Abstract
Although known for the important function in the immune system, MHC class I molecules are increasingly ascribed an alternative role in modifying signal transduction. In medulloblastoma, HLA class I molecules are associated with poor prognosis, and can induce ERK1/2 activation upon engagement with ligands that bind to incompletely assembled complexes (so called open conformers). We here demonstrate that ERK1/2 activation in medulloblastoma can occur in the absence of endogenously synthesized β2m, formally excluding involvement of closed HLA class conformation. In addition, several experimental observations suggest that heterogeneity of HLA class I expression may be a reflection of the status of original cells before transformation, rather than a consequence of immune-based selection of HLA-loss mutants. These results contribute to our understanding of an immune system-independent role of HLA class I in the pathology of medulloblastoma, and cancer in general.
Keywords: HLA, Medulloblastoma, Cerebellum, Signaling
Introduction
MHC class I molecules are heterodimers formed by a single transmembrane polypeptide chain non-covalently bound to a beta-2-microglobulin (β2m) subunit [1, 2]. Heavy chain/β2m dimers bind peptides generated in the cytosol via proteasome-mediated protein degradation. These peptides are translocated into the lumen of the ER by the aid of transporter associated with antigen processing (TAP), formed by two subunits, TAP1 and TAP2. Peptides are then loaded onto nascent heavy chain/β2m dimers. The completion of this loading allows for MHC class I molecules to exit the ER through the secretory pathway and reach the cell surface. Once at the cell surface, MHC class I molecules may present peptides to activate CD8+ T-cells.
Besides their role in the immune system, MHC class I molecules may also be involved in altering signal transduction by virtue of cis-associations with cell surface receptors [3]. This may have important consequences, as demonstrated by negative prognostic impact of HLA class I expression in several types of cancer [4–7]. This function contrasts with the implied role of HLA class I molecules in enabling tumor destruction by the immune system, where expression of HLA class I is a favorable prognostic sign [8–11]. In this case, HLA class I-loss variants arise through mutations in the genes encoding one or more components required for the assembly of HLA class I, followed by unopposed growth of the mutated cancer cells [12, 13]. Different roles of HLA class I do not arise simply due to factors related to individual experimental conditions, as both positive and negative association with disease outcome were observed in a single study of colon carcinoma [14]. Here, low levels of HLA class I were associated with poor outcome, whereas complete loss of expression was associated with extended survival. Our recent studies on medulloblastoma provided an insight into the mechanism of the contribution of HLA class I to the poor prognosis of the disease [7]. These studies showed that HLA class I was expressed in a subset of medulloblastomas with poor prognosis, and that interaction of two subunits of MHC class I induced ERK1/2 phosphorylation and increased the migratory potential of medulloblastoma cells [7]. We here extend these findings to formally show that fully assembled HLA class I molecules are not required for the signaling function and that heterogeneity of HLA class I expression reflects the dynamic developmental pattern of HLA expression by neurons of the external granular layer in cerebellum, the presumed precursor cell of most medulloblastomas.
Methods
Immunohistochemistry
Following IRB approval for the construction and analysis of tissue microarrays and normal cerebellar tissue specimens, medulloblastoma and adult glioblastoma tissue microarrays were constructed as previously described [7, 15]. Deidentified cerebellum tissue was obtained post mortem from six patients from Children's National Medical Center, age ranging from prenatal 28 weeks to postnatal 3 years. The cause of death was not related to damage to the brain. Tissues were paraformaldehyde fixed and preserved in paraffin blocks. 4–8 μm sections were cut from these blocks and adhered to slides. Staining with anti-human HLA heavy chain (HC-10), anti-TAP1 (NOB-1) and TAP2 (NOB-2) antibodies [16] and polyclonal rabbit anti-human β2m antibodies and analysis of deparaffinized and rehydrated slides was previously described [7].
Cell lines and IFN-γ treatment
The medulloblastoma cell lines DAOY and D283 were maintained at 37°C in a humidified atmosphere containing 5% CO2 in RPMI media supplemented with 10% Fetal calf serum, 2 mM l-glutamine, 1 mM 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin. For MHC class I induction, cells were treated with 100 U/ml IFN-γ for 72 h at 37°C.
Flow cytometry
MHC class I expression was detected by flow cytometry using the monoclonal mouse anti-human W6/32. This antibody is specific for HLA A, B and C and specifically to the combined epitope contributed by the α2 and α3 domains of the heavy chain and β2m [17–19]. Cells were collected and washed with PBS. Samples were incubated in 5% FBS in PBS for 15 min and then incubated with either 5% FBS in PBS (controls) or W6/32 supernatant for 1 h. After 3 rinses, cells were incubated with PE labeled donkey anti-mouse IgG (H + L) (eBiosciences, San Diego, CA) for 30 min at 4°C. Cells were rinsed 3 times and then fixed in cytofix (BD Sciences, San Diego, CA).
RT-PCR
Total cellular RNA was subjected to the SuperScript First-strand synthesis system for RT-PCR (Invitrogen, Carlsbad, CA) to generate the cDNA for the PCR reactions. RT-PCR was performed for TAP1, TAP2, β2m and β-actin using 35 cycles of 30 s at 94°C, 1 min at the annealing temperature and 1 min at 72°C. HLA-A and HLA-B PCR reactions was performed using 35 cycles of 30 s at 94°C, 1 min at the annealing temperature and 1 min 30 s at 72°C. The primers were previously reported [7]. Size fractionation of products on a 1% agarose gel identified the products.
