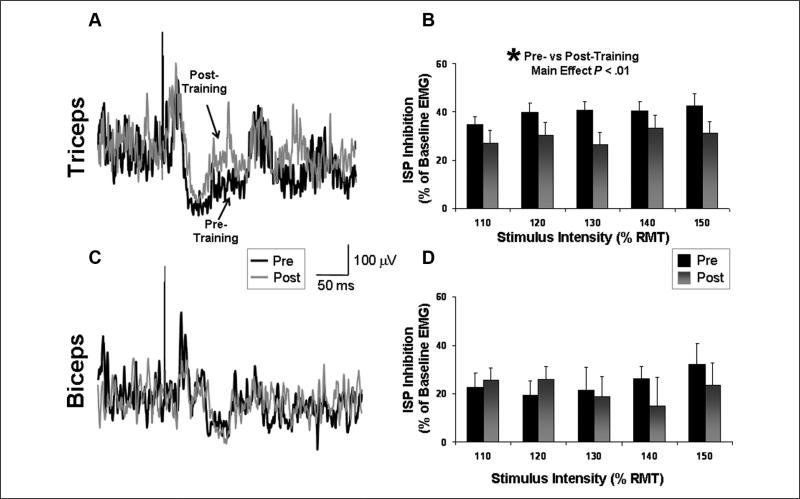
Repeated-measures ANOVA showed a significant main effect of Time for triceps ISP, indicating a significant difference between pretesting versus posttesting (F = 16.90; P < .01). The magnitude of ISP inhibition targeting the affected triceps muscle averaged across all stimulation intensities decreased from 39.7% ± 3.0% to 29.7% ± 4.3% after training, an effect that was observed at all 5 stimulation intensities tested (Figure 6B). The main effect of Intensity and the Time × Intensity interaction were not significant. On average, the triceps ISP latencies were 33.5 ± 1.4 and 34.2 ± 1.4 ms immediately before and after training, respectively (P = ns). Triceps ISP durations (measured from ISP onset to offset) were 38.8 ± 4.5 and 46.9 ± 7.4 ms immediately before and after training, respectively (P = ns).
Figure 6.
Ipsilateral silent period (ISP) recorded from the affected arm: ISP inhibition before and after a session of affected-arm reaching training. ISP inhibition is calculated as (1 – [EMG during ISP/EMG prestimulation]) × 100. The average of 20 trials is shown for the triceps (A) and biceps (C) for a single participant before (thick line) and after (thin line) training. Mean values (±standard error) for the group significantly decreased in the triceps (B), but no significant pre–post difference was detected in the biceps (D)
In contrast to the consistent and relatively strong ISP inhibition observed in the triceps, ISP inhibition targeting the affected biceps muscle tended to be less pronounced and was unchanged after training (Pre = 24.3% ± 5.8%; Post = 21.8% ± 6.6%). Repeated-measures ANOVA indicated no significant effect of Time, Stimulation Intensity, or Time × Intensity interaction (P = ns; Figures 6C and 6D). On average, the biceps ISP latencies were 47.2 ± 3.3 and 46.1 ± 3.6 ms immediately before and after training, respectively (P = ns). Biceps ISP durations were 45.5 ± 4.4 and 47.5 ± 4.9 ms immediately before and after training, respectively (P = ns).
