Abstract
Objective
Exposure to dioxins has been shown to contribute to the development of inflammatory diseases such as atherosclerosis. Macrophage-mediated inflammation is a critical event in the initiation of atherosclerosis. Previously we showed that treatment of macrophages with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) leads to aryl hydrocarbon receptor (AhR)-dependent activation of inflammatory mediators and the formation of cholesterol laden foam cells. However, the mechanisms responsible for the formation of atherosclerotic lesions mediated through AhR have not been identified.
Methods and Results
An in vitro macrophage and an ApoE−/− mouse model were used to determine whether chemokines and their receptors are responsible for the AhR-mediated atherogenesis. Exposure of ApoE−/− mice to TCDD caused a time-dependent progression of atherosclerosis, which was associated with induction of inflammatory genes including Interleukin (IL)-8 as well as F4/80 and matrix metalloproteinase (MMP)-12. High fat diet enhanced the TCDD-mediated inflammatory response and deteriorated the formation of complex atheromas. Treatment with a CXCR2 inhibitor and an AhR antagonist reduced the TCDD-induced progression of early atherosclerotic lesions in ApoE−/− mice.
Conclusions
The results suggest that CXCR2 mediates the atherogenic activity of environmental pollutants, such as dioxins, and contributes to the development of atherosclerosis through the induction of a vascular inflammatory response by activating the AhR-signaling pathway.
Keywords: AhR, IL-8, TCDD, MMP, CXCR2
Epidemiological studies on a population heavily exposed to dioxin in 1976 in Seveso, Italy, indicate that coronary and hypertensive diseases are increasing in recent years among the exposed population.1 A more recent study confirms a higher incidence of hyperlipidaemia, atherosclerotic plaques, increased intima-media thickness, and ischaemic heart disease in a group of former dioxin workers.2 These observations agree well with the results of experimental animal studies3–4 as well as other epidemiological evidence from factory workers exposed to chlorinated organic compounds5 that these AhR ligands indeed cause hyperlipidemia. Hyperlipidemia, particularly hypercholesterolemia, has been described to be associated with a significant reduction in binding of low-density lipoprotein (LDL) to its receptor on the hepatic plasma membrane observed in guinea pigs as a result of TCDD treatment.6 Dalton et al. reported that TCDD promotes an earlier onset and greater severity of atherosclerotic lesions in ApoE−/− mice.7 Their study provides further evidence that atherogenesis is a major risk factor for ischemic heart diseases that are known to develop in ApoE−/− mice as an end result of TCDD exposure and AhR activation.
Atherosclerosis is considered a chronic inflammatory disease of the vessel wall characterized by chemokine-driven mononuclear cell recruitment entering the subendothelial space where they differentiate into macrophages.8 These macrophages promote plaque development by secreting numerous inflammatory mediators, such as cytokines and chemokines, that sustain the inflammatory milieu, recruit leukocytes to the vascular wall and regulate immune functions. All these functions make the plaque macrophage an interesting target for prevention and therapy of atherosclerotic disease.
Our previous studies have shown that air pollutants, like urban dust particles or particles from diesel engine exhaust as well as the prototypical AhR ligand TCDD, activate an inflammatory response in macrophages and may lead to the formation of foam cells.9–10 Another major risk factor for cardiovascular diseases is cigarette smoke, which contains many compounds that activate the AhR, such as polycyclic aromatic hydrocarbon (PAH). Thus, it is important to understand how pathological stimuli like air pollutants may affect macrophage phenotype and function and how these mechanisms lead to atherosclerosis.
L-8 has been implicated as a contributing factor to atherosclerosis and has been considered to play a pivotal role in pathogenesis of vascular diseases.11 Thus, increased expression of IL-8 was found to be associated with the presence of macrophage-derived foam cells in human atheroma.12 Macrophages are considered as a major source to contribute to overexpression of IL-8 in atherosclerotic tissue. Our previous studies show that the most consistently observed phenomenon accompanying induction of foam cell formation triggered by oxidized LDL, lipopolysaccaride (LPS), or TCDD in U937 macrophages is an early and sustained upregulation of IL-8.10, 13 The discovery, that IL-8 also acts as a mediator of cholesterol loading in macrophages makes IL-8 one of the most important players in atherogenesis. In this regard, it is interesting to learn that recently Gargalovic et al. found atherogenesis induced by oxidized phospholipids in endothelial cells can be attenuated by silencing the IL-8 pathway through the use of siRNA technology.14 CXCR2, a G protein-coupled receptor mediating chemotaxis in immune responses, is believed to play a key role in several inflammatory diseases including atherosclerosis. CXCR2 is expressed in neutrophils, monocytes and macrophages and binds several chemokines including CXCL8 (IL-8), CXCL1 (GROα), and CXCL5.15 CXCR2 has been shown to have a major impact on macrophage accumulation in advanced lesions.16 Activation of the chemokine receptor CXCR2 enhances monocyte-endothelial adhesion and promotes angiogenesis by increasing VEGF expression.17–18 Thus, IL-8 and its receptor CXCR2 appear to be at least one of the essential players in this cell model of atherogenesis. CCR2 is a receptor for monocyte chemoattractant protein-1 (MCP-1), a chemokine, which specifically mediates monocyte chemotaxis. In the current study we investigate how chemokine receptors CXCR2 and CCR2 contribute to the development of atherosclerotic plaques mediated by AhR activation.
Methods
Cell differentiation, treatment and transient siRNA transfection
U937 monocytic cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA) (3 µg/mL) and allowed to adhere for 48 h in a 5% CO2 tissue culture incubator at 37°C, after which they were fed with TPA-free medium, to promote differentiation into macrophages.
Cigarette smoke extract (CSE) was prepared as described earlier.19 Macrophages were treated for 24 h with 10 nM TCDD, 10% CSE or 0.1 % Me2SO as vehicle control in absence or presence of 10 µM AhR antagonist 3'-methoxy-4'nitroflavone (MNF).
Transfection of siRNA into U937 macrophages was performed via Nucleofector technology as previously described.13 Twenty four hours after transfection, cells were treated with 10 nM TCDD, 10% CSE, or 0.1% Me2SO (control) for another 24 h. The reduction of the target RNA was detected by quantitative real-time RT-PCR.
Quantitative real-time RT-PCR
The RNA isolation from macrophage cells was carried out using a high pure RNA isolation kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. For preparation of total RNA from the aorta of ApoE−/− mice, the frozen tissue samples were homogenized first in TRIzol using a TissueLyser (Qiagen). The RNA was extracted with chloroform and further purified with the RNA isolation kit. cDNA synthesis was performed as previously described.13 Quantitative real-time PCR was run with a LightCycler Instrument (Roche Diagnostics) using the Fast Real-Time SYBR Green PCR Kit (Qiagen). All PCR assays were performed in duplicate or triplicate. For quantification, data were analyzed with the LightCycler analysis software.