Western blot analysis
DAOY cells were cultured in serum-free media for 18 h at 37°C. Cells were washed twice and then treated with serum-free RPMI with or without purified human β2m (Lee Biosolutions, Inc, St. Louis, Missouri) or monoclonal antibodies for 12 min or longer, as indicated, at 37°C. Signaling was halted by the addition of ice cold PBS and cells were lysed with 1× cell lysis buffer (Cell Signaling, Beverly, CA) supplemented with PhosSTOP phosphatase inhibitor cocktail and complete mini protease inhibitor cocktail (Roche, Indianapolis, Indiana). Lysates were collected and centrifuged at 14,000 rpm for 15 min to remove cell debris. Lysates were boiled for 5 min in loading buffer and separated by a 4–12% Bis-Tris Gel (Invitrogen). Membranes were blocked in 5% milk in TBST for 1 h at room temperature. Primary and secondary antibodies were diluted in 3% BSA in TBST and incubated with the membrane at 4°C overnight and at room temperature for 1 h, respectively. Signal was detected using the enhanced chemiluminescence system (Pierce, Rockford, IL).
β2m knock-down
Cells were washed twice with OptiMem media and then maintained in RPMI supplemented with 10% FBS and l-glutamine (no antibiotics). Cells were transfected with either ON-TARGETplus SMARTpool siRNA human β2m, non-targeting siRNA or siGLO transfection indicator (Dharmacon, Lafayette, CO). Briefly, siRNA was diluted in 1× siRNA buffer (Dharmacon) and combined with Opti-Mem. In a separate tube, lipofectamine was mixed with OptiMem. Both tubes were placed at room temperature for 5 min to allow for complex formation. Equal volumes of the siRNA solution and the lipofectamine solution were then combined and allowed to sit at room temperature in the dark for 20 min. The siRNA/lipofectamine mixture was added to DAOY cells in a 6-well plate at a final concentration of 100 nM. After 24 h, cells were serum starved overnight and treated the following day with various experimental conditions.
Results
Fully assembled HLA complexes are not required for open conformer engagement-mediated ERK1/2 activation
Fully assembled MHC class I molecules, known as closed conformers and consisting of heavy chain, β2m and peptide subunits, at the cell surface are in equilibrium with open conformers, complexes that have lost β2m and/or peptide subunits. Engagement of open HLA class I conformers with β2m or a specific antibody (HC-10) leads to ERK1/2 activation in DAOY medulloblastoma cell line [7]. It is unclear whether the signaling is a result of direct engagement of open conformers, or the formation of additional closed conformers. To exclude the potential role of closed conformers in signal transduction, we down-modulated the levels of β2m using siRNA treatment. Western blot analysis showed no detectable levels of endogenous β2m 48 h post transfection, while exogenously added β2m was readily detected (Fig. 1a). Consequently, there was a 14-fold reduction in mean fluorescent intensity of cell surface MHC class I closed conformers and undetectable levels of open conformation MHC class I (Fig. 1b). Despite undetectable levels of open conformers by flow cytometry, HLA heavy chains were present at the cell surface, as suggested by the following observation. The addition of exogenous β2m resulted in β2m detection that could be partially inhibited by the HC-10 antibody, specific for open conformers, and not by W6/32 antibody, specific for closed conformers (Fig. 1c). HC-10 antibody is specific for an epitope hidden in the HLA class I structure, requiring complete unfolding of the α-helix [20]. Hence, HC-10 antibody most likely does not recognize all open conformers and its effect is partial. ERK1/2 phosphorylation occurred in β2m-deficient cells in response to both β2m and HC-10 antibody (Fig. 1c), as previously observed in DAOY cells with unmanipulated levels of β2m [7]. These findings suggest that ERK1/2 phosphorylation does not occur through fully assembled MHC class I molecules at the cell surface.
Fig. 1.
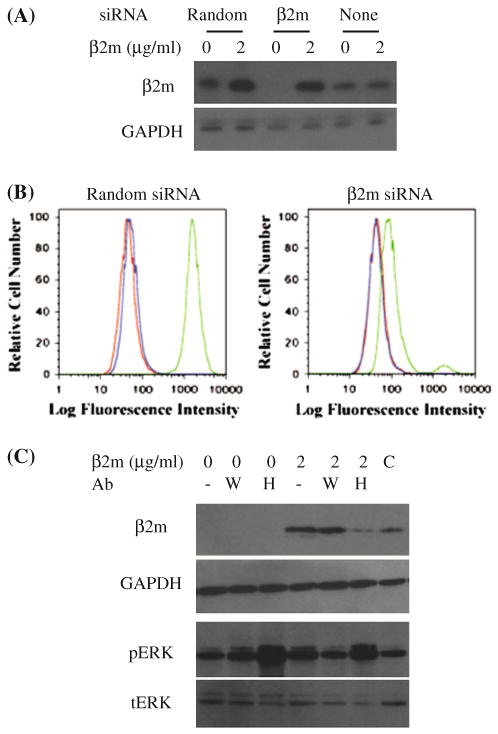
Endogenously synthesized β2m is not required for open conformer-dependent signaling in DAOY cells. a DAOY cells were treated with either β2m-specific siRNA or random siRNA control. Exogenous β2m was added 48 h later and lysates were subjected to the Western blot analysis using β2m- or GAPDH-specific antibodies, as indicated. b DAOY cells treated in the same manner as in a were stained with W6/32 (green lines; right), HC-10 (blue lines; middle) monoclonal antibodies, or secondary antibody alone (red lines; left), and analyzed by flow cytometry. c Exogenous β2m in the absence or presence of W6/32 (W) or HC-10 (H) monoclonal antibodies was added to DAOY cells pretreated with β2m-specific siRNA, as indicated. Cell lysates were probed by β2m-, GAPDH-, phospho-ERK1/2, or total ERK1/2-specific antibodies. C-control cells treated with neither siRNA nor exogenous β2m
HLA class I expression in CNS tumors does not correlate with the extent of leukocyte infiltration
Absence of HLA class I expression in medulloblastoma may be a result of selection mediated by the immune system. In this case, one would expect an inverse correlation between leukocyte infiltration of the tumor and HLA class I expression. We have previously examined leukocyte infiltration of medulloblastoma with the goal of excluding leukocyte contamination in assigning HLA class I expression to medulloblastoma cells [7]. We here extended this analysis to establish correlation between the two parameters and compare the findings with another CNS tumor with pronounced HLA class I expression. Of the 106 evaluable specimens, 87% of medulloblastomas showed absent or faint heavy chain positivity (56% scored 0 and 31% scored 1), while scores 2 and 3 were observed in 5% and 8% of tissues, respectively (Fig. 3). In contrast, the MHC class I heavy chain in an adult glioma array of 248 tissues, showed 0.4% negative tumors, 7% ‘1’ positive tumors, 15% ‘2’ positive tumors and 78% ‘3’ positive tumors, (Fig. 2), as previously reported [21, 22].