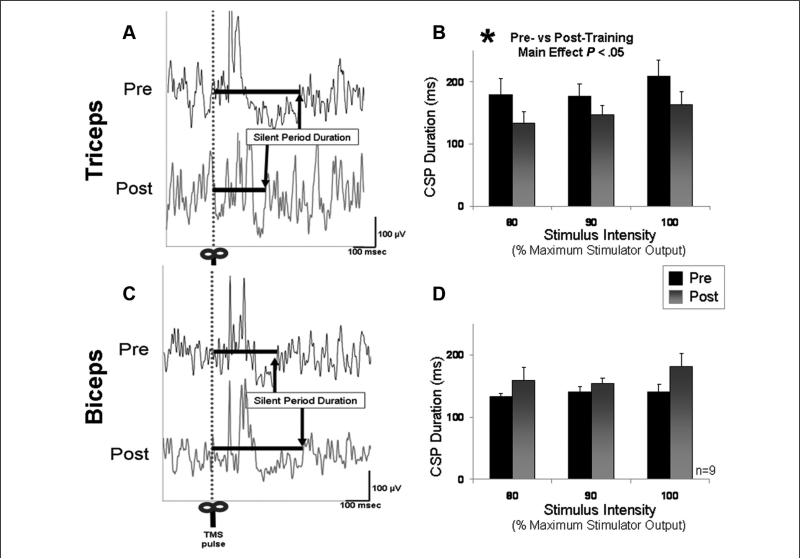
Contralateral silent period
The CSP duration targeting the affected triceps muscle increased with increasing stimulus intensity (F = 9.67; P < .01) and, more important, was shorter in duration after training (F = 5.79; P < .05). For the group, the duration of the CSP decreased in the affected triceps muscle from an average at baseline of 184.8 ± 22.0 ms to 150.5 ± 16.0 ms after training (Figures 7A and 7B). The percentage inhibition of background EMG during the triceps CSP averaged across the 3 stimulation intensities was 38.9% ± 4.1% before and 31.1% ± 4.4% after training. Repeated-measures ANOVA revealed a trend toward a posttraining decrease in this measure as well (F = 5.06; P = .07). Biceps CSP duration tended to increase from an average at baseline of 138.0 ± 6.3 to 160.9 ± 13.9 ms after training (P > .1; Figures 7C and 7D). The percentage inhibition of background EMG during the biceps CSP averaged across the 3 stimulation intensities was 11.7% ± 5.5% before and 20.9% ± 5.9% after training (P = ns).
Figure 7.
Cortical silent period (CSP) recorded from affected arm: duration of the CSP with lesioned hemisphere stimulation. (A) The average of 10 trials in the affected triceps muscle is shown for a single patient before and after training. Whereas the levels of prestimulus EMG were similar before and after training, the duration of the CSP decreased after training. (B) Displays the group means for intracortical inhibition targeting the affected triceps muscle. The triceps CSP duration significantly decreased after training (P < .05). (C) The average of 10 trials in the biceps muscle of the same patient displayed in A. (D) The group means for CSP in the affected biceps muscle before and after training. Though there was a tendency for longer biceps CSP durations after training, this tendency did not reach statistical significance (P > .10)
Control Data
No difference in corticospinal excitability of the nonlesioned hemisphere M1 (as measured by the slope of the recruitment curve) was observed after training for either biceps or triceps (Table 2, row A). The amount of background EMG produced was ~10% of that produced during a maximum voluntary isometric contraction. There were no significant differences in the level of background EMG activity produced before versus after training or between tests at different stimulation intensities (Table 2, rows B and C). In addition, unaffected-arm “mirror” activation during contralesional and ipsilesional stimulation when present was comparable for pretraining versus posttraining measurements (Table 2, rows D and E).
Table 2.
Control Data
| Triceps |
Biceps |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre |
Post |
Pre |
Post |
|||||||
| Control Variable | Mean | SD | Mean | SD | P Value | Mean | SD | Mean | SD | P Value |
| A. Slope of unaffected-arm recruitment curve | 1.57 | 1.20 | 1.54 | 1.44 | ns | 4.32 | 3.55 | 7.63 | 9.08 | ns |
| B. Affected-arm background EMG (contralesional stimulation) | 6.41 | 0.98 | 6.36 | 1.26 | ns | 5.31 | 1.82 | 6.23 | 1.99 | ns |
| C. Affected-arm background EMG (ipsilesional stimulation) | 9.71 | 2.09 | 9.69 | 2.21 | ns | 8.26 | 3.43 | 8.83 | 4.15 | ns |
| D. Unaffected-arm mirror EMG (contralesional stimulation; n = 3) | 3.47 | 4.25 | 3.24 | 2.54 | ns | 11.25 | 15.92 | 7.04 | 6.93 | ns |
| E. Unaffected-arm mirror EMG (ipsilesional stimulation; n = 3) | 3.42 | 3.63 | 4.07 | 3.27 | ns | 5.85 | 6.48 | 9.02 | 10.38 | ns |
Abbreviations: Pre, immediately before training; Post, immediately after training; EMG, mean rectified electromyographic activity (in μV) calculated over 100 ms before stimulus delivery; SD, standard deviation.