Measurement of cholesterol
Cellular cholesterol and protein determinations were performed as described previously.10 Free and esterified cholesterol (total cholesterol) was extracted directly from in situ macrophage monolayers in the cell culture dish. Cells were treated for 5 days with 10 nM TCDD or 10% CSE in absence or presence of AhR antagonist MNF (10 µM), CCR2 inhibitor INCB3344 (10 µM), and CXCR2 inhibitor SB225002 (10 µM) before cholesterol measurement.
Cholesterol from mice aorta was extracted by isopropyl alcohol: acetonitrile: water treatment followed by a derivatization reaction and was measured by gas chromatography mass spectrometry (GC/MS) as previously described.20
Animal treatment and immunohistochemistry
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication No 82–23, revised 1996) and was approved (Protocol #13165) by the University of California Davis Animal Use and Care committee.
Mice were fed a regular chow (diet No. 5001, LabDiet) or a High-Fat Diet (HFD) (diet No. D12079B, Research Diets, Inc.). ApoE−/− mice (Jackson Laboratory, Sacramento, CA) were pretreated with 5 mg/kg CXCR2 inhibitor SB220005 or AHR antagonist CH223191 for 24h before TCDD treatment and co-treatment with inhibitors plus TCDD. Control animals received an equal amount of corn oil (5 ml/kg) or inhibitor alone. Eight week old C57BL/6J and ApoE−/− mice received a single intra-peritoneal (i.p.) injection of 15 µg/kg TCDD and a weekly maintenance dose of 1 µg/kg TCDD or 5 mg/kg SB225002/CH223191. If not otherwise stated, eight animals from each group were killed at day 60 and organs were excised, weighed and quickly frozen in liquid nitrogen and stored at −80 °C for analysis. Aortas were cleaned and stripped of fat on the adventitia before being excised.
Aortas were processed in serial sections at 3 µm and stained with hematoxylin eosin (H&E). Further aortic sections were used for immunohistochemistry. Immunohistochemical stains to detect MMP-12 (Santa Cruz Biotech, Santa Cruz, CA) and identify macrophages and foam cells. Briefly, endogenous peroxidase was blocked with 3% hydrogen peroxidase/methanol. Slides were incubated in fresh citrate buffer, pH 6.0, at 25°C for antigen retrieval. The primary antibodies were diluted in 0.5% phosphate-buffered saline and ovalbumin and incubated at room temperature overnight. Detection was performed using a biotinylated secondary antibody followed by the Avidin-Biotin-Peroxidase Complete ABC kit (Vector Laboratories, Burlingame, CA) and developed with diaminobenzidine chromogen substrate.
Statistical analysis
To detect fold differences in gene expression, all expression values were logarithm-transformed for analysis, with results expressed as backtransformed means ± standard deviations. Data were analyzed by one-way ANOVA as appropriate. If statistical significance (P ≤0.05) was determined by ANOVA, the data were further analyzed by Students-Newman-Keuls procedure to detect specific differences between treatments.
Results
Effect of CSE and TCDD on expression of inflammatory genes in macrophages
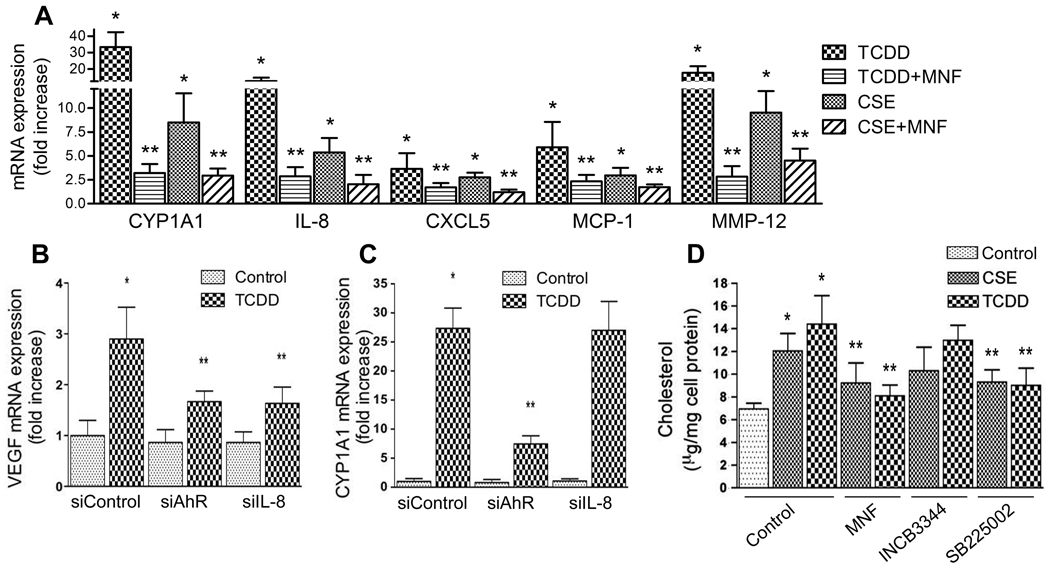
A number of pro-inflammatory genes play important roles in vascular inflammation and the development of atherosclerosis. Here, we investigated the effect of two environmental pollutants including CSE and TCDD on pro-inflammatory marker genes in macrophages derived from U937 cells. We selected CSE since it is well known to contain AhR-activating components and to cause cardiovascular diseases including atherosclerosis.21–22 As shown in Figure 1A, TCDD significantly increased the expression of IL-8, MCP-1 and MMP-12 by 13-fold, 6-fold, and 18-fold, respectively. The expression of the chemokine CXCL5, which is co-regulated with IL-8, was increased approximately 3-fold by TCDD. Treatment with 10% CSE also increased the mRNA level of IL-8, CXCL5, MCP-1 and MMP-12 but the effect was less pronounced than after TCDD exposure. Increased expression of MMP-12 in atherosclerotic lesions has been shown to be associated with foam cell generation.23 CYP1A1 gene expression was induced by TCDD- and CSE-treated U937 macrophages 33- and 8-fold, respectively (Fig. 1A). To test, if the effects of TCDD and CSE are mediated through AhR signaling, cells were co-treated with CSE or TCDD in the presence of AhR antagonist MNF. As expected, TCDD-induced target genes were significantly suppressed by MNF significantly by about 80–90%. CSE-induced target genes were suppressed by MNF to a lesser degree (~50%).
Figure 1.
(A) Expression of foam cell marker genes in macrophages treated with TCDD or CSE in absence or presence of MNF. Significantly different from control (*) or from CSE and TCDD alone (**). (B–C) Expression of (B) VEGF and (C) CYP1A1 in macrophages treated with TCDD for 24h after transient transfection with siRNA against AhR or IL-8 for 48h. Significantly different from control (*) or from TCDD treated siControl (**). (D) Cholesterol accumulation in macrophages treated with TCDD or CSE in the absence or presence of MNF, INCB3344 or SB225002. Significantly different from control (*) or from corresponding CSE or TCDD-treated cells (**). *P< 0.05.