Fig. 3.
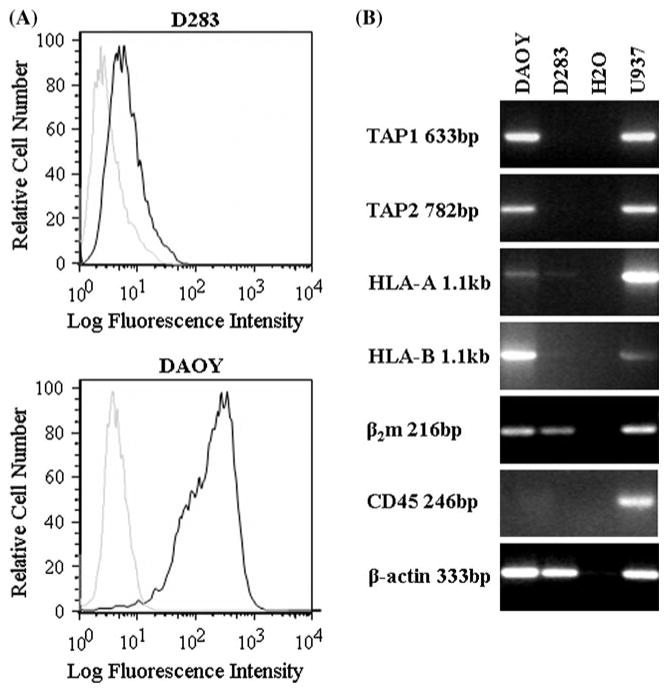
HLA class I expression in medulloblastoma cell lines. a Flow cytometry analysis of three medulloblastoma cell lines stained with W6/32 monoclonal antibody followed by FITC conjugated anti-mouse Ig (bold lines) or control samples with primary antibody omitted (plain lines). b RT-PCR analysis using TAP1, TAP2, β2m, HLA-A, HLA-B, and CD45 specific primers. cDNAs obtained from the DAOY, D283 medulloblastoma and U937 leukemia (positive control) cell lines were used as templates. Negative control consisted of samples with no cDNA (H2O)
Fig. 2.
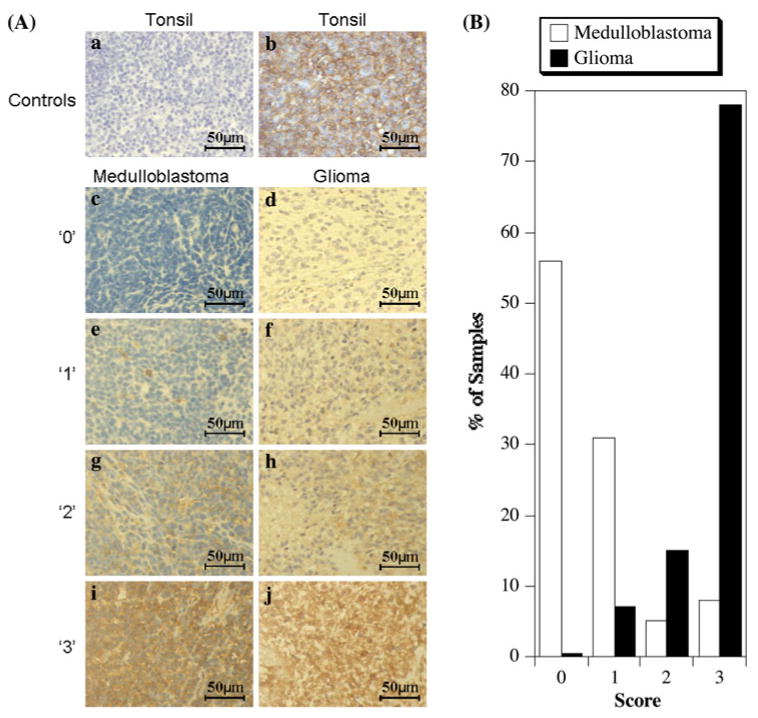
Immunohistochemistry analysis of medulloblastoma and adult glioma for HLA class I. a Representative sections showing staining with the heavy chain specific monoclonal antibody HC-10 (×40 magnification). Control tonsil tissue sections processed identically except for the absence (a) or presence (b) of primary HC-10 monoclonal antibody. Examples of medulloblastoma (c, e, g, i) or glioblastoma (d, f, h, j) sections graded as ‘0’ (c, d), ‘1’ (e, f), ‘2’ (g, h) and ‘3’ (i, j). b Relative distribution of HC-10 staining intensities in medulloblastoma and adult glioblastoma microarrays
Both medulloblastomas and gliomas had low levels of infiltration, with only slight differences in the distribution of grades ‘0’ and ‘1’: the proportion of grade ‘1’ being greater in glioma than in medulloblastoma. Of the 103 evaluable medulloblastoma specimens, 58.3% were negative for infiltration, with 39.8% receiving a score of 1 and 0.95% a score of 2 and 3 each. Out of 248 adult glioblastoma samples, 48% were negative for infiltration, 43% ‘1’ positive, 8.5% ‘2’ positive and 0.5% ‘3’ positive (Table 1). Interestingly, the MHC class I positive medulloblastomas also have a slightly higher level of infiltration than the MHC class I negative medulloblastoma, similar to the levels detected in adult glioblastoma (Fig. 2). Taken together, these results show no correlation between the levels of tumor infiltration by leukocytes and absence of HLA class I expression. If anything, HLA class I appears to correspond to mildly increased infiltration. These data do not support the concept of HLA loss tumor variants arising as a result of selection imposed by the immune system.