Discussion
Patient Population
The patient group included in the present study is not customarily targeted for mechanistic studies of rehabilitation training effects. They had substantial motor impairments (Upper-Extremity F-M score = 26.7 ± 6.0, corresponding to 40.5% ± 9.1% of the maximum score) and little or no voluntary finger or wrist movement but were able to perform some movements of the more proximal elbow and shoulder joints. In contrast, previous investigations into the mechanisms of upper-extremity rehabilitative training strategies have largely focused on relatively mildly affected and/or well-recovered individuals performing motor tasks with the wrist, hand, or fingers.15,16
Behavioral Results
A major challenge for the patient group we studied is moving the stroke-affected hand out away from the body and into a more functional workspace. The affected elbow tends to remain flexed, often with overactivation of the affected biceps and underactivation of the affected triceps muscle.12,43,44 Therefore, we chose to evaluate and train a reaching task because it is both functionally important and because it engages proximal arm muscles over which patients with severe motor deficits still exert some control. We observed a significant improvement in reaching response time after training, with no decrement in reaching accuracy. The relative improvement in response time for the group was 14.3% ± 4.2%, in line with the amount of improvement reported in previous single-session training studies performed in less-impaired stroke patients practicing a distal arm task.45-47 Patients who had the most impaired reaching response times at baseline made the largest training-induced improvements, a finding that further supports the potential usefulness of rehabilitative training strategies in more impaired individuals after stroke.13,14 We do not believe that this finding was a result of a ceiling effect of the task because our pilot recordings of healthy volunteers performing this task demonstrated much shorter response times despite much larger reach amplitudes.
Motor Cortex Function at Baseline
We found that patients with greater interhemispheric inhibition (ISP) at baseline, partly reflecting stronger inhibition from the nonlesioned to the lesioned hemisphere, had more severe arm impairment as measured by the F-M score (Figure 3E). This suggestion of a mechanistic link seems to be present also in patients with more successful motor recovery in whom abnormal persistence of premovement interhemispheric inhibition targeting the affected hand was present among those with less finger strength (lower MRC scores) and slower finger tapping speeds.48,49 These correlations identify an exciting area of investigation but stop short of identifying a cause–effect link. They provide further support for the notion that interhemispheric inhibition targeting the affected arm, alone or in combination with intracortical GABAergic circuits within the lesioned hemisphere,50,51 is a factor that may influence the severity of motor impairment after stroke.
Changes in Motor Cortex Function With Training
Corticospinal excitability
Training-induced improvements in reaching were accompanied by an increase in lesioned hemisphere M1 corticospinal excitability targeting the triceps, but no such increase was observed in the biceps muscle, a differential training effect that is consistent with previous findings in healthy participants performing a distal hand training task52 and that may have contributed to training-dependent performance improvements in this study. This finding is in agreement with previous work conducted in less-impaired patients performing distal hand tasks.45,46,53-55 Trained-induced changes in corticospinal excitability, or the lack thereof, in muscles antagonist to those engaged in performance of these distal tasks in chronic stroke patients have not, to our knowledge, been previously reported. Therefore, an additional conclusion from our data is that excitability changes demonstrated after motor training in stroke patients with more severe impairment are likely to be specific to the role that each muscle plays in relation to the trained motor tasks.