Induction of VEGF mRNA expression in U937 macrophages by TCDD is AhR and IL-8 dependent
Vascular endothelial growth factor (VEGF) has been described as a downstream target of CXCR2 activation.18 In order to test if CXCR2 signaling is stimulated by TCDD, the expression of VEGF was analyzed. The results in supplemental Figure SI show that silencing of AhR and IL-8 decreased their mRNA level specifically by 70% and 75%, respectively. As shown in Figure 1B, suppression of AhR as well as IL-8 expression through gene silencing decreased the TCDD-stimulated induction of VEGF indicating that this effect is AhR-dependent and mediated through IL-8 which binds to and activate CXCR2. CSE-induced expression of VEGF was also inhibited by AhR antagonist MNF (data not shown). The expression of CYP1A1 was analyzed as a control and found to be suppressed after transient transfection with siAhR, but not after silencing IL-8 (Fig. 1C).
Stimulation of cholesterol accumulation in U937 macrophages by TCDD and CSE
Foam cells are primarily macrophages laden with cholesterol ester-rich cytoplasmic lipid inclusions. To quantify the total amount of cholesterol in U937 macrophages, we used a colorimetric method in the presence of cholesterol oxidase and cholesterol esterase. Exposure of U937 macrophages for 5 days to 10 % CSE or 10 nM TCDD resulted in the accumulation of cholesterol by 1.8- and 2.1-fold, respectively (Fig. 1D). In order to test whether the effect of CSE and TCDD exposure on cholesterol accumulation is AhR-dependent and involves the chemokine receptors CCR2 or CXCR2, cells were treated with CSE and TCDD in the presence of MNF (10 µM), INCB3344 (10 µM), or SB225002 (10 µM), respectively. INCB3344, kindly provided by Pfizer, is a potent and selective small molecule antagonist of the mouse CCR2 receptor. INCB3344 inhibits CCL2-mediated functional responses such as ERK phosphorylation and chemotaxis.24 SB225002 is a potent and selective CXCR2 chemokine receptor antagonist and prevents IL-8-induced neutrophil chemotaxis in vitro and sequestration in vivo.25 Results from cholesterol assay show that AhR-antagonist MNF as well as the CXCR2 antagonist SB225002 efficiently blocked CSE- and TCDD-stimulated cholesterol accumulation. On the other hand, the CCR2 antagonist INCB3344 did not decrease the accumulation of cholesterol in CSE- and TCDD-treated cells (Fig. 1D). These data suggest that CXCR2 signaling is involved in CSE- and TCDD-stimulated cholesterol accumulation.
TCDD promotes the development of atherosclerotic lesions in ApoE−/− mice
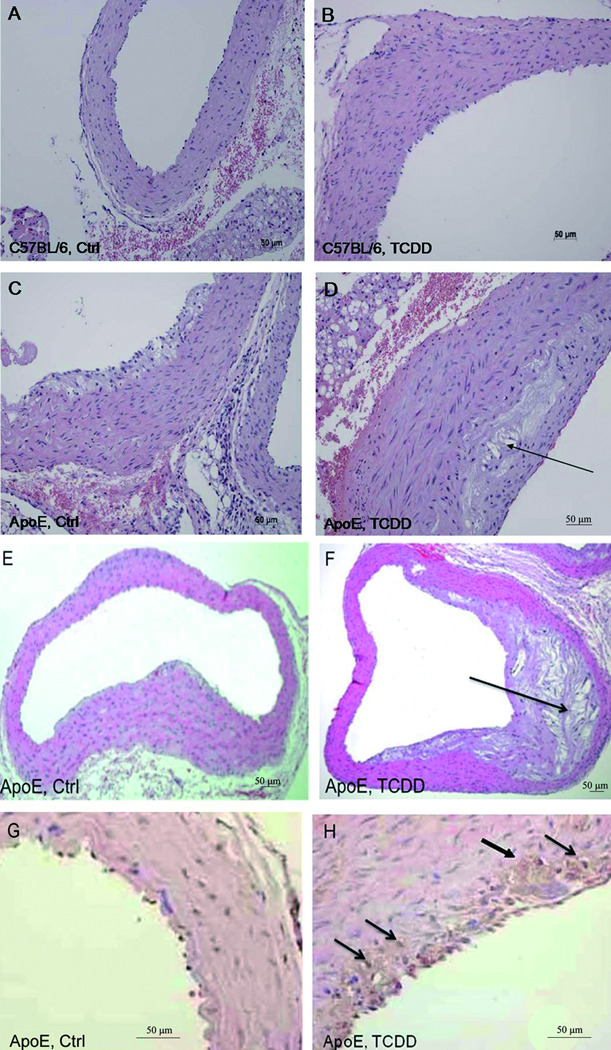
In vivo effects of TCDD on the formation of atherosclerotic plaques were studied in ApoE−/− mice. As expected no lesions were observed in C57BL/6 wild type control (Fig. 2A and SIIA) or TCDD-treated mice (Fig. 2B and SIIB). However, H&E-staining of aorta sections showed clear signs of atherosclerotic lesions with larger accumulation of lipid droplets in ApoE−/− mice treated with TCDD for 60 days (Fig. 2D and SIID) compared to untreated control ApoE−/− mice (Fig. 2C and SIIC). After 7 months of the initial treatment with TCDD, ApoE−/− developed a complex atheroma with extensive accumulation of extracellular lipid particles forming a large lipid core (Fig. 2F and SIIF) even without being fed a high-fat diet (HFD). Activation of macrophages in atherosclerotic lesions and formation of foam cells has been shown to be associated with increased expression of MMP-12 in macrophages9 (Fig. 1A) and aorta of ApoE−/− after TCDD treatment (Table 1). In order to examine if macrophages are present in early atherosclerotic lesions, aorta sections were stained with a MMP-12 specific antibody. As shown in Figure 2G and SIIG expression of MMP-12 was not detectable in 3 months old ApoE−/− mice fed a regular diet. In contrast, treatment with TCDD for 40 days resulted in the expression of MMP-12 in macrophages and macrophage-derived foam cells appearing in early atherosclerotic lesions (Fig. 2H and SIIH).
Figure 2.
Development of atherosclerotic lesions in ApoE−/− mice fed on regular chow after treatment with TCDD. (A–B) C57BL/6 and (C–D) ApoE−/− mice received (A&C) corn oil (Ctrl) or (B&D) TCDD for 60 days. (E–F) ApoE−/− mice received (E) corn oil or (F) TCDD for 7 months. Shown are representative cross sections from serial sections of aorta arches visualized by H&E staining. In each group, eight mice were evaluated showing similar phenotypes (See also Fig. SII). Arrows indicate formation of atherosclerotic lesions. (G–H) Expression of foam cell marker MMP-12 in aorta of (G) control and (H) 40-day TCDD treated ApoE−/− mice. Arrows indicate staining of MMP-12.