Table 1. CD45 staining scores for medulloblastoma and glioblastoma.
| Tumor | HC-10 staining scores | Immunohistochemistry scores (CD45) | Total | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Glioma | 0–3 | 119 (48.0%) | 106 (42.7%) | 21 (8.5%) | 1 (0.5%) | 237 (100%) |
| 2–3 | 101 (47.6%) | 90 (42.5%) | 20 (9.4%) | 1 (0.5%) | 212 (100%) | |
| Medullo | 0–3 | 60 (58.3%) | 41 (39.8%) | 1 (0.95%) | 1 (0.95%) | 103 (100%) |
| 2–3 | 6 (42.9%) | 6 (42.9%) | 1 (7.1%) | 1 (7.1%) | 14 (100%) | |
IFNγ treatment restores expression in HLA class I negative cell line
The loss of class I expression due to selection by the immune system is associated with mutations that prevent normally regulated expression of components required for class I expression, such as heavy chain, TAP1, TAP2 and β2m [12, 13]. The D283 medulloblastoma cell line is HLA class I negative, and if this is due to the mutation in a gene encoding a particular component required for HLA class I expression, we would expect unaltered levels of mRNAs for all components required for HLA class I expression, except perhaps one. Furthermore, mutation would prevent upregulation of HLA class I by IFNγ. In contrast to these predictions, low levels of constitutive MHC class I expression in D283 cells are due to low levels of mRNA for both heavy chains and TAP1 and TAP2 components of the antigen processing machinery. Only β2m expression was noted in this cell line (Fig. 3). Treatment with 100 U/ml IFN-γ upregulated mRNA levels of all components (Fig. 4), and restored cell surface expression of HLA class I in D283 cells. These results suggest that HLA class I deficiency in D283 cells is unlikely a consequence of mutation in one or more components of HLA class I processing machinery.
Fig. 4.
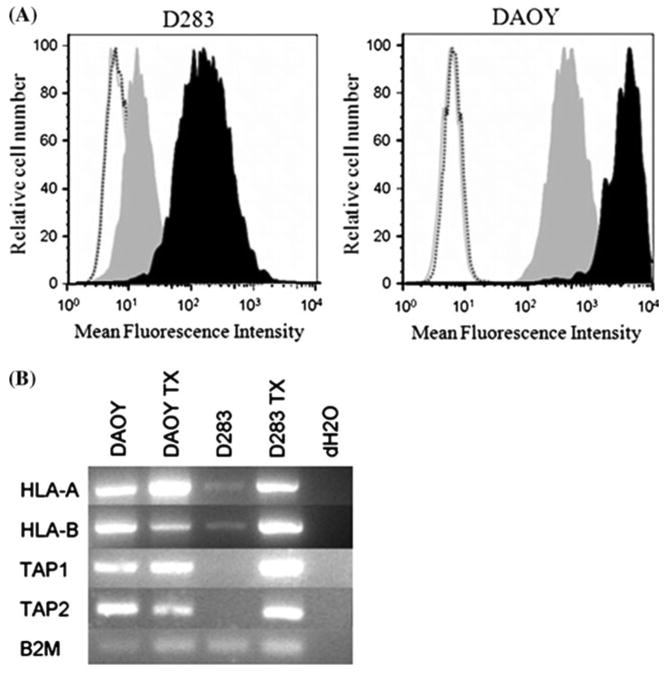
Upregulation of MHC class I by IFNγ treatment. Cells were incubated with media alone or supplemented with 100 U/ml IFNγ for 72 h at 37°C. a Induction of MHC class I was evaluated by flow cytometry using the W6/32 antibody. The shaded gray curve represents levels of MHC class I prior to IFNγ treatment. Shaded black curve represents the levels of MHC class I following IFNγ treatment. Controls include unstained cells (gray solid line) and secondary only (black dashed line). b RT-PCR using the heavy chain, TAP1, TAP2 and β2m primers for MHC class I machinery confirmed induction of MHC class I components
Changes in HLA class I expression during development of human cerebellum
An alternative explanation for heterogenous HLA class I expression in medulloblastoma would be that it simply reflects the status of HLA class I expression in the tumor originating cells at the time of transformation. About 75% of medulloblastomas are believed to originate from the external granular layer neurons of cerebellum. To determine the expression of MHC class I in the developing cerebellar cortex human brain tissue samples from two prenatal (28, 35 gestational weeks), one perinatal and three postnatal periods (6 months, 1 and 3 years) were analyzed. These samples were stained for the HLA class I heavy chain, TAP1, TAP2 and β2m (Fig. 5a).
Fig. 5.