Interhemispheric inhibition
Training-induced improvements in affected-arm reaching were also accompanied by decreased ISP, partially reflecting interhemispheric inhibition targeting the affected triceps, whereas no change was detected in the biceps. This finding, to our knowledge, represents the first demonstration of a training-induced modulation of the nonlesioned- to lesioned-hemisphere inhibitory influence in stroke patients. The slope of the recruitment curve for the unaffected triceps muscle showed no changes, indicating that there was no overall decrease in excitability in the nonlesioned M1 that could have accounted for this pre–post difference (Table 2, row A). Previous work has demonstrated that 1 month of motor training accompanied by a priming task induced changes in interhemispheric inhibition from the lesioned to the nonlesioned M1 in stroke patients who were better recovered than those in our study,56 but no data were reported on inhibition from the nonlesioned to the lesioned M1, the focus of our investigation. Though not training related, a decrease in interhemispheric inhibition from the nonlesioned to the lesioned M1 has been reported after a single session of transient ischemic cutaneous anesthesia of the unaffected hand57 and after a single session of inhibitory repetitive TMS applied to the contralesional hemisphere.58
Intracortical inhibition
Because affected-arm MEPs could not be elicited at rest and were not consistently elicited even with background activation, commonly used paired-pulse approaches to measuring intracortical inhibition could not be implemented in this patient group. Instead, we measured the CSP because changes in the CSP reflect changes in cortical inhibitory mechanisms, including intracortical inhibition (CSP).36,42 Although there is probably a spinal inhibitory component during the first 50 to 100 ms of the CSP, there is strong evidence that the CSP is largely mediated by GABAB intracortical inhibitory circuits, and it is an accepted method of measuring the excitability of these circuits.36,37,41,42 Similar to other measures of intracortical inhibition, the CSP has been shown to be disrupted after stroke.59-61 Reaching training led to decreases in the CSP consistent with decreased intracortical inhibition in the affected triceps but not the biceps muscle. Previous work has shown that CSP targeting distal muscles in the affected arm, which reflects activity in GABAB inhibitory circuits,40 is prolonged in acute stroke patients59,62,63 relative to healthy controls.60 Over time, CSP shortens in association with functional recovery.59
A comparison of our physiological findings with those in healthy volunteers or with other reports on stroke patients is challenging because few other studies have reported these measurements from the more proximal arm muscles studied here. It is, however, well known that in healthy individuals, MEP amplitudes are lower and motor thresholds higher in proximal than in distal arm muscles. Previous studies have reported average peak-to-peak amplitudes of 150 to 200 μV in the biceps and triceps muscles of healthy controls.64,65 Gerachshenko et al43 were unable to elicit MEPs in the affected biceps muscle of stroke patients (F-M = 37.4; 16 participants; data included from 8). Instead, they applied TMS just before EMG onset while participants were performing repetitive, rhythmic, externally paced movements. The MEP amplitudes recorded were surprisingly large (480 ± 130 μV prior to flexor and 350 ± 140 μV prior to pronator activation). What was interesting was that the values in healthy volunteers were lower (210 ± 70 μV before flexor and 30 ± 7 μV before pronation), leading the authors to suggest possible abnormalities in inhibition of the biceps muscle after stroke. In the present study, with low-level background activation and high stimulus intensities, MEP amplitudes recorded from the biceps and triceps were around 85 to 105 μV—somewhat lower than what has been reported in healthy volunteers.
CSP duration in the biceps of healthy volunteers has been reported to be around 190 to 200 ms,66,67 similar to values reported in more distal muscles. Abnormally long CSPs have been reported in the hand muscles of patients with stroke-related motor impairment, and shortening of the CSP tends to occur in parallel with clinical recovery.68 The average CSPs we recorded from the biceps and triceps were around 150 to 200 ms, apparently within normal limits yet malleable to training. The area of the ISP expressed as a percentage of prestimulus EMG activity has been reported to be around 40% to 60% inhibition in the distal muscles of healthy volunteers.20,21,69 Similar levels have been reported in the unaffected hand muscles of stroke patients.70 We recorded 20% to 40% inhibition in the affected biceps and triceps of significantly impaired stroke patients.