Table 1. Induction of inflammatory marker genes in aorta of ApoE−/− mice.
Mice were treated with a single i.p. injection of 15 µg/kg and a weekly dose of 1 µg/kg TCDD for 60 days. ApoE−/− mice were fed a regular chow (reg. chow) or a high-fat diet (HFD). mRNA levels of CYP1A1, CXCL5, CXCR2, KC, MCP-1, MMP-12, F4/80, P-Selectin, and VEGF were quantified using real-time PCR. Values represent means of three animals per group.
| ApoE−/− (reg. chow) | ApoE−/− (HFD) | |||
|---|---|---|---|---|
| Gene | Control | TCDD | Control | TCDD |
| CYP1A1 | 1.2 ± 0.5 | 207.7 ± 6.5* | 1.5 ± 0.9 | 210.0 ± 9.0* |
| CXCL5 | 1.5 ± 0.6 | 3.8 ± 1.2* | 3.7 ± 0.4** | 12.5 ± 0.9* |
| CXCR2 | 1.1 ± 0.3 | 1.2 ± 0.4 | 1.3 ± 0.3 | 1.2 ± 0.6 |
| KCa | 1.1 ± 0.4 | 8.3 ± 0.6* | 11.7 ± 0.7** | 28.4 ± 3.1* |
| MCP-1 | 1.2 ± 0.2 | 5.7 ± 0.9* | 3.7 ± 0.4** | 18.4 ± 0.8* |
| MMP-12 | 1.3 ± 0.4 | 29.2 ± 2.3* | 27.00 ± 2.1** | 52.1 ± 3.6* |
| F4/80 | 1.0 ± 0.2 | 3.6 ± 0.4* | 2.6 ± 0.4** | 7.7 ± 0.6* |
| P-Selectin | 1.2 ± 0.3 | 2.7 ± 0.3* | 2.8 ± 0.4** | 3.4 ± 0.5 |
| VEGF | 1.2 ± 0.5 | 2.9 ± 0.4* | 2.5 ± 0.3** | 3.2 ± 0.8 |
Values are significantly different from control;
values are different from ApoE−/− control mice fed a regular chow, (p < 0.01).
KC is the orthologue of human IL-8.
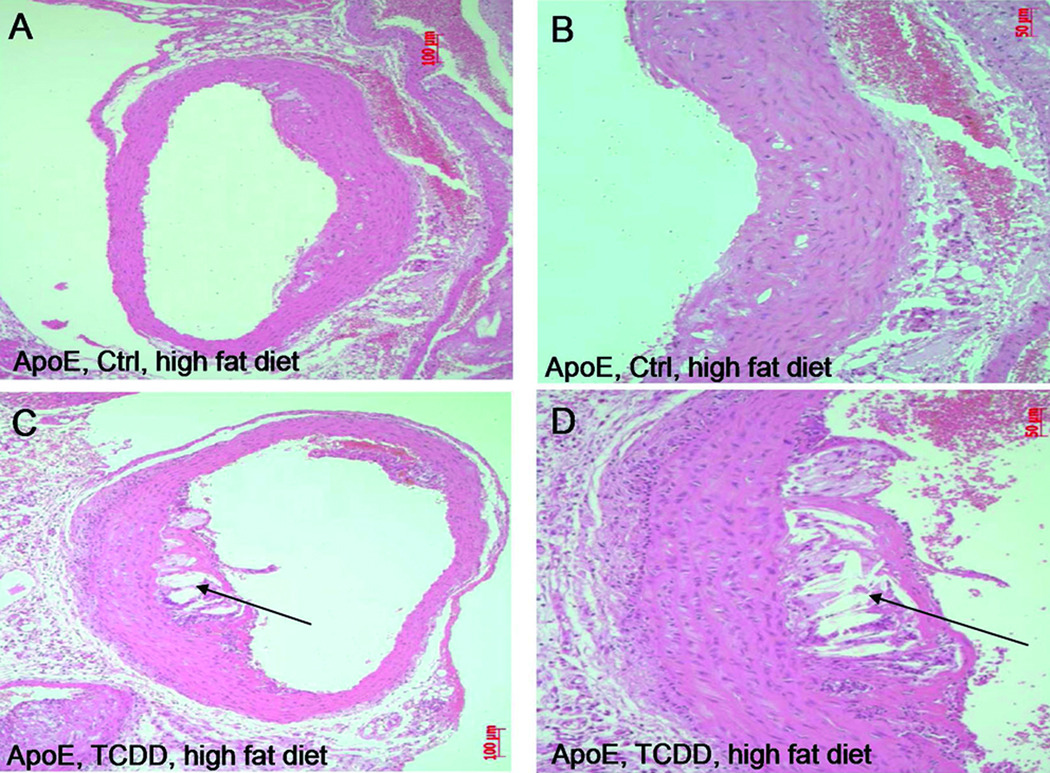
To test the contribution of AhR activation by TCDD on the formation of atherosclerotic lesion induced through HFD, mice were fed a Western diet for a period of 60 days after treatment with a single injection of 15 µg/kg TCDD. The hearts and aortas were excised from the mice to examine the extent of atherosclerosis in aorta. Representative examples of the aorta arches are shown in Figure 3A–D. The development of atherosclerotic lesions with mainly intracellular lipid accumulation occurred in ApoE−/− control mice fed a HFD diet (Fig. 3A and B, Fig. SIIIA and B). Furthermore, the area of atherosclerotic lesion with extensive lipid accumulation and a large lipid core in the serial sections of the aortic arches was advanced in TCDD-treated ApoE−/− mice, which were also fed a HFD (Fig. 3C and D, Fig. SIIIC and D).
Figure 3.
Development of atherosclerotic lesions in ApoE−/− mice fed a high fat diet. (A–B) ApoE−/− mice received coin oil (Ctrl) or (C–D) a single injection of 15 µg/kg TCDD. Representative images of eight mice are shown of serial tissue sections of aorta arches prepared 60 days after treatment with TCDD and stained with H&E. All animals in each group showed a similar phenotype (see also Fig. SIII).
