Abstract
Objective-
Vascular gene transfer is a powerful tool for investigating and treating vascular diseases; however, its utility is limited by brevity of transgene expression and vector-associated inflammation. Helper-dependent adenovirus (HDAd), an advanced-generation Ad that lacks all viral genes, is superior to first-generation Ad (FGAd) in normal rabbit arteries. We compared HDAd to FGAd in arteries of cholesterol-fed rabbits, a model of early atherogenesis in which transgene expression might be decreased, and inflammation increased.
Methods and Results-
Carotid arteries of chow- and cholesterol-fed rabbits were infused with FGAd, HDAd, or medium. HDAd expressed a transgene at least as well in arteries of cholesterol-fed rabbits as in arteries of chow-fed rabbits and expressed more durably than FGAd. In arteries of cholesterol-fed rabbits, HDAd stimulated less intimal growth, lipid deposition, and inflammation than FGAd. Neither vector affected phenylephrine-induced contraction or nitroprusside-mediated relaxation; however, both vectors decreased maximal acetylcholine-stimulated vasorelaxation. Relative absence of intimal growth in HDAd arteries could interfere with the utility of this model for testing atheroprotective genes; however, both co-infusion of FGAd and extension of cholesterol feeding yielded larger intimal lesions, on which atheroprotective genes could be tested.
Conclusions-
HDAd is superior to FGAd for expression of transgenes in atherosclerosis-prone arteries.
Keywords: atherosclerosis, vascular gene therapy, helper-dependent adenovirus, intima, rabbit
In vivo vascular gene transfer is a powerful tool for investigating the roles of genes and their protein products in vascular homeostasis and disease.1, 2 Vascular gene transfer has several advantages compared to the alternative approach of germ-line transgenesis with vascular targeting using cell-type-specific promoters. First, because vascular gene transfer is site-specific, un-manipulated vascular segments in the same animal can serve as experimental controls. Second, vascular gene transfer confines transgene expression to a specific site. In contrast, germ line transgenesis yields expression throughout the vasculature, making it difficult to determine the precise location(s) at which the transgene acts. Third, because vascular gene transfer cannot cause embryonic lethality, it can be used reliably to express transgenes in blood vessels of adult animals. Fourth, it is far easier to adjust gene expression levels using vascular gene transfer than with germ-line transgenesis. Finally, vascular gene transfer can be applied as a human therapy3, 4 whereas germ-line transgenesis cannot.
Despite its promise, application of vascular gene transfer for both investigational and therapeutic purposes has been limited by brevity of transgene expression and vector-associated inflammation.5-7 These are significant limitations because many vascular diseases and biologically important phenotypes (e.g. atherosclerosis and aneurysm formation) develop over prolonged periods and are affected by inflammation. Accordingly, a vector used to investigate or treat these diseases must achieve prolonged transgene expression and should not cause significant vascular inflammation. Until recently, no such vector was available.1, 8
Helper-dependent adenoviral (HDAd) vectors, advanced-generation Ad vectors that lack all viral genes,9 are promising agents for vascular gene transfer. Recombinant HDAd are relatively easily prepared10, 11 and transfer genes efficiently to many tissues.12 HDAd can express therapeutic genes for 2 – 3 yr in rodents (essentially a rodent “lifetime”) and for over 2 yr in livers of nonhuman primates.12-14 To begin to test the suitability of HDAd for vascular gene transfer, several years ago we tested the performance of HDAd in normal rabbit carotid arteries. HDAd expressed a transgene far longer than first-generation (FG) Ad (≥ 8 wk versus 1 – 2 wk for FGAd)5, 6 and caused only minimal vascular inflammation.15 These data were promising and suggested that HDAd will be broadly useful for both investigational and therapeutic purposes. However, atherosclerosis and other vascular diseases develop and progress on a background of hyperlipidemia and associated arterial pathology. Before using HDAd to investigate or treat these diseases, the performance of HDAd in arteries of hyperlipidemic animals must be tested. Ideally, HDAd would express a transgene persistently and at high levels in arteries of hyperlipidemic animals, with little or no associated inflammation and no detrimental effects on arterial physiology. However, studies with FGAd suggest that—in arteries with early atherosclerosis—vector-mediated transgene expression could be significantly lower16 and vector-related inflammation and lesion formation significantly higher than in normal arteries.17 Here we report experiments that test the performance of HDAd in a rabbit model of early atherosclerosis.