Although it has been shown that even severely impaired patients can make improvements with intensive training,13,71,72 the mechanisms of training-induced upper-extremity motor recovery remain poorly explained for these patients because they are difficult to evaluate appropriately with fMRI and TMS investigations that require intact MEP responses to TMS. Here, we demonstrate that even in stroke patients with relatively severe motor impairment, plastic changes can be elicited in association with a single session of intense, repetitive motor practice. Previous studies have shown that changes in affected-hemisphere physiology in response to a single training session could predict response to longer-term training.73 The plastic changes we observed were similar to those of use-dependent plasticity studies in which the kinematic details of the trained movement seem to become encoded in M1, and excitability of the trained (agonist) muscle is enhanced.74 In addition, there appears to be a particularly prominent role of interhemispheric inhibition targeting the affected triceps in these patients as it (1) correlates with arm impairment severity and (2) significantly changes with triceps motor practice. Because our primary behavioral measure was response time, which encompasses movement and reaction time, other brain areas, such as those involved in motor preparation and planning (prefrontal and premotor areas), may also have contributed.
In summary, our findings suggest that training-induced improvements in functional reaching movements come about in association with training-specific changes in intracortical circuits of M1 consisting of corticospinal excitability and diminished interhemispheric and intracortical inhibition targeting the predominantly agonist triceps muscle with no such changes observed in the primarily antagonist biceps. More broadly, our findings suggest that intracortical mechanisms controlling different muscle groups in a stroke-affected arm can show training-related changes in one muscle group and not another, based on the training task performed—an effect that could have contributed to the significant variability identified in some of these measurements in previous investigations.
Acknowledgments
We thank the study participants for their time and effort as well as Michelle Sarin, Chris Nicholas, Tre Zimmermann, and Reginald Kapteyn for their assistance with the project. We also thank Dr Michael Dimyan for helpful comments on an earlier version of the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: this work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS), Intramural Program (MH-L, MAP, LGC), and the National Institute of Child Health and Development K01 HD050369 (SMM).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Crammond DJ, Kalaska JF. Differential relation of discharge in primary motor cortex and premotor cortex to movements versus actively maintained postures during a reaching task. Exp Brain Res. 1996;108:45–61. doi: 10.1007/BF00242903. [DOI] [PubMed] [Google Scholar]
- 2.Kawashima R, Roland PE, O'Sullivan BT. Fields in human motor areas involved in preparation for reaching, actual reaching, and visuomotor learning: a positron emission tomography study. J Neurosci. 1994;14:3462–3474. doi: 10.1523/JNEUROSCI.14-06-03462.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalaska JF, Sergio LE, Cisek P. Cortical control of whole-arm motor tasks. Novartis Found Symp. 1998;218:176–190. doi: 10.1002/9780470515563.ch10. discussion 190-201. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MT, Ebner TJ. Processing of multiple kinematic signals in the cerebellum and motor cortices. Brain Res Brain Res Rev. 2000;33:155–168. doi: 10.1016/s0165-0173(00)00027-8. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AB, Moran DW. Arm trajectory and representation of movement processing in motor cortical activity. Eur J Neurosci. 2000;12:1851–1856. doi: 10.1046/j.1460-9568.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 6.Sergio LE, Hamel-Paquet C, Kalaska JF. Motor cortex neural correlates of output kinematics and kinetics during isometric-force and arm-reaching tasks. J Neurophysiol. 2005;94:2353–2378. doi: 10.1152/jn.00989.2004. [DOI] [PubMed] [Google Scholar]