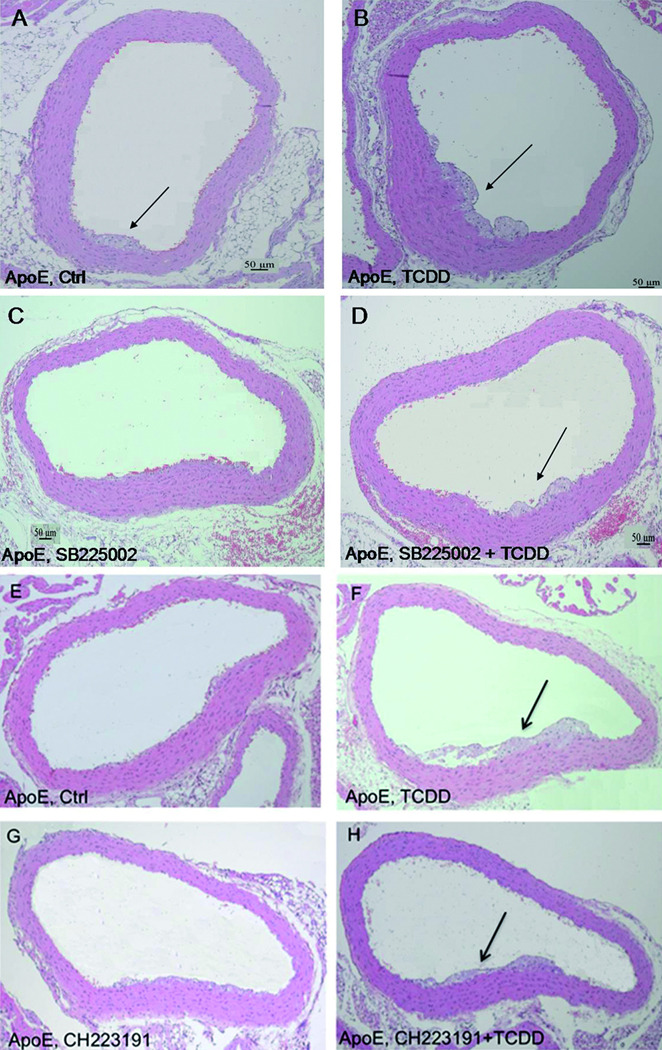
In accordance with the in vitro findings we tested the effect of SB225002 was tested for inhibition of CXRC2 in ApoE−/− mice that were fed a regular chow and treated with TCDD as described in Figure 4 and Figure SIV. Small early atherosclerotic lesions were detectable in control ApoE−/− mice (Fig. 4A and SIVA). TCDD, as shown earlier, enhanced the development of the lesions (Fig. 4B and F, Fig. SIVB and F). Aorta sections of ApoE−/− mice treated with SB225002 showed less accumulation of lipid droplets and lesion formation induced by activation of AhR after treatment with TCDD (Fig. 4D and SIVD).
Figure 4.
Inhibition of CXCR2 and AhR decreases plaque formation in ApoE−/− mice. Mice fed regular chow and received (A&E) corn oil (Ctrl) or (B&F) TCDD for 60 days. ApoE−/− mice were treated with (C) SB225002 in the absence or (D) presence of TCDD or treated with (G) CH223191 in the absence or (H) presence of TCDD. Serial cross sections of representative aorta arches from six mice were visualized by H&E staining. The animals in each group showed a similar phenotype (see also Fig. SIV). Arrows indicate formation of early atherosclerotic lesions.
In order to investigate the AhR-dependent effect in vivo, we co-treated mice with MNF, which antagonized the TCDD- and CSE-mediated effects in U937 macrophages (Fig. 1). However, experiments with MNF in vivo were not successful to block TCDD-induced CYP1A1 expression or the development of atherosclerotic lesions in ApoE−/− mice (data not shown). This result was unexpected since a previous report showed the successful inhibition of AhR-mediated induction of CYP1A1 by MNF in vivo.26 One explanation is that MNF is metabolized more rapidly in vivo and not a suitable AhR antagonist for long-term treatment in vivo. On the other hand, CH223191 a structurally unrelated AhR antagonist was used, since it has been demonstrated to prevent toxic effects of TCDD in vivo.27 CH223191 inhibited the TCDD-mediated induction of CYP1A1 by about 40% in liver of TCDD-treated ApoE−/− mice (data not shown). Furthermore, the development of atherosclerotic lesions was reduced by co-treatment with CH223191 (Fig. 4H and SIVH) compared to mice treated with TCDD alone (Fig. 4F and SIVF).
Assessment of atherosclerosis
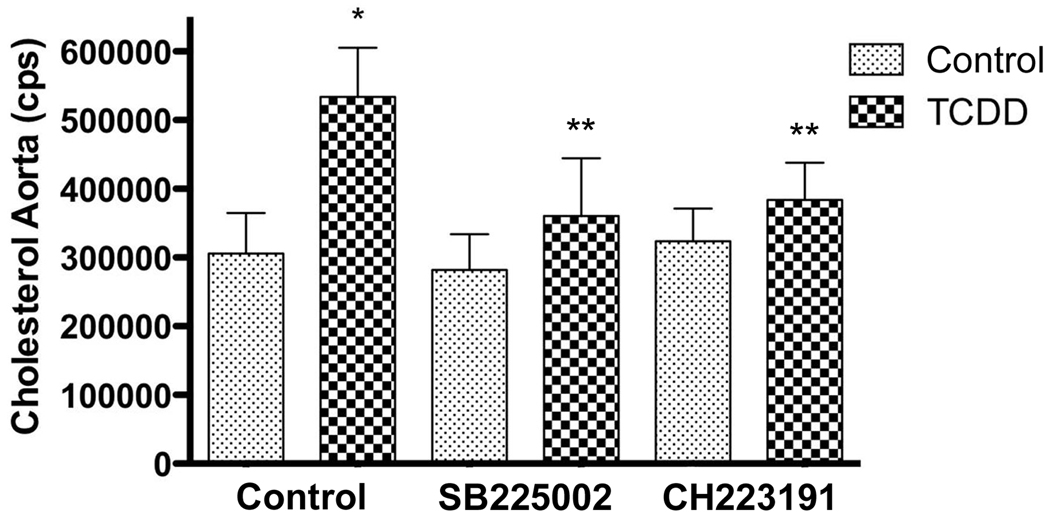
In order to quantify the lesion of the atherosclerotic plaques, a quantitative analysis of the amount of cholesterol accumulated in the aorta of the ApoE−/− mice was done via GC/MS. The mean aorta cholesterol level of the TCDD-treated group was increased approximately 1.7-fold compared to the control group (Fig. 5). The concentrations of cholesterol found in mice treated with the CXCR2 inhibitor SB225002 or the AhR antagonist CH223191 were similar to those in control mice. Interestingly, in agreement with the in vitro macrophage model the TCDD-induced cholesterol level in aorta from mice co-treated with SB225002 was not increased compared to the control group and lower than that in mice treated with TCDD alone. Furthermore, TCDD did not increase the level of cholesterol in the aorta of mice co-treated with CH223191 suggesting that the development of atherosclerotic lesions is AhR-dependent (Fig. 5).
Figure 5.
TCDD increased the amount of cholesterol accumulated in the aorta. ApoE−/− mice were treated with TCDD for 60 days in the absence or presence of SB225002 or CH223191 as described in Fig. 4. The amount of cholesterol accumulated in the aorta was measured via GC/MS from five animals in each group. Significantly different from vehicle control (*) or from TCDD-treated mice (**) (P < 0.05).