Materials and Methods
Adenoviral Vectors
We used 6 vectors: FGAduPA, FGAdNull, FGAdCMVnlacZ, HDAduPA, HDAdNull, and HDAdGFP. FGAduPA, FGAdNull, and FGAdCMVnlacZ are first-generation E1/E3-deleted vectors that contain a rabbit urokinase plasminogen activator (uPA) cDNA,18 an identical expression cassette without the uPA transgene,18 and a nucleus-localized β-galactosidase gene,19 respectively. HDAduPA,15 HDAdNull20 and HDAdGFP20 are helper-dependent vectors, lacking all viral open reading frames. HDAduPA and HDAdNull contain the same expression cassettes as FGAduPA and FGAdNull, respectively. HDAdGFP expresses green fluorescent protein. All 6 vectors include the cytomegalovirus (CMV) promoter and SV40 polyadenylation signal. Viral stocks were propagated in either human embryonic kidney 293 cells (for FGAd) or 293 Cre cells (for HDAd).21 Viral concentrations [viral particles (vp)/mL] were measured with a spectrophotometer.22 E1A-containing genomes and helper virus contamination (for HDAd) were measured by real-time polymerase chain reaction (PCR).20 Viral preparations were used only if E1A-containing genomes were below 1 in 106 vector genomes. Helper virus concentrations were below 1% in all HDAd preparations.
Rabbit Common Carotid Artery Gene Transfer
Male New Zealand White rabbits (2.5 – 3.5 kg, Western Oregon Rabbit Co.) were fed either normal chow or a high-fat diet containing 0.25% cholesterol and 3% soybean oil (Dyets, Inc.). Plasma cholesterol levels were measured with a colorimetric assay (Abbott Laboratories). In rabbits fed the high-fat diet, cholesterol levels were measured after 4 wk on diet, and rabbits were assigned to the experimental groups in a manner that the mean cholesterol levels of rabbits in each group did not differ significantly. Gene transfer surgery was performed 4 wk after starting the high-fat diet. Endothelium-specific gene transfer to common carotid arteries of hyperlipidemic rabbits (atherosclerosis-prone arteries) or chow-fed rabbits (normal arteries) was performed as described.17 For infusion in vivo, viral stocks were diluted to 2 – 7.5 × 1011 vp/mL with phenol red-free DMEM containing 1mg/mL rabbit serum albumin (Sigma). Control arteries were infused with this diluent alone. Arteries were harvested 3, 14, 28, or 56 d after infusion and for most experiments were either perfusion-fixed in situ with 10% neutral-buffered formalin and embedded in paraffin, placed in OCT medium for frozen sectioning, snap-frozen for RNA extraction, or placed in explant culture for measurement of uPA activity. Arteries infused with HDAdGFP were fixed ex vivo with 4% paraformaldehyde for 1 h, rinsed with PBS, placed in 30% sucrose at 4 °C overnight, then embedded in OCT medium. Arteries infused with FGAdCMVnlacZ were fixed for 2 hr in 0.2% glutaraldehyde in 0.1 M PBS with 5 mM EGTA, stained with X-gal for 4 h at 37 °C, then embedded in paraffin. For vascular reactivity measurement, arteries were removed, rinsed, and placed immediately in ice-cold physiological saline solution (PSS; see below).
Histochemistry, Immunohistochemical Staining and Fluorescence Microscopy
Sections of paraffin-embedded arteries were stained with Verhoeff van Gieson stain for planimetry of intimal and medial areas. Serial frozen sections were stained with antibodies to macrophages (RAM-11, 1:50 dilution, DAKO), VCAM-1, or ICAM-1 (Rab1/9 and Rab2/3, respectively, at 1:50 dilutions; from Dr. Myron Cybulsky, University of Toronto).23 Bound antibodies were detected with the Vectastain ABC kit (PK-4002, Vector Laboratories) and aminoethyl carbazole (AEC) substrate (Invitrogen). The specificity of primary antibodies was confirmed by substituting an isotype-matched primary antibody (eBiosciences). Lipid was visualized by oil red O staining of frozen sections.24 GFP expression was detected by fluorescence microscopy of frozen sections. β-galactosidase expression was detected by light microscopic examination of nuclear fast red-counterstained sections.