- 7.Gandolfo F, Li C, Benda BJ, Schioppa CP, Bizzi E. Cortical correlates of learning in monkeys adapting to a new dynamical environment. Proc Natl Acad Sci U S A. 2000;97:2259–2263. doi: 10.1073/pnas.040567097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson AG, Overduin SA, Valero-Cabre A, et al. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci. 2006;26:12466–12470. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desrosiers J, Noreau L, Rochette A, Bourbonnais D, Bravo G, Bourget A. Predictors of long-term participation after stroke. Disabil Rehabil. 2006;28:221–230. doi: 10.1080/09638280500158372. [DOI] [PubMed] [Google Scholar]
- 10.Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127(pt 5):1035–1046. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]
- 11.Canning CG, Ada L, O'Dwyer NJ. Abnormal muscle activation characteristics associated with loss of dexterity after stroke. J Neurol Sci. 2000;176:45–56. doi: 10.1016/s0022-510x(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 12.Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 13.Michaelsen SM, Dannenbaum R, Levin MF. Task-specific training with trunk restraint on arm recovery in stroke: randomized control trial. Stroke. 2006;37:186–192. doi: 10.1161/01.STR.0000196940.20446.c9. [DOI] [PubMed] [Google Scholar]
- 14.Michaelsen SM, Levin MF. Short-term effects of practice with trunk restraint on reaching movements in patients with chronic stroke: a controlled trial. Stroke. 2004;35:1914–1919. doi: 10.1161/01.STR.0000132569.33572.75. [DOI] [PubMed] [Google Scholar]
- 15.Hodics T, Cohen LG, Cramer SC. Functional imaging of intervention effects in stroke motor rehabilitation. Arch Phys Med Rehabil. 2006;87(suppl 2):S36–S42. doi: 10.1016/j.apmr.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Richards LG, Stewart KC, Woodbury ML, Senesac C, Cauraugh JH. Movement-dependent stroke recovery: a systematic review and meta-analysis of TMS and fMRI evidence. Neuropsychologia. 2008;46:3–11. doi: 10.1016/j.neuropsychologia.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhamoon MS, Moon YP, Paik MC, et al. Long-term functional recovery after first ischemic stroke: the Northern Manhattan Study. Stroke. 2009;40:2805–2811. doi: 10.1161/STROKEAHA.109.549576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. Compensation in recovery of upper extremity function after stroke: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75:852–857. doi: 10.1016/0003-9993(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 19.Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blicher JU, Jakobsen J, Andersen G, Nielsen JF. Cortical excitability in chronic stroke and modulation by training: a TMS study. Neurorehabil Neural Repair. 2009;23:486–493. doi: 10.1177/1545968308328730. [DOI] [PubMed] [Google Scholar]
- 21.Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123(pt 5):940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 23.Trombly CA. Deficits of reaching in subjects with left hemiparesis: a pilot study. Am J Occup Ther. 1992;46:887–897. doi: 10.5014/ajot.46.10.887. [DOI] [PubMed] [Google Scholar]
- 24.Wolf SL, Catlin PA, Blanton S, Edelman J, Lehrer N, Schroeder D. Overcoming limitations in elbow movement in the presence of antagonist hyperactivity. Phys Ther. 1994;74:826–835. doi: 10.1093/ptj/74.9.826. [DOI] [PubMed] [Google Scholar]
- 25.Barker RN, Brauer S, Carson R. Training-induced changes in the pattern of triceps to biceps activation during reaching tasks after chronic and severe stroke. Exp Brain Res. 2009;196:483–496. doi: 10.1007/s00221-009-1872-8. [DOI] [PubMed] [Google Scholar]
- 26.Woodbury ML, Howland DR, McGuirk TE, et al. Effects of trunk restraint combined with intensive task practice on post-stroke upper extremity reach and function: a pilot study. Neurorehabil Neural Repair. 2009;23:78–91. doi: 10.1177/1545968308318836. [DOI] [PubMed] [Google Scholar]