Gene expression analysis in the aorta arch of ApoE−/− mice
The effects of TCDD were first analyzed in ApoE−/− mice exposed to regular chow. Results from mRNA analysis of the aortas of ApoE−/− mice showed a distinct increase in CXC chemokines KC (mouse orthologue of human IL-8) and CXCL5; both of these chemokines bind to and activate the receptor CXCR2 (homolog of human IL-8R). An increased level of the CC chemokine MCP-1 was also found. Besides CYP1A1, the greatest gene expression increase was found for MMP-12, which is considered an indicator of activated macrophages and foam cells.23 Atherogenic marker genes P-Selectin and F4/80 were also elevated. F4/80 is a specific cell surface marker of macrophages, and elevated levels of P-Selectin imply the recruitment of macrophages/foam cells to the atherogenic site. In parallel, mRNA levels of the same marker genes were compared in ApoE−/− mice fed a HFD. The mRNA levels of all marker genes, except CYP1A1, of these mice were further increased by TCDD exposure. Interestingly, the expression of the examined genes, again with the exception of CYP1A1, was increased in control ApoE−/− mice on a HFD compared to the control group of ApoE−/− mice on a regular chow (Table 1).
Discussion
Atherosclerosis is an inflammatory disease of the arterial wall characterized by monocytes entering the subendothelial space followed by differentiation into macrophages and the development of foam cells. We have shown previously that environmental pollutants including TCDD, diesel engine exhaust and urban dust lead to an AhR-dependent elevated level of cholesterol in macrophages, which is associated with a dose-dependent increase in IL-8 as well as other inflammatory markers, including MCP-1 and COX-2, and the formation of foam cells.9–10 Here, we show that treatment of macrophages not only with TCDD, but also with CSE led to increased expression of the chemokines IL-8, MCP-1 and foam cell markers, such as MMP-12 in an AhR-dependent manner. Treatment with CSE, similar to TCDD, led to an elevated level of cholesterol in macrophages, which can be suppressed by an AhR antagonist. Interestingly, an AhR-dependent lipid accumulation stimulated by TCDD and benzo(a)pyrene (B[a]P) has been shown to involve the induction of the Niemann-Pick type C1 protein (NPC1) in macrophages.28
The current study shows that inhibition of the chemokine receptor CXCR2 but not CCR2 abolished the enhanced accumulation of cholesterol by both TCDD and CSE. This result agrees with previous findings reporting that progression of early atherosclerotic lesions still occurred in ApoE and CCR2 double-knockout mice on an atherogenic diet.29 These data indicate that TCDD as well as CSE stimulate formation of foam cells in an AhR-dependent manner and that this effect is mediated by CXCR2 through an elevated level of IL-8. This hypothesis is supported by results showing that gene silencing of AhR and IL-8 suppressed the increase of VEGF, which is a downstream target of CXCR2 signaling. Furthermore, these data suggest that AhR is a critical mediator of activating macrophages and forming foam cells not only for TCDD, but also for components of cigarette smoke. This supports a previous report showing that the increased risk of coronary heart disease induced by cigarette smoke does not correlate with the amount of tar, nicotine or carbon monoxide but rather other components of CSE, such as B[a]P and other AhR-activating PAHs.30 Similar to B[a]P, PAHs like B[e]P may increase inflammation and plaque formation independently from the AhR.31 This would explain the potency of cigarette smoke in promoting atherogenesis since cigarette smoke is a major source of exposure to PAHs. PAHs are also known to form DNA adducts, which must be considered as a contributing factor to the development of atherosclerotic plaques in humans.32–33
Inhibition of CXCR2 in vitro as well as in vivo in the ApoE−/− mice by SB225002 prevented macrophages from accumulating cholesterol and reduced the formation of aortic plaque in TCDD-treated mice. Several CXC chemokines including IL-8, KC or CXCL5 can bind to and activate CXCR2. Thus, there is a possibility that besides KC or IL-8 other chemokines like CXCL5, are responsible for the development of early atherosclerotic lesions. However, the TCDD-mediated increase of CXCL5 in U937 macrophages was relatively low compared to the clear induction of IL-8 or the induction of KC in ApoE−/− mice. Therefore, the effect of enhanced lesions in vivo is more likely to be mediated through KC rather than an elevated level of CXCL5 observed in aortas of ApoE−/− mice. Furthermore, mice co-treated with the AhR antagonist CH223191 accumulated less cholesterol and had reduced atherosclerotic lesions compared to TCDD treated mice, supporting the concept that this process is AhR-dependent.
The expression of genes analyzed in the aorta of ApoE−/− mice revealed that the selected inflammatory genes especially KC and MMP-12 were significantly increased in ApoE−/− mice fed a HFD compared to ApoE−/− mice on a regular chow. Previous reports indicate that the TCDD- and B[a]P-induced profile of pro-inflammatory factors in macrophages might be more complex and may involve transforming growth factor (TGF)β, tumor necrosis factor (TNF)α, and CCL-1.31, 34–35 The results from the current study indicate that activation of KC and MMP-12 is involved in the development of atherosclerosis mediated through a HFD. On the other hand, the mRNA level of CYP1A1 was not affected by the HFD in ApoE−/− mice compared to mice on a regular chow, which suggests that CYP1A1 is unlikely to be a key player in the formation of early atherosclerotic lesions induced by a HFD. This is not a trivial point since modified LDL has been found to bind to and activate the AhR followed by an AhR-mediated signaling like CYP1A1 induction.36
Other investigators have found that TCDD binds and disrupts the secondary and tertiary lipoprotein structure, which leads to a significant reduction in its ability to bind lipoprotein lipase.37 The altered lipoprotein structure impairs the cellular uptake of LDL and VLDL, leading to an increased accumulation of serum lipoproteins that could accelerate the development of coronary artery disease. The TCDD-induced changes in the structure of LDL and VLDL were found to be different from other modified LDL or VLDL, which may bind to and activate scavenger receptors.37 In this study, TCDD was found to be physically associated with LDL particles in TCDD treated ApoE−/− mice fed a HFD.7 These findings suggest that LDL might also serve as a vehicle to deliver the compound to atherosclerotic lesions, which may lead to more severe atherosclerotic plaques when mice are fed a HFD. Although TCDD can physically associate with LDL, we found similar effects in vitro after treatment with CSE, which suggests that this mechanism is unlikely and induction of pro-inflammatory stimuli is the more probable mechanism. CSE and components of CSE like peroxynitrite38 have been reported to be involved in oxidative modification of LDL,39–41 which can induce pro-inflammatory responses and promotion of atherosclerosis.
Data from the current study support the assumption showing an earlier onset and greater severity of atherosclerotic lesions induced by TCDD in ApoE−/− mice fed a HFD compared to a regular chow. The fact that there was an earlier onset of atherosclerotic lesions after two months of TCDD exposure and the formation of severe atheroma after 7 months of TCDD exposure without feeding a HFD is noteworthy. Although, the lesions induced by TCDD exposure alone were smaller compared to mice treated with TCDD and HFD, the current data suggest that induction of an inflammatory response by TCDD is sufficient to cause accumulation of cholesterol and the formation of atherosclerotic lesions in ApoE−/− mice.