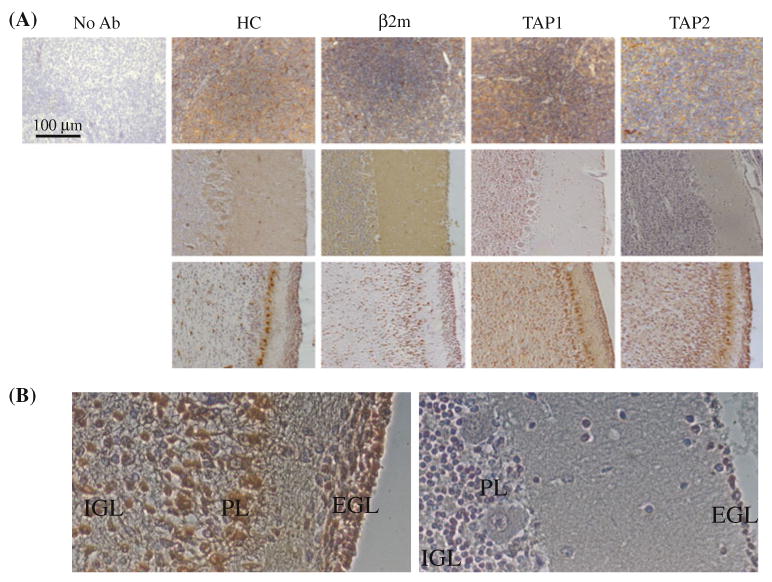
HLA class I expression in developing cerebellum. a Tissues from adult tonsil (top row), perinatal/early childhood (40 weeks of gestation through 3 years after the birth- middle row) or prenatal cerebellum were stained with heavy chain (HC)-, β2m, TAP1- and TAP2-specific antibodies and viewed at 20× magnification. For the perinatal/early childhood group, examples of negative staining were deliberately chosen, even though not all antibodies stained negative. b Higher magnification (×40) view of the prenatal (35 weeks of gestation; left) and 1 year old cerebellum specimens stained with HC-10 monoclonal antibody
The cerebellar cortex is composed of four layers during development. From outer to inner, the layers are the external granular layer, the molecular layer, the Purkinje cell layer and the internal granular layer. The cells of the external granular layer migrate into the inner layers. In the first postnatal year, the width of the external granular layer begins decreasing until it finally disappears around the 11th postnatal month [23, 24]. All four components of MHC class I were expressed only in the prenatal cerebellum, while later in development, at least one of the components was missing (Table 2). This pattern of staining was observed in the internal granular, and Purkinje cell layer and external granular layer (visible in all ages except for 3 years). Heterogeneity of HLA class I expression can be observed even in the samples designated as positive. Thus, under higher magnification, the outer layers of the EGL appear positive, while the inner layers and cells that appear migrating through the molecular layer are negative (Fig. 5b). Taken together, these results support the possibility that HLA class I expression in medulloblastoma may be a reflection of the HLA class I status of precursor cells in the external granular layer.
Table 2. Summary of MHC class I component expression during prenatal and perinatal/early childhood development.
| Age | EGL | PCL | IGL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC10 | TAP1 | TAP2 | β2m | HC10 | TAP1 | TAP2 | β2m | HC10 | TAP1 | TAP2 | β2m | |
| 28WG | + | + | + | + | + | + | + | + | + | + | + | + |
| 35WG | + | + | + | + | + | + | + | + | + | + | + | + |
| NB | − | + | + | − | + | + | + | − | − | + | + | − |
| 6M | + | + | − | − | + | + | − | + | + | + | − | + |
| 1Y | + | + | − | + | + | + | − | + | + | + | − | + |
| 3Y | − | + | − | + | − | + | − | + | − | + | + | + |
EGL External granular layer, PCL Purkinje cellular layer, IGL Internal granular layer, WG Weeks gestation; NB Newborn
Discussion
Initiation of a rapid, robust, and effective immune response against a particular tumor is the best therapeutic strategy for the long-term survival of cancer patients. CD8+ T cells are particularly effective in the prevention and treatment of tumors through a direct cytotoxic action on the tumor cells [Rouvier, 1993 #332; Kagi, 1994 #333; Lowin, 1994 #334]. In addition to cytotoxic activity, CD8+ T cells also secrete lymphokines, such as interferon-γ and TNF-α, which activate macrophages, neutrophils, or NK cells. It is therefore not surprising that a great effort has been made to study immune responses (especially CD8+ T cell responses) in cancer patients, with the aim to induce, or potentiate existing inefficient immune responses. Understanding the expression of immune markers such as MHC class I in brain tumors and normal brain, as well as the ability of immune cells to reach the tumor site are integral factors in determining the feasibility of CD8+ T cell-mediated immunotherapy. Although HLA class I expression has previously been assessed in both medulloblastoma [7, 25, 26] and glioma [21, 22], the present study is the largest tumor series evaluated to date. Our results suggest that key components of the natural immune response to medulloblastoma are missing and that therapeutic attempts using T cells that recognize antigens presented by MHC class I will have to include strategies that by-pass or up-regulate the expression of these molecules.
The importance of MHC class I in an immune response is well documented. However, the role of these molecules outside the immune system is less well understood. Open conformers are the product of the natural turnover of closed conformer MHC class I molecules that begins with the loss of β2m and/or peptide from the complex [27, 28]. The resulting free heavy chains can form homodimers or heterodimers. Thus far, homodimers have not been assigned a specific function, aside from targeted removal and degradation [29, 30]. On the other hand, binding of heavy chain to heterologous cell surface receptors, can lead to receptor modification that affects ligand binding and/or receptor internalization [31–35]. These events may impact cell growth [31] or migration [7]. The involvement of open, and not closed conformers in modification of signal transduction was assumed because addition of exogenous β2m impaired the cis-association of the insulin receptor to the open conformers [36, 37]. However, involvement of closed conformers needs to be formally excluded, especially in the light of the fact that cross-linking of MHC class I molecules by antibodies specific for closed conformers (W6/32) or β2m can induce TCR activation similar to that induced by TCR engagement [38, 39]. Our experiments using β2m siRNA treatment show that a drastic reduction of MHC class I closed conformers did not significantly reduce signaling involving activation of ERK1/2 in DAOY medulloblastoma. The antibody blocking of open conformers partially inhibited, while antibody to closed conformers had no effect on binding of exogenously added β2m to siRNA-treated cells, suggesting that β2m was binding to open conformers. In addition, the antibody to open conformation of MHC class I was also able to mimic the effects of β2m addition in siRNA-treated cells on ERK1/2 activation. It therefore appears that β2m and HC-10 compete for the binding site implicating open conformation in MHC class I signaling in medulloblastoma.
The association of HLA class I with tumor outcome may be due to immune selection mediated by CD8+ T cells selecting for HLA class I negative tumor variants [12, 13]. Two predictions can be made if selective pressure successfully changes the phenotype of the tumor. First, one would expect more abundant leukocyte infiltration of tumors that do not express HLA class I. This, however, is not the case in our experimental system. Second, HLA class I-loss mutant cells that result from selection would be expected to have structural mutations preventing successful assembly. This is, again, not the case in a representative HLA-negative cell line D283, as IFNγ treatment successfully restored HLA class I expression.