- 27.Prange GB, Jannink MJ, Stienen AH, van der Kooij H, Ijzerman MJ, Hermens HJ. Influence of gravity compensation on muscle activation patterns during different temporal phases of arm movements of stroke patients. Neurorehabil Neural Repair. 2009;23:478–485. doi: 10.1177/1545968308328720. [DOI] [PubMed] [Google Scholar]
- 28.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 29.Levy AS, Kelly BT, Lintner SA, Osbahr DC, Speer KP. Function of the long head of the biceps at the shoulder: electromyographic analysis. J Shoulder Elbow Surg. 2001;10:250–255. doi: 10.1067/mse.2001.113087. [DOI] [PubMed] [Google Scholar]
- 30.Halder AM, Halder CG, Zhao KD, O'Driscoll SW, Morrey BF, An KN. Dynamic inferior stabilizers of the shoulder joint. Clin Biomech. 2001;16:138–143. doi: 10.1016/s0268-0033(00)00077-2. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125(pt 8):1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- 32.Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118(pt 2):429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- 33.Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- 34.Dimyan MA, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of functional recovery mechanisms after stroke. Neurorehabil Neural Repair. 2010;24:125–135. doi: 10.1177/1545968309345270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curra A, Modugno N, Inghilleri M, Manfredi M, Hallett M, Berardelli A. Transcranial magnetic stimulation techniques in clinical investigation. Neurology. 2002;59:1851–1859. doi: 10.1212/01.wnl.0000038744.30298.d4. [DOI] [PubMed] [Google Scholar]
- 36.Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- 37.Davey NJ, Romaiguere P, Maskill DW, Ellaway PH. Suppression of voluntary motor activity revealed using transcranial magnetic stimulation of the motor cortex in man. J Physiol. 1994;477(pt 2):223–235. doi: 10.1113/jphysiol.1994.sp020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Priori A, Berardelli A, Inghilleri M, Accornero N, Manfredi M. Motor cortical inhibition and the dopaminergic system. Pharmacological changes in the silent period after transcranial brain stimulation in normal subjects, patients with Parkinson's disease and drug-induced parkinsonism. Brain. 1994;117(pt 2):317–323. doi: 10.1093/brain/117.2.317. [DOI] [PubMed] [Google Scholar]
- 39.Ziemann U, Lonnecker S, Paulus W. Inhibition of human motor cortex by ethanol: a transcranial magnetic stimulation study. Brain. 1995;118(pt 6):1437–1446. doi: 10.1093/brain/118.6.1437. [DOI] [PubMed] [Google Scholar]
- 40.Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 41.Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517(pt 2):591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol. 2007;583(pt 3):971–982. doi: 10.1113/jphysiol.2007.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerachshenko T, Rymer WZ, Stinear JW. Abnormal corticomotor excitability assessed in biceps brachii preceding pronator contraction post-stroke. Clin Neurophysiol. 2008;119:683–692. doi: 10.1016/j.clinph.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lum PS, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle Nerve. 2003;27:211–221. doi: 10.1002/mus.10305. [DOI] [PubMed] [Google Scholar]
- 45.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31:1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]
- 46.Muellbacher W, Richards C, Ziemann U, et al. Improving hand function in chronic stroke. Arch Neurol. 2002;59:1278–1282. doi: 10.1001/archneur.59.8.1278. [DOI] [PubMed] [Google Scholar]
- 47.Zittel S, Weiller C, Liepert J. Citalopram improves dexterity in chronic stroke patients. Neurorehabil Neural Repair. 2008;22:311–314. doi: 10.1177/1545968307312173. [DOI] [PubMed] [Google Scholar]
- 48.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55:400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 49.Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28:940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 50.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543(pt 1):317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci. 2008;28:5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- 53.Liepert J, Uhde I, Graf S, Leidner O, Weiller C. Motor cortex plasticity during forced-use therapy in stroke patients: a preliminary study. J Neurol. 2001;248:315–321. doi: 10.1007/s004150170207. [DOI] [PubMed] [Google Scholar]