In summary, the results reported in this study suggest that activation of macrophage-mediated inflammation followed by accumulation of cholesterol is a potential target of AhR-activating pollutants, such as dioxin and cigarette smoke. Findings also include the identification of the important role that CXCR2 ligands KC and IL-8 play in atherogenesis induced by AhR activation and support that environmental pollutants like TCDD may trigger vascular inflammation by activating signaling pathways involved in inflammatory responses and atherosclerosis. Although, the toxicity of AhR-activating environmental pollutants in regards to cardiovascular disease warrants further study, the implications of CXC chemokines and their receptors for human health could be significant.
Supplementary Material
Acknowledgements
We thank Dr. Phillip Fujiyoshi for helping with statistical analysis of the data and Anna Lollies, and Shannon Murphy for technical assistance. We also like to thank Jane Qian Chen and Katie Bell from the Center for Comparative Medicine at UC Davis for technical support. We like to thank Monika Heitmeier and Pfeizer for providing INCB3344. This work was supported by American Heart Association Western Affiliate 0765056Y and R21ES15846 from the National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest is declared.
References
- 1.Bertazzi PA, Bernucci I, Brambilla G, Consonni D, Pesatori AC. The seveso studies on early and long-term effects of dioxin exposure: A review. Environ Health Perspect. 1998;106 Suppl 2:625–633. doi: 10.1289/ehp.98106625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelclova D, Fenclova Z, Preiss J, Prochazka B, Spacil J, Dubska Z, Okrouhlik B, Lukas E, Urban P. Lipid metabolism and neuropsychological follow-up study of workers exposed to 2,3,7,8- tetrachlordibenzo- p-dioxin. Int Arch Occup Environ Health. 2002;75 Suppl:S60–S66. doi: 10.1007/s00420-002-0350-4. [DOI] [PubMed] [Google Scholar]
- 3.Brewster DW, Matsumura F, Akera T. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on guinea pig heart muscle. Toxicol Appl Pharmacol. 1987;89:408–417. doi: 10.1016/0041-008x(87)90160-8. [DOI] [PubMed] [Google Scholar]
- 4.Arsenescu V, Arsenescu RI, King V, Swanson H, Cassis LA. Polychlorinated biphenyl-77 induces adipocyte differentiation and proinflammatory adipokines and promotes obesity and atherosclerosis. Environ Health Perspect. 2008;116:761–768. doi: 10.1289/ehp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flesch-Janys D, Berger J, Gurn P, Manz A, Nagel S, Waltsgott H, Dwyer JH. Exposure to polychlorinated dioxins and furans (pcdd/f) and mortality in a cohort of workers from a herbicide-producing plant in hamburg, federal republic of germany. Am J Epidemiol. 1995;142:1165–1175. doi: 10.1093/oxfordjournals.aje.a117575. [DOI] [PubMed] [Google Scholar]
- 6.Bombick DW, Matsumura F, Madhukar BV. Tcdd (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes reduction in the low density lipoprotein (ldl) receptor activities in the hepatic plasma membrane of the guinea pig and rat. Biochem Biophys Res Commun. 1984;118:548–554. doi: 10.1016/0006-291x(84)91337-8. [DOI] [PubMed] [Google Scholar]
- 7.Dalton TP, Kerzee JK, Wang B, Miller M, Dieter MZ, Lorenz JN, Shertzer HG, Nerbert DW, Puga A. Dioxin exposure is an environmental risk factor for ischemic heart disease. Cardiovasc Toxicol. 2001;1:285–298. doi: 10.1385/ct:1:4:285. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Vogel CF, Sciullo E, Matsumura F. Activation of inflammatory mediators and potential role of ah-receptor ligands in foam cell formation. Cardiovasc Toxicol. 2004;4:363–373. doi: 10.1385/ct:4:4:363. [DOI] [PubMed] [Google Scholar]
- 10.Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and c-reactive protein in human macrophage cell line u937 exposed to air pollution particulates. Environ Health Perspect. 2005;113:1536–1541. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin WS, Szuba A, Rockson SG. The role of chemokines in human cardiovascular pathology: Enhanced biological insights. Atherosclerosis. 2002;160:91–102. doi: 10.1016/s0021-9150(01)00571-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Tabas I, Winchester R, Ravalli S, Rabbani LE, Tall A. Interleukin 8 is induced by cholesterol loading of macrophages and expressed by macrophage foam cells in human atheroma. J Biol Chem. 1996;271:8837–8842. doi: 10.1074/jbc.271.15.8837. [DOI] [PubMed] [Google Scholar]
- 13.Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. Relb, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gargalovic PS, Imura M, Zhang B, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Patel S, Nelson SF, Horvath S, Berliner JA, Kirchgessner TG, Lusis AJ. Identification of inflammatory gene modules based on variations of human endothelial cell responses to oxidized lipids. Proc Natl Acad Sci U S A. 2006;103:12741–12746. doi: 10.1073/pnas.0605457103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boisvert WA, Rose DM, Johnson KA, Fuentes ME, Lira SA, Curtiss LK, Terkeltaub RA. Up-regulated expression of the cxcr2 ligand kc/gro-alpha in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am J Pathol. 2006;168:1385–1395. doi: 10.2353/ajpath.2006.040748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boisvert WA, Santiago R, Curtiss LK, Terkeltaub RA. A leukocyte homologue of the il-8 receptor cxcr-2 mediates the accumulation of macrophages in atherosclerotic lesions of ldl receptor-deficient mice. J Clin Invest. 1998;101:353–363. doi: 10.1172/JCI1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadopoulou C, Corrigall V, Taylor PR, Poston RN. The role of the chemokines mcp-1, gro-alpha, il-8 and their receptors in the adhesion of monocytic cells to human atherosclerotic plaques. Cytokine. 2008;43:181–186. doi: 10.1016/j.cyto.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang G, Rosen DG, Liu G, Yang F, Guo X, Xiao X, Xue F, Mercado-Uribe I, Huang J, Lin SH, Mills GB, Liu J. Cxcr2 promotes ovarian cancer growth through dysregulated cell cycle, diminished apoptosis, and enhanced angiogenesis. Clin Cancer Res. 2010;16:3875–3886. doi: 10.1158/1078-0432.CCR-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taegue S, Pinkerton K, Goldsmit M, Gebremichael A, Chang S, Jenkins R, Moneyhun J. Sidestream cigarette smoke generation and exposure system for environmental tobacco smoke studies. Inhal Toxicol. 1994;6:79–93. [Google Scholar]