In the absence of evidence for selection of HLA class I loss variants by the immune system, we decided to seek an alternative explanation for heterogenous HLA class I expression in medulloblastomas. We considered the possibility that expression may reflect developmental changes in the external granular layer cells, which are thought to be the origin of ∼75% of medulloblastomas [40, 41]. Indeed, we found that neurons in the external granular layer, but also in the internal granular layer and Purkinje cells express HLA class I in a developmentally controlled fashion. Regions that were less mature, as determined by the thicker EGL or less orderly structured Purkinje cell layer, expressed more heavy chain, β2m, TAP1 and TAP2 positive cells. This finding is in agreement with previous findings of MHC class I expression in the Purkinje cells in the mouse [42] and supports the general pattern observed in other areas of the CNS where MHC class I expression appeared in a developmentally regulated fashion, the highest levels observed during the late stages of fetal development [43]. The specific pattern of MHC class I expression in the cerebellum may serve a specific functional role in neuronal development. Two major characteristics of the external granular layer are high cell proliferation and migration of the cells into the inner cortex layers. In humans, both of these activities peak between the 28th and 34th week of gestation [23], at the time when highest MHC class I expression is observed. Given the ability of HLA class I molecules to induce ERK1/2 phosphorylation and to promote migration in medulloblastoma [7], it is tempting to speculate that signaling induced by the binding of β2m to the MHC class I heavy chain in vivo may help regulate migration and/or proliferation of external granular layer neurons.
MHC class I was originally thought to be expressed in the CNS only under pathological conditions associated with inflammation and infection [44–53]. More recent studies, however, have shown that MHC class I is expressed under physiological conditions in neurons of both the developing and adult brain, albeit at a lower level than in cells outside the CNS [54–58]. The presence of MHC class I in brain has been reported in the developing lateral geniculate nucleus neurons, developing and adult hippocampal neurons, hippocampal pyramidal cells, adult brainstem, the pars compacta of the substantia nigra, dorsal root ganglion cells, spinal motoneurons, primary somatosensory cortex and layer IV of the primary visual cortex [54–56, 59, 60]. MHC class I remains expressed in the adult retinal ganglion cells, but the levels are notably decreased [61]. The visual cortex also shows levels of MHC class I expression during segregation of the LGN axons of layer 4 neurons [62, 63]. MHC Class I is also expressed in hippocampal neurons of adult brain, as suggested by the findings that these cells have longer potentiation and long-term depression activity in mice with genetically deleted β2m [64–66].
In conclusion, we have shown that properly assembled HLA class I molecules (closed conformers) are not involved in the signaling function in medulloblastoma. Furthermore, heterogenous expression of HLA class I appears not to be a consequence of selection mediated by the immune system, but more likely reflects a dynamic developmental pattern of expression in the cerebellar cortex. These findings contribute to our understanding of the role of HLA class I in the pathology of medulloblastoma.
Acknowledgments
This work was partially supported by national Institutes of Health grant (R01 CA111835) awarded to TJM.
Abbreviations
- β2m
β2 microglobulin
- IHC
Immunohistochemistry
- TAP
Transporter associated with antigen processing
Contributor Information
Courtney Smith, Center for Cancer and Immunology Research, Children's Research Institute, Children's National Medical Center, 111 Michigan Avenue NW, Washington, DC 20010-2970, USA; Department of Pathology, Children's National Medical Center, 111 Michigan Avenue NW, Washington, DC, USA.
Mariarita Santi, Department of Pathology, Children's National Medical Center, 111 Michigan Avenue NW, Washington, DC, USA.
Elisabeth J. Rushing, Department of Neuropathology, Armed Forces Institute of Pathology, Washington, DC, USA
Robert Cornelison, Cancer Genetics Branch, National Human Genome Research Institute NIH, Bethesda, MD, USA.
Tobey J. MacDonald, Center for Cancer and Immunology Research, Children's Research Institute, Children's National Medical Center, 111 Michigan Avenue NW, Washington, DC 20010-2970, USA; Department of Pathology, Children's National Medical Center, 111 Michigan Avenue NW, Washington, DC, USA
Stanislav Vukmanovic, Email: svukmano@cnmc.org, Center for Cancer and Immunology Research, Children's Research Institute, Children's National Medical Center, 111 Michigan Avenue NW, Washington, DC 20010-2970, USA, Department of Pathology, Children's National Medical Center, 111 Michigan Avenue NW, Washington, DC, USA.