Histological and Morphometric Analyses
Planimetry of intimal and medial areas was performed with ImagePro Plus 5.0 (Bethesda, MD). At least 4 evenly spaced sections from each artery were measured and mean areas computed. Intimal area was calculated by subtracting the lumen area from the area within the internal elastic lamina. Medial area was calculated by subtracting the area with the internal elastic lamina from the area within the external elastic lamina. Percentages of intimal RAM-11 and oil red O staining were calculated by dividing the stained areas by the total intimal area. The intensity of antibody staining for VCAM-1 and ICAM-1 was graded semiquantitatively using the scale: 0, no staining; 1, rare positive cells or staining barely visible at low power (100 X); 2, focal staining or faint diffuse staining clearly visible at low power; 3, multifocal staining or moderate diffuse staining; 4, intense diffuse staining. In most experiments grading was done by two independent observers, whose scores were highly correlated (r2 = 0.82 for VCAM-1 and r2 = 0.84 for ICAM-1). The remainder of the scoring was done by a single observer, whose results were highly reproducible (r2 > 0.8 for 2 independent sets of scores). All observers were blinded to the identity of the specimens.
RNA Extraction and Quantitative Real-Time PCR
Total RNA was extracted with the RNeasy Fibrous Tissue Mini Kit (Qiagen). Specific RNA were measured using quantitative reverse transcriptase-mediated PCR (qRT-PCR) with an Applied Biosystems 7500 Real-Time PCR System, the Verso 1-step QRT-PCR low ROX mix kit (Thermo Scientific), and 100 ng of RNA template in a 25 μL reaction. A no-template control was included in all experiments, and a no-reverse-transcriptase control in many experiments. Baseline levels of cytokine expression were determined by qRT-PCR performed on RNA extracted from unmanipulated arteries of chow-fed rabbits. RNA levels were calculated using the ΔΔCT method25 and were normalized to GAPDH mRNA. Primer and probe sequences are in Supplemental Table I.
Plasminogen Activator (PA) Activity Assay
To measure PA activity in medium conditioned by explanted arteries, aliquots of conditioned medium were incubated with human Glu-plasminogen (0.87 μM; American Diagnostica) and the plasmin substrate S-2251 (0.8 mM; diaPharma) at 37 °C. The change in absorbance at 405 nm was measured, and uPA concentration calculated by reference to a human scuPA standard (American Diagnostica), with normalization to wet artery weight.
Measurement of Vascular Reactivity
Two weeks after infusion, carotid arteries were excised and connective tissue removed with the aid of a dissecting microscope. Arteries were cut into 3 mm rings and transferred to an organ bath containing PSS, equilibrated with 95% O2 and 5% CO2. PSS contains (in mM): NaCl 119; KCl 4.7; MgSO4 2.4; KH2PO4 1.2; CaCl2 3.3; NaHCO3 25; EDTA 0.03; Dextrose 6. Buffer was maintained at 37 °C, pH 7.4. Arterial rings were attached to two probes in a Multi Wire Myograph System - 610M (DMT) and the transducer interfaced to a Powerlab 4/26 recorder for measurement of isometric force. Rings were placed under an initial tension of 20 mN and equilibrated for 1 hr. Ring contraction was measured using phenylephrine hydrochloride (Sigma), and endothelium-dependent and -independent relaxations were measured using acetylcholine and sodium nitroprusside, respectively. Vessel relaxation was expressed as the percentage of contraction induced by phenylephrine.26
Statistical Analysis
Results are reported as mean ± SEM. For comparison of 2 groups, we used an unpaired t test for normally distributed data with equal group variances and a Mann-Whitney rank-sum test for other data. Comparison of three or more groups (for plasma cholesterol values) was by one-way ANOVA, with Dunn’s correction for multiple comparisons. Vascular reactivity was analyzed by repeated measurement two-way ANOVA. Concentration-response curves were fitted with a nonlinear regression program (GraphPad Prism) to obtain values of maximal effect (Emax). Emax values were compared by one-way ANOVA.