- 54.Sawaki L, Butler AJ, Leng X, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22:505–513. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 56.Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain. 2008;131(pt 5):1381–1390. doi: 10.1093/brain/awn051. [DOI] [PubMed] [Google Scholar]
- 57.Floel A, Hummel F, Duque J, Knecht S, Cohen LG. Influence of somatosensory input on interhemispheric interactions in patients with chronic stroke. Neurorehabil Neural Repair. 2008;22:477–485. doi: 10.1177/1545968308316388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- 59.Classen J, Schnitzler A, Binkofski F, et al. The motor syndrome associated with exaggerated inhibition within the primary motor cortex of patients with hemiparetic. Brain. 1997;120(pt 4):605–619. doi: 10.1093/brain/120.4.605. [DOI] [PubMed] [Google Scholar]
- 60.Kukowski B, Haug B. Quantitative evaluation of the silent period, evoked by transcranial magnetic stimulation during sustained muscle contraction, in normal man and in patients with stroke. Electromyogr Clin Neurophysiol. 1992;32:373–378. [PubMed] [Google Scholar]
- 61.Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- 62.Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000;23:1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 63.Ahonen JP, Jehkonen M, Dastidar P, Molnar G, Hakkinen V. Cortical silent period evoked by transcranial magnetic stimulation in ischemic stroke. Electroencephalogr Clin Neurophysiol. 1998;109:224–229. doi: 10.1016/s0924-980x(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 64.Krutky MA, Perreault EJ. Motor cortical measures of use-dependent plasticity are graded from distal to proximal in the human upper limb. J Neurophysiol. 2007;98:3230–3241. doi: 10.1152/jn.00750.2007. [DOI] [PubMed] [Google Scholar]
- 65.Fujiwara K, Tomita H, Kunita K. Increase in corticospinal excitability of limb and trunk muscles according to maintenance of neck flexion. Neurosci Lett. 2009;461:235–239. doi: 10.1016/j.neulet.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 66.Loscher WN, Nordlund MM. Central fatigue and motor cortical excitability during repeated shortening and lengthening actions. Muscle Nerve. 2002;25:864–872. doi: 10.1002/mus.10124. [DOI] [PubMed] [Google Scholar]
- 67.Todd G, Petersen NT, Taylor JL, Gandevia SC. The effect of a contralateral contraction on maximal voluntary activation and central fatigue in elbow flexor muscles. Exp Brain Res. 2003;150:308–313. doi: 10.1007/s00221-003-1379-7. [DOI] [PubMed] [Google Scholar]
- 68.van Kuijk AA, Pasman JW, Geurts AC, Hendricks HT. How salient is the silent period? The role of the silent period in the prognosis of upper extremity motor recovery after severe stroke. J Clin Neurophysiol. 2005;22:10–24. doi: 10.1097/01.wnp.0000150975.83249.71. [DOI] [PubMed] [Google Scholar]
- 69.Cincotta M, Borgheresi A, Balestrieri F, et al. Mechanisms underlying mirror movements in Parkinson's disease: a transcranial magnetic stimulation study. Mov Disord. 2006;21:1019–1025. doi: 10.1002/mds.20850. [DOI] [PubMed] [Google Scholar]
- 70.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- 71.Lo AC, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–1783. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. 2008;22:706–714. doi: 10.1177/1545968308317436. [DOI] [PubMed] [Google Scholar]
- 73.Koski L, Mernar TJ, Dobkin BH. Immediate and long-term changes in corticomotor output in response to rehabilitation: correlation with functional improvements in chronic stroke. Neurorehabil Neural Repair. 2004;18:230–249. doi: 10.1177/1545968304269210. [DOI] [PubMed] [Google Scholar]
- 74.Duque J, Mazzocchio R, Stefan K, Hummel F, Olivier E, Cohen LG. Memory formation in the motor cortex ipsilateral to a training hand. Cereb Cortex. 2008;18:1395–1406. doi: 10.1093/cercor/bhm173. [DOI] [PubMed] [Google Scholar]