References
- 1.Pamer E, Cresswell P. Mechanisms of MHC class I—restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 2.Vukmanovic S, et al. Peptide loading of nascent MHC class I molecules. Arch Immunol Therap Exp. 2001;49:195–201. [PubMed] [Google Scholar]
- 3.Arosa FA, Santos SG, Powis SJ. Open conformers: the hidden face of MHC-I molecules. Trends Immunol. 2007;28:115–123. doi: 10.1016/j.it.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Ericsson C, et al. Association of HLA class I and class II antigen expression and mortality in uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:2153–2156. [PubMed] [Google Scholar]
- 5.Madjd Z, et al. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer. 2005;117:248–255. doi: 10.1002/ijc.21163. [DOI] [PubMed] [Google Scholar]
- 6.Ramnath N, et al. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother. 2006;55:891–899. doi: 10.1007/s00262-005-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith C, et al. A novel role of HLA class I in the pathology of medulloblastoma. J Transl Med. 2009;7:59. doi: 10.1186/1479-5876-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delp K, et al. Functional deficiencies of components of the MHC class I antigen pathway in human tumors of epithelial origin. Bone Marrow Transplant. 2000;25:S88–S95. doi: 10.1038/sj.bmt.1702363. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal S, Kishore MC. MHC class I gene expression and regulation. J Hematother Stem Cell Res. 2000;9:795–812. doi: 10.1089/152581600750062237. [DOI] [PubMed] [Google Scholar]
- 10.Nacht M, et al. Molecular characteristics of non-small cell lung cancer. Proc Natl Acad Sci USA. 2001;98:15203–15208. doi: 10.1073/pnas.261414598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamarashev J, et al. TAP1 down-regulation in primary melanoma lesions: an independent marker of poor prognosis. Int J Cancer. 2001;95:23–28. doi: 10.1002/1097-0215(20010120)95:1<23::aid-ijc1004>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera T, et al. Analysis of HLA expression in human tumor tissues. Cancer Immunol Immunother. 2003;52:1–9. doi: 10.1007/s00262-002-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227–236. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson NFS, et al. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer. 2006;118:6–10. doi: 10.1002/ijc.21303. [DOI] [PubMed] [Google Scholar]
- 15.Sallinen SL, et al. Identification of differentially expressed genes in human gliomas by DNA microarray and tissue chip techniques. Cancer Res. 2000;60:6617–6622. [PubMed] [Google Scholar]
- 16.Albers A, et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:11146–11455. doi: 10.1158/0008-5472.CAN-05-0772. [DOI] [PubMed] [Google Scholar]
- 17.Maziarz RT, et al. The human HLA-specific monoclonal antibody W6/32 recognizes a discontinuous epitope within the alpha 2 domain of murine H-2Db. Immunogenetics. 1986;24:206–208. doi: 10.1007/BF00364750. [DOI] [PubMed] [Google Scholar]
- 18.Jefferies WA, MacPherson GG. Expression of the W6/32 HLA epitope by cells of rat, mouse, human and other species: critical dependence on the interaction of specific MHC heavy chains with human or bovine beta 2-microglobulin. Eur J Immunol. 1987;17:1257–1263. doi: 10.1002/eji.1830170907. [DOI] [PubMed] [Google Scholar]
- 19.Tanabe M, et al. Structural and functional analysis of monomorphic determinants recognized by monoclonal antibodies reacting with the HLA class I alpha 3 domain. J Immunol. 1992;148:3202–3209. [PubMed] [Google Scholar]
- 20.Perosa F, et al. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–1926. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 21.Miyaki K, et al. Immunohistochemical detection and correlation between MHC antigen and cell-mediated immune system in recurrent glioma by APAAP method. Neurol Med Chir (Tokyo) 1990;30:649–653. doi: 10.2176/nmc.30.649. [DOI] [PubMed] [Google Scholar]
- 22.Saito T, et al. Immunohistochemical analysis of tumor-infiltrating lymphocytes and major histocompatibility antigens in human gliomas and metastatic brain tumors. Surg Neurol. 1988;29:435–442. doi: 10.1016/0090-3019(88)90137-1. [DOI] [PubMed] [Google Scholar]
- 23.Abraham H, et al. Cell formation in the cortical layers of the developing human cerebellum. Int J Dev Neurosci. 2001;19:53–62. doi: 10.1016/s0736-5748(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 24.Rakic P, Sidman RL. Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J Comp Neurol. 1970;139:473–500. doi: 10.1002/cne.901390407. [DOI] [PubMed] [Google Scholar]
- 25.Bodey B, Bodey BJ, Siegel SE. Immunophenotypic characterization of infiltrating polynuclear and mononuclear cells in childhood brain tumors. Mod Pathol. 1995;8:333–338. [PubMed] [Google Scholar]
- 26.Raffaghello L, et al. Expression and functional analysis of human leukocyte antigen class I antigen-processing machinery in medulloblastoma. Cancer Res. 2007;67:5471–5478. doi: 10.1158/0008-5472.CAN-06-4735. [DOI] [PubMed] [Google Scholar]
- 27.Demaria S, Schwab R, Bushkin Y. The origin and fate of beta 2m-free MHC class I molecules induced on activated T cells. Cell Immunol. 1992;142:103–113. doi: 10.1016/0008-8749(92)90272-q. [DOI] [PubMed] [Google Scholar]
- 28.Santos SG, Powis SJ, Arosa FA. Misfolding of major histocompatibility complex class I molecules in activated T cells allows cis-interactions with receptors and signaling molecules and is associated with tyrosine phosphorylation. J Biol Chem. 2004;279:53062–53070. doi: 10.1074/jbc.M408794200. [DOI] [PubMed] [Google Scholar]
- 29.Matko J, et al. Clustering of class I HLA molecules on the surfaces of activated and transformed human cells. J Immunol. 1994;152:3353–3360. [PubMed] [Google Scholar]
- 30.Bodnar A, et al. Class I HLA oligomerization at the surface of B cells is controlled by exogenous beta(2)-microglobulin: implications in activation of cytotoxic T lymphocytes. Int Immunol. 2003;15:331–339. doi: 10.1093/intimm/dxg042. [DOI] [PubMed] [Google Scholar]
- 31.Schreiber AB, Schlessinger J, Edidin M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J Cell Biol. 1984;98:725–731. doi: 10.1083/jcb.98.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claas FH, et al. The interaction between gamma-type endorphins and HLA class I antigens. Hum Immunol. 1986;15:347–356. doi: 10.1016/0198-8859(86)90011-x. [DOI] [PubMed] [Google Scholar]
- 33.Mommaas AM, et al. Internalization of MHC class I molecules is a prerequisite for endocytosis of endorphin by lymphocytes. Clin Exp Immunol. 1991;84:170–174. doi: 10.1111/j.1365-2249.1991.tb08143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bushkin Y, et al. A new HLA-linked T cell membrane molecule, related to the beta chain of the clonotypic receptor, is associated with T3. J Exp Med. 1986;164:458–473. doi: 10.1084/jem.164.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bene L, et al. Lateral organization of the ICAM-1 molecule at the surface of human lymphoblasts: a possible model for its co-distribution with the IL-2 receptor, class I and class II HLA molecules. Eur J Immunol. 1994;24:2115–2123. doi: 10.1002/eji.1830240928. [DOI] [PubMed] [Google Scholar]
- 36.Due C, Simonsen M, Olsson L. The major histocompatibility complex class I heavy chain as a structural subunit of the human cell membrane insulin receptor: implications for the range of biological functions of histocompatibility antigens. Proc Natl Acad Sci USA. 1986;83:6007–6011. doi: 10.1073/pnas.83.16.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramalingam TS, Chakrabarti A, Edidin M. Interaction of class I human leukocyte antigen (HLA-I) molecules with insulin receptors and its effect on the insulin-signaling cascade. Mol Biol Cell. 1997;8:2463–2474. doi: 10.1091/mbc.8.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geppert TD, et al. Activation of human T cell clones and Jurkat cells by cross-linking class I MHC molecules. J Immunol. 1989;142:3763–3772. [PubMed] [Google Scholar]
- 39.Skov S, Bregenholt S, Claesson MH. MHC class I ligation of human T cells activates the ZAP70 and p56lck tyrosine kinases, leads to an alternative phenotype of the TCR/CD3 zeta-chain, and induces apoptosis. J Immunol. 1997;158:3189–3196. [PubMed] [Google Scholar]
- 40.Kadin ME, Rubinstein LJ, Nelson JS. Neonatal cerebellar medulloblastoma originating from the fetal external granular layer. J Neuropathol Exp Neurol. 1970;29:583–600. doi: 10.1097/00005072-197010000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Reddy AT, Packer RJ. Medulloblastoma. Curr Opin Neurol. 1999;12:681–685. doi: 10.1097/00019052-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 42.McConnell MJ, et al. H2-K(b) and H2-D(b) regulate cerebellar long-term depression and limit motor learning. Proc Natl Acad Sci USA. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- 44.Lampson LA, Fisher CA. Weak HLA and beta-2-microglobulin expression of neuronal cell lines can be modulated by interferon. Proc Natl Acad Sci USA. 1984;81:6476–6480. doi: 10.1073/pnas.81.20.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong GH, Bartlett PF, Clark-Lewis I, Battye F, Schrader JW. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984;310:688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- 46.Maehlen J, Schroder HD, Klareskog L, Olsson T, Kristensson K. Axotomy induces MHC class I antigen expression on rate nerve cells. Neurosci Lett. 1998;92:8–13. doi: 10.1016/0304-3940(88)90733-1. [DOI] [PubMed] [Google Scholar]
- 47.Streit WJ, Graeber MB, Kreutzberg GW. Peripheral nerve lesion produces increased levels of major histocompatiblity complex antigens in the central nervous system. J Neuroimmunol. 1989;21:117–123. doi: 10.1016/0165-5728(89)90167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pereira RA, Tscharke DC, Simmons A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells, and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. J Exp Med. 1994;180:841–850. doi: 10.1084/jem.180.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neumann H, Cavalie A, Jenne D, Wekerle H. Induction of MHC class I genes in neurons. Science. 1995;269:549–552. doi: 10.1126/science.7624779. [DOI] [PubMed] [Google Scholar]
- 50.Fujimaki H, Hikawa N, Nagoya M, Nagata T, Minami M. IFN-gamma induces expression of MHC class I molecules in adult mouse dorsal root ganglion neurones. Neuroreport. 1996;7:2951–2955. doi: 10.1097/00001756-199611250-00030. [DOI] [PubMed] [Google Scholar]
- 51.Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major Histocompatibility Complex (MHC) Class I Gene Expression in Single Neurons of the Central Nervous System: Differential REgulation by Interferon (IFN)-gamma and tumor Necrosis FActor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Redwine JM, Buchmeier MJ, Evans CF. In vivo expression of major histocompatibility complex molecules on oligodendrocytes and neurons during viral infection. Am J Pathol. 2001;159:1219–1224. doi: 10.1016/S0002-9440(10)62507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foster JA, Quan N, Stern EL, Kristensson K, Herkenham M. Induced neuronal expression of class I major histoompatibility complex mRNA in acute and chronic inflammation models. J Neuroimmunol. 2002;131:83–91. doi: 10.1016/s0165-5728(02)00258-8. [DOI] [PubMed] [Google Scholar]
- 54.Corriveau R, Huh GS, Shatz CJ. Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- 55.Lidman O, Olsson T, Piehl F. Expression of nonclassical MHC class I (RT1-U) in certain neuronal populations of the central nervous system. Eur J Neurosci. 1999;11:4468–4472. doi: 10.1046/j.1460-9568.1999.00904.x. [DOI] [PubMed] [Google Scholar]
- 56.Linda H, Hammarberg H, Piehl F, Khademi M, Olsson T. Expression of MHC class I heavy chain and beta-2-microglobulin in rat brainstem motoneurons and nigral dopaminergic neurons. J Neuroimmunol. 1999;101:76–86. doi: 10.1016/s0165-5728(99)00135-6. [DOI] [PubMed] [Google Scholar]
- 57.Huh G, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;15:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miralvès J, et al. High levels of MeCP2 depress MHC class I expression in neuronal cells. PLOS One. 2007;2:e1354. doi: 10.1371/journal.pone.0001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishii T, Hirota J, Mombaerts P. Combinatorial coexpression of neural and immune multigene families in mouse vomeronasal sensory neurons. Curr Biol. 2003;13:394–400. doi: 10.1016/s0960-9822(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 60.Loconto J, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–618. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 61.Sur M, Weller RE, Sherman SM. Development of X- and Y-cell retinogeniculate terminations in kittens. Nature. 1984;310:246–249. doi: 10.1038/310246a0. [DOI] [PubMed] [Google Scholar]
- 62.LeVay S, Stryker MP, Shatz CJ. Ocular dominance columns and their development in layer IV of the cat's visual cortex: a quantitative study. J Comp Neurol. 1978;179:223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- 63.Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- 64.Malenka RC. Synaptic plasticity in the hippocampus: LTP and LTD. Cell. 1994;78:535–538. doi: 10.1016/0092-8674(94)90517-7. [DOI] [PubMed] [Google Scholar]
- 65.Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 66.Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]