Results
Transgene Expression by HDAd and FGAd in Arteries of Chow-Fed and Cholesterol-Fed Rabbits
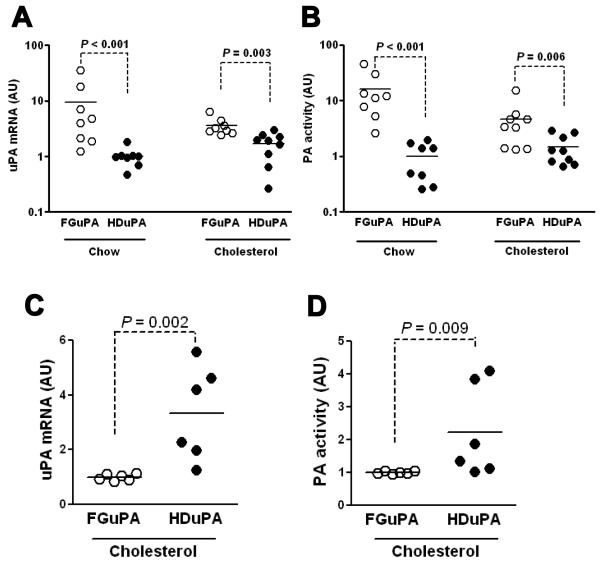
Three days after infusion of FGAduPA or HDAduPA in arteries of chow-fed rabbits, uPA mRNA levels in FGAduPA arteries were 10-fold higher than in HDAduPA arteries (Figure 1A; P < 0.001). In cholesterol-fed rabbits the increase in uPA mRNA in FGAduPA versus HDAduPA arteries was far more modest (2-fold; P = 0.003). Measurements of PA activity in medium conditioned by explanted arteries yielded similar results: PA activity was 16-fold higher in medium from FGAduPA versus HDAduPA arteries of chow-fed rabbits but only 3-fold higher in medium from arteries of cholesterol-fed rabbits (Figure 1B; P ≤ 0.006 for both). The smaller difference in transgene expression from FGAduPA versus HDAduPA in arteries of cholesterol-fed versus chow-fed rabbits appeared to result both from reduced expression by FGAduPA (60 – 80% less) and increased expression by HDAduPA (50 – 70% more) in arteries of cholesterol-fed rabbits. By 28 d after gene transfer, uPA expression was significantly higher in HDAduPA than FGAduPA arteries (Figure 1C – D), similar to results obtained in chow-fed rabbits.15 Repeat qRT-PCR (in a single batch) of the 3 d and 4 wk cholesterol-fed rabbit arteries confirmed relatively persistent transgene expression from HDAduPA (Supplemental Figure I). Differences in transgene expression level and persistence between FGAd and HDAd are not due to transduction of different cell types because FGAd and HDAd both primarily transduce luminal endothelial cells (Supplemental Figure II).
Figure 1.
Transgene expression from FGAd and HDAd in carotid arteries of chow-fed and cholesterol-fed rabbits. FGAduPA and HDAduPA, each containing an identical uPA expression cassette, were infused at 5 × 1011 vp/mL in arteries of rabbits fed either a chow or cholesterol-enriched diet. Arteries were harvested 3 d (A, B) and 4 wk (C, D) later. A and C, uPA mRNA was measured in arterial extracts by quantitative RT-PCR. B and D, uPA activity was measured in conditioned medium from carotid artery explant cultures. In each of the 4 panels, results are expressed as arbitrary units (AU), with the mean of the group with the lowest expression assigned a value of 1. Data points are from individual arteries; bars are group means.
Intimal Growth, Macrophage, and Lipid Accumulation Are Increased by FGAd, Not HDAd
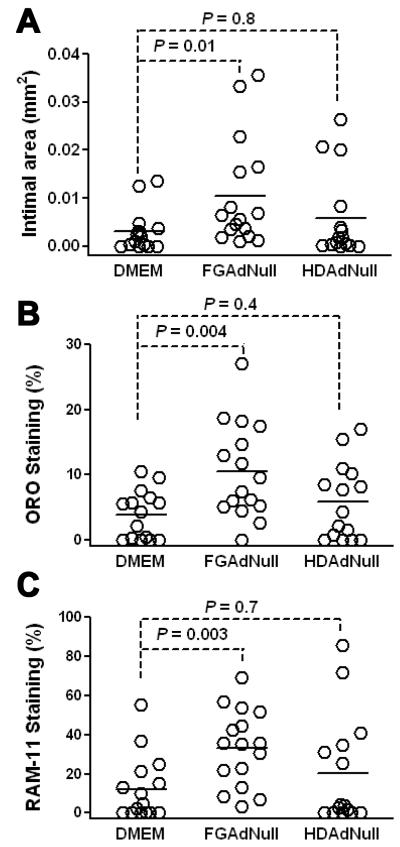
2 wk after infusion of FGAdNull in arteries of cholesterol-fed rabbits, intimal lesions were 3 – 4-fold larger than in control DMEM-infused arteries (Figure 2A; 0.011 ± 0.003 versus 0.003 ± 0.001 mm2; P = 0.01). In contrast, HDAdNull infusion did not significantly increase intimal area (0.006 ± 0.002 mm2; P = 0.8 versus DMEM-infused arteries). Infusion of FGAdNull also increased the percentage of intimal lesions occupied by lipid and macrophages (2 – 3-fold; Figures 2B – C and Supplemental Figure III; P ≤ 0.004). Infusion of HDAdNull did not increase macrophage and lipid accumulation significantly (P ≥ 0.4 for both). At the time of DMEM or vector infusion, the mean cholesterol levels of the 3 groups of rabbits did not differ significantly (Supplemental Table II).
Figure 2.
FGAd—but not HDAd—accelerates intimal growth, lipid and macrophage accumulation 2 wk after infusion. Rabbits were fed a cholesterol-enriched diet for 1 mo and their carotid arteries infused with either DMEM, FGAdNull, or HDAdNull. Arteries were harvested 2 wk later. A, Intimal area. B, Staining for lipid with oil red O (ORO). ORO-positive area is expressed as a percentage of total intimal area. C, Staining for macrophages using the RAM-11 antibody. RAM-11 positive area is expressed as a percentage of total intimal area. Data points are from individual arteries; bars are group means.
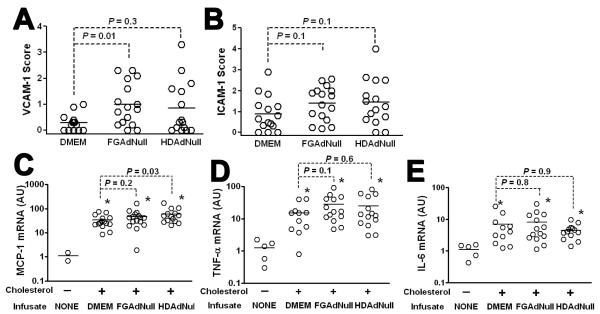
Expression of Adhesion Molecules and Atherogenic Cytokines in Arteries Infused with FGAd or HDAd
Separate segments of the same arteries were used to measure expression of VCAM-1, ICAM-1, MCP-1, TNF-α, and IL-6. Arteries infused with FGAdNull had significantly higher expression of VCAM-1 than control DMEM-infused arteries (Figure 3A and Supplemental Figure III; P = 0.01). ICAM-1 expression was also modestly increased by FGAdNull; however, this increase was not statistically significant (Figure 3B and Supplemental Figure III; P = 0.1). HDAdNull-infused arteries of cholesterol-fed rabbits tended to have higher expression of VCAM-1 and ICAM-1 than control DMEM-infused arteries but these increases were also not statistically significant (Figure 3A – B; P = 0.3 and 0.1, respectively).
Figure 3.
Expression of adhesion molecules and atherogenic cytokines 2 wk after infusion of DMEM, FGAdNull, or HDAdNull. Segments from the same arteries reported in Figure 2 were analyzed for: A and B, VCAM-1 and ICAM-1 expression, using semiquantitative immunostaining; C – E) Expression of MCP-1, TNF-α, and IL-6, using quantitative RT-PCR. Data points are from individual arteries; bars are group means. * = P < 0.05 versus unoperated, chow-fed arteries.
Compared to un-operated arteries from chow-fed rabbits, expression of the atherogenic cytokines MCP-1, TNF-α, and IL-6 was substantially elevated in arteries harvested from cholesterol-fed, operated rabbits, regardless of whether arteries were infused with DMEM, FGAdNull, or HDAdNull (6 – 60-fold mean increases; Figure 3C – E; P < 0.05 for all). Compared to DMEM-infused arteries, FGAdNull-infused arteries had modest, statistically insignificant increases in MCP-1 and TNF-α mRNA (1.5 – 2-fold; P = 0.2 and 0.1, respectively). Infusion of HDAdNull significantly increased expression of MCP-1 (2-fold; P = 0.03) but not TNF-α. IL-6 expression was similar among all 3 groups of infused arteries.
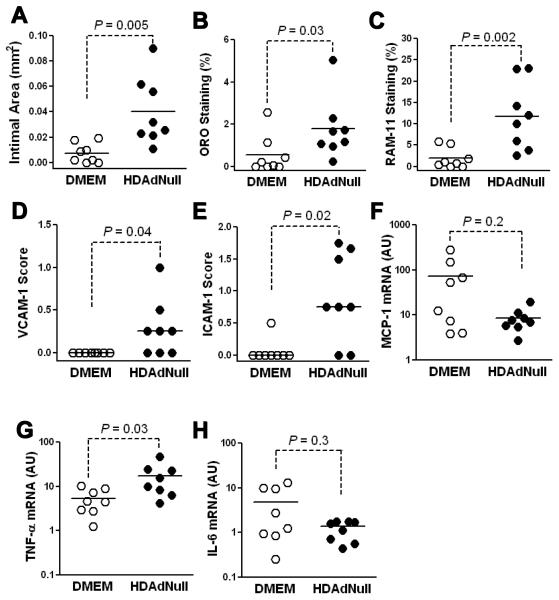
To determine whether HDAd-induced intimal growth and inflammation becomes more evident at a later time point, we infused arteries of cholesterol-fed rabbits with HDAdNull (7.5 × 1011 vp/mL) or DMEM alone and harvested the arteries 4 wk later. Compared to DMEM arteries, HDAdNull arteries had larger intimas, a higher percentage of lesion occupied by macrophages and lipid, increased expression of intimal VCAM-1 and ICAM-1, and higher expression of TNF-α mRNA (Figure 4; P ≤ 0.04 for all). These results were striking because we found no appreciable intimal growth in a separate study in which HDAdNull was infused a lower dose (2 × 1011 vp/mL).27 Therefore, to test the hypothesis that HDAdNull-induced intimal growth at 4 wk is dose-dependent, we cut new sections of arteries both from that study27 and from the present experiment and stained them all in a single batch. Analysis of these sections confirmed that intimal area, macrophage and lipid content, and ICAM-1 and VCAM-1 expression were all increased by the higher dose of HDAd (P = 0.06 – 0.008), but were not significantly increased by the lower dose (P = 0.3 – 0.6; Supplemental Figure IV).
Figure 4.
High-concentration HDAd increases intimal growth, lipid accumulation, and artery wall inflammation 4 wk after infusion. Rabbits were fed a cholesterol-enriched diet for 1 mo and their carotid arteries infused with either DMEM or HDAdNull (7.5 × 1011 vp/mL) and harvested 4 wk later. A, Intimal area; B, Percentage of oil-red O (ORO)-positive area in the intima; C, percentage of RAM-11 positive area in the intima; D and E, VCAM-1 and ICAM-1 expression, using semiquantitative immunostaining; F - H, Expression of MCP-1, TNF-α, and IL-6, using quantitative RT-PCR. Data points are from individual arteries; bars are group means.
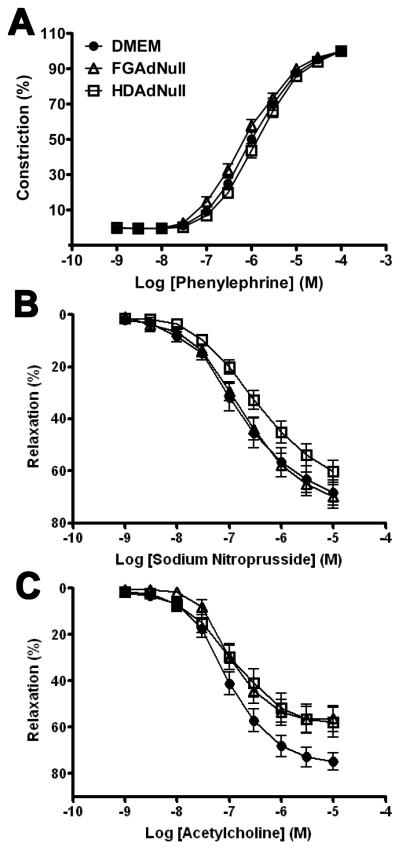
Similar Vascular Reactivity in Arteries Infused with FGAd and HDAd.
Vascular reactivity was measured in rings cut from the same arteries in which we measured inflammation, lipid accumulation, and intimal growth. Arteries infused with DMEM, FGAdNull, or HDAdNull (both at 5 × 1011 vp/mL) contracted similarly to phenylephrine and relaxed similarly to sodium nitroprusside (Figure 5A – B). Arteries of all 3 groups also relaxed significantly in response to acetylcholine (P < 0.001 for acetylcholine dose-responsive relaxation for all 3 groups), confirming the presence of functional endothelium in arteries from all groups (Figure 5C). Across the full range of acetylcholine doses, the responsiveness of the 3 groups of arteries to acetylcholine did not differ significantly (P > 0.05 by 2-way repeated measures ANOVA). Nevertheless, at higher acetylcholine doses, arteries infused with FGAdNull or HDAdNull relaxed less completely than did DMEM-infused arteries. Comparison of Emax among the 3 groups revealed less relaxation for both FGAdNull and HDAdNull-infused arteries compared to DMEM-infused arteries (P < 0.05 for both).
Figure 5.
Vascular reactivity in arteries of cholesterol-fed rabbits infused with DMEM, FGAdNull, or HDAdNull. Arteries were harvested 6 wk after beginning a cholesterol-enriched diet and 2 wk after infusion of DMEM or Ad (5 × 1011 vp/mL). Reactivity of vascular rings was measured in response to: A, Phenylephrine; B, Sodium nitroprusside; or C, Acetylcholine. Data are mean ± SEM of 14-16 arteries/group.
Increased Intimal Growth in HDAd-Infused Arteries 8 Weeks after Infusion or 4 Weeks after Co-Infusion of FGAd
Analysis of arteries harvested 4 wk after vector infusion (Figure 4 and Supplemental Figure IV) suggested that infusion of HDAd in arteries of cholesterol-fed rabbits causes dose-dependent intimal growth, macrophage and lipid accumulation, and adhesion molecule expression. However, even at the higher dose the effect of HDAd on intimal growth and inflammation is small compared to effects we found previously in 4 wk FGAd arteries.17, 18 Relatively limited inflammation and intimal growth in HDAd arteries (especially at 2 × 1011 vp/mL) bodes well for HDAd as a clinical and experimental tool. However, there is a problematic aspect to these results. The utility of this cholesterol-fed rabbit carotid artery model for rapidly testing atheroprotective gene therapy depends on its ability to generate a lesion soon after vector infusion. This lesion, which forms in response to the combination of hyperlipidemia and inflammation associated with Ad vector infusion,17 is the target on which atheroprotective gene(s) expressed from the infused vector are tested. If—unlike FGAd-infused arteries—HDAd-infused arteries of cholesterol-fed rabbits form only small intimal lesions, it will be difficult to use this model to test the efficacy of HDAd-expressed atheroprotective transgenes.
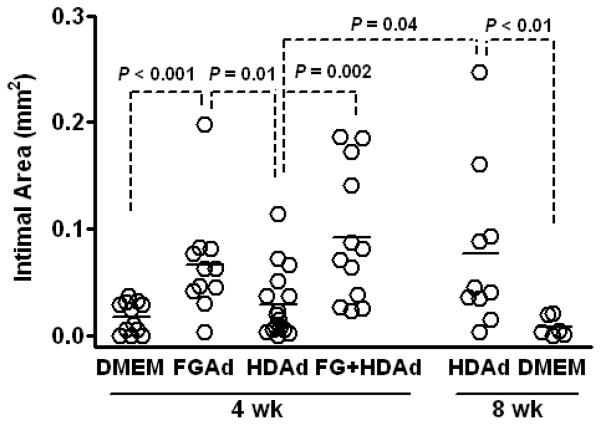
We therefore formally compared FGAd and HDAd (both at 7.5 × 1011 vp/mL) for their ability to induce lesions at 4 wk, and we tested whether HDAd-induced lesions would become larger by 8 wk. Mean cholesterol levels in the 4 wk groups did not differ significantly either at the time of DMEM/Ad infusion or at the time of harvest (Supplemental Table II and data not shown). Intimal area was significantly increased by FGAdNull compared both to DMEM and HDAdNull (0.067 ± 0.015 vs 0.017 ± 0.004 mm2 and 0.029 ± 0.008 mm2 for DMEM and HDAdNull-infused arteries, respectively; P < 0.001 and 0.01; Figure 6). By 8 wk after infusion, intimal areas of HDAdNull arteries had increased (0.077 ± 0.024 mm2; P = 0.04 versus 4-wk HDAdNull) and were similar to intimas of 4-wk FGAdNull arteries. Intimal growth in 8-wk HDAdNull arteries was not due only to prolonged cholesterol feeding and the surgical procedure because DMEM-infused carotid arteries of hyperlipidemic rabbits have virtually no intimal growth 8 wk after infusion (Figure 6).
Figure 6.
Intimal growth 4-8 wk after infusion of FGAd and HDAd. Rabbits were fed a cholesterol-enriched diet for 4 wk and their carotid arteries infused with DMEM, FGAdNull, HDAdNull (both at 7.5 × 1011 vp/mL), or a mixture of HDAdNull (2 × 1011 vp/mL) and FGAdNull (7.5 × 1011 vp/mL). Arteries were harvested at the indicated times, sectioned, stained, and intimal area was measured. Data points are from individual arteries; bars are group means.
To enable testing of HDAd-expressed atheroprotective genes within 4 wk of vector infusion, we tested whether coinfusion of FGAdNull (7.5 × 1011 vp/mL) with an HDAd (HDAdNull, 2.0 × 1011 vp/mL) would accelerate intimal growth at 4 wk. This experiment is based on our previous work showing that human-like early atherosclerotic lesions form in arteries of hyperlipidemic rabbits infused with FGAdNull.17 Intimal areas of the co-infused arteries (Figure 6) were similar to intimal areas of 4-wk arteries infused with FGAdNull alone, and were significantly larger than intimas of 4-wk HDAdNull arteries (3-fold; P = 0.002).
Transgene expression in arteries co-infused with FGAdNull
We next asked whether co-infusion of an HDAd with FGAdNull would cause rapid loss of HDAd-mediated gene expression, as reported by others in a hepatic gene transfer model.13 Chow-fed rabbits were used for this experiment. As expected,15 when HDAd-mediated uPA expression was measured 3 d after infusion (HDAduPA was infused at 2.0 × 1011 vp/mL), co-infusion of FGAdNull (7.5 × 1011 vp/mL) increased uPA expression 20 – 30-fold at both RNA and protein levels (Supplemental Figure V). After 4 wk, uPA expression in HDAduPA arteries co-infused with FGAdNull decreased substantially but was still detectable above background (P = 0.04 for uPA mRNA and P = 0.01 for uPA activity versus control, HDAdNull-infused arteries). Moreover, at 4 wk both uPA mRNA and activity were higher in HDAduPA arteries co-infused with FGAdNull than in HDAduPA arteries co-infused with HDAdNull. Although these increases were not statistically significant (P ≥ 0.09), the higher levels of uPA mRNA and activity in FGAdNull-infused arteries do not support the hypothesis that co-infusion of FGAdNull will cause rapid loss of HDAd-mediated transgene expression.
Discussion
We tested the performance of HDAd in a rabbit model of early atherosclerosis. Our major findings were: 1) HDAd expresses a transgene at least as well in atherosclerosis-prone arteries as it does in normal arteries; 2) Transgene expression from HDAd is more durable than from FGAd; 3) HDAd stimulates less intimal growth, lipid and macrophage accumulation, and VCAM-1 expression than FGAd; 4) HDAd induces only minimal lesion formation and inflammation, especially when it is infused at a submaximal dose; 5) Both FGAd and HDAd have similar, relatively minor effects on vascular reactivity; and 6) Limited intimal growth in HDAd-infused arteries may decrease the utility of this animal model for testing atheroprotective genes expressed by HDAd; however, this limitation may be circumvented by examining later time points or by “spiking” HDAd with FGAd. Our results support the utility of HDAd as a tool for investigation and treatment of atherosclerosis and other vascular diseases.
To be useful for investigating, preventing and treating atherosclerosis, a vector must be able to express transgenes at high levels in arteries that are at risk for developing atherosclerosis. An earlier in vivo study reported lower transgene expression from FGAd in atherosclerotic versus non-atherosclerotic balloon-injured rabbit arteries;16 however, a subsequent ex vivo study reported increased expression from FGAd in atherosclerotic versus normal rabbit arteries.28 In the present study HDAd expressed at least as much transgene mRNA and protein in atherosclerosis-prone as in normal arteries (Figure 1). Our results are therefore similar to the ex vivo results cited above, potentially because in both this earlier study and the present study the transduced cells were endothelial cells and the vectors contained the CMV promoter.28 In contrast, in the in vivo study the transduced cells were likely smooth muscle cells and the vector contained a different promoter.16 The relatively robust expression of HDAd in atherosclerosis-prone versus normal arteries (Figure 1) is encouraging; however, as we found earlier,15 HDAd-mediated transgene expression was initially far less than was obtained with the same cassette in FGAd (Figure 1). Increased expression from FGAd is likely due to upregulation of the CMV promoter by FGAd-stimulated, host-derived inflammatory factors because HDAd expression is increased in vivo (but not in vitro) by co-delivery of FGAd (reference #15 and Supplemental Figure V). It was possible that vascular inflammation in cholesterol-fed rabbits would upregulate HDAd expression as effectively as FGAd; however, it did not (Figure 1). Achievement of higher expression from HDAd in this model will likely require new expression cassettes that function independently of local inflammatory stimuli.29
The most important and encouraging findings of this study are that in arteries of hyperlipidemic rabbits (a setting that accentuates pathogenic effects of FGAd)17 HDAd causes less intimal growth, lipid and macrophage accumulation, and VCAM-1 expression than FGAd (Figures 2, 3 and Supplemental Figure III). Moreover, when infused at a submaximal dose (2 × 1011 vp/mL, a dose that yields near-maximal transgene expression)27 HDAd causes little if any significant arterial pathology (Supplemental Figure IV). Therefore, HDAd is both a “cleaner” experimental tool than FGAd and a safer vector for gene therapy. Improved performance of HDAd in this model is likely due to deletion of all viral genes in HDAd, which conceals transduced cells from the adaptive immune system and avoids cellular toxicities of viral gene products