- 20.Huster D, Purnat TD, Burkhead JL, Ralle M, Fiehn O, Stuckert F, Olson NE, Teupser D, Lutsenko S. High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of wilson disease. J Biol Chem. 2007;282:8343–8355. doi: 10.1074/jbc.M607496200. [DOI] [PubMed] [Google Scholar]
- 21.Kasai A, Hiramatsu N, Hayakawa K, Yao J, Maeda S, Kitamura M. High levels of dioxin-like potential in cigarette smoke evidenced by in vitro and in vivo biosensing. Cancer Res. 2006;66:7143–7150. doi: 10.1158/0008-5472.CAN-05-4541. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura M, Kasai A. Cigarette smoke as a trigger for the dioxin receptor-mediated signaling pathway. Cancer Lett. 2007;252:184–194. doi: 10.1016/j.canlet.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto S, Kobayashi T, Katoh M, Saito S, Ikeda Y, Kobori M, Masuho Y, Watanabe T. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: Relationship to lesion development. Am J Pathol. 1998;153:109–119. doi: 10.1016/s0002-9440(10)65551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodmerkel CM, Huber R, Covington M, Diamond S, Hall L, Collins R, Leffet L, Gallagher K, Feldman P, Collier P, Stow M, Gu X, Baribaud F, Shin N, Thomas B, Burn T, Hollis G, Yeleswaram S, Solomon K, Friedman S, Wang A, Xue CB, Newton RC, Scherle P, Vaddi K. Discovery and pharmacological characterization of a novel rodent-active ccr2 antagonist, incb3344. J Immunol. 2005;175:5370–5378. doi: 10.4049/jimmunol.175.8.5370. [DOI] [PubMed] [Google Scholar]
- 25.White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM. Identification of a potent, selective non-peptide cxcr2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 26.Nazarenko DA, Dertinger SD, Gasiewicz TA. In vivo antagonism of ahr-mediated gene induction by 3'-methoxy-4'-nitroflavone in tcdd-responsive lacz mice. Toxicol Sci. 2001;61:256–264. doi: 10.1093/toxsci/61.2.256. [DOI] [PubMed] [Google Scholar]
- 27.Kim SH, Henry EC, Kim DK, Kim YH, Shin KJ, Han MS, Lee TG, Kang JK, Gasiewicz TA, Ryu SH, Suh PG. Novel compound 2-methyl-2h-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide (ch-223191) prevents 2,3,7,8-tcdd-induced toxicity by antagonizing the aryl hydrocarbon receptor. Mol Pharmacol. 2006;69:1871–1878. doi: 10.1124/mol.105.021832. [DOI] [PubMed] [Google Scholar]
- 28.Podechard N, Le Ferrec E, Rebillard A, Fardel O, Lecureur V. Npc1 repression contributes to lipid accumulation in human macrophages exposed to environmental aryl hydrocarbons. Cardiovasc Res. 2009;82:361–370. doi: 10.1093/cvr/cvp007. [DOI] [PubMed] [Google Scholar]
- 29.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in ccr2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman DW, Helmrich SP, Rosenberg L, Miettinen OS, Shapiro S. Nicotine and carbon monoxide content of cigarette smoke and the risk of myocardial infarction in young men. N Engl J Med. 1983;308:409–413. doi: 10.1056/NEJM198302243080801. [DOI] [PubMed] [Google Scholar]
- 31.Curfs DM, Knaapen AM, Pachen DM, Gijbels MJ, Lutgens E, Smook ML, Kockx MM, Daemen MJ, van Schooten FJ. Polycyclic aromatic hydrocarbons induce an inflammatory atherosclerotic plaque phenotype irrespective of their DNA binding properties. FASEB J. 2005;19:1290–1292. doi: 10.1096/fj.04-2269fje. [DOI] [PubMed] [Google Scholar]
- 32.Ramos KS, Moorthy B. Bioactivation of polycyclic aromatic hydrocarbon carcinogens within the vascular wall: Implications for human atherogenesis. Drug Metab Rev. 2005;37:595–610. doi: 10.1080/03602530500251253. [DOI] [PubMed] [Google Scholar]
- 33.Thirman MJ, Albrecht JH, Krueger MA, Erickson RR, Cherwitz DL, Park SS, Gelboin HV, Holtzman JL. Induction of cytochrome cypia1 and formation of toxic metabolites of benzo[a]pyrene by rat aorta: A possible role in atherogenesis. Proc Natl Acad Sci U S A. 1994;91:5397–5401. doi: 10.1073/pnas.91.12.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lecureur V, Ferrec EL, N'Diaye M, Vee ML, Gardyn C, Gilot D, Fardel O. Erk-dependent induction of tnfalpha expression by the environmental contaminant benzo(a)pyrene in primary human macrophages. FEBS Lett. 2005;579:1904–1910. doi: 10.1016/j.febslet.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 35.N'Diaye M, Le Ferrec E, Lagadic-Gossmann D, Corre S, Gilot D, Lecureur V, Monteiro P, Rauch C, Galibert MD, Fardel O. Aryl hydrocarbon receptor- and calcium-dependent induction of the chemokine ccl1 by the environmental contaminant benzo[a]pyrene. J Biol Chem. 2006;281:19906–19915. doi: 10.1074/jbc.M601192200. [DOI] [PubMed] [Google Scholar]
- 36.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc Natl Acad Sci U S A. 2007;104:1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arehart E, Giasson G, Walsh MT, Patterson H. Dioxin alters the human low-density and very low-density lipoprotein structure with evidence for specific quenching of trp-48 in apolipoprotein c-ii. Biochemistry. 2004;43:8503–8509. doi: 10.1021/bi036190i. [DOI] [PubMed] [Google Scholar]
- 38.Yamaguchi Y, Matsuno S, Kagota S, Haginaka J, Kunitomo M. Peroxynitrite-mediated oxidative modification of low-density lipoprotein by aqueous extracts of cigarette smoke and the preventive effect of fluvastatin. Atherosclerosis. 2004;172:259–265. doi: 10.1016/j.atherosclerosis.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Santanam N, Sanchez R, Hendler S, Parthasarathy S. Aqueous extracts of cigarette smoke promote the oxidation of low density lipoprotein by peroxidases. FEBS Lett. 1997;414:549–551. doi: 10.1016/s0014-5793(97)01067-3. [DOI] [PubMed] [Google Scholar]
- 40.Yokode M, Ueyama K, Arai NH, Ueda Y, Kita T. Modification of high- and low-density lipoproteins by cigarette smoke oxidants. Ann N Y Acad Sci. 1996;786:245–251. doi: 10.1111/j.1749-6632.1996.tb39067.x. [DOI] [PubMed] [Google Scholar]
- 41.Yokode M, Kita T, Arai H, Kawai C, Narumiya S, Fujiwara M. Cholesteryl ester accumulation in macrophages incubated with low density lipoprotein pretreated with cigarette smoke extract. Proc Natl Acad Sci U S A. 1988;85:2344–2348. doi: 10.1073/pnas.85.7.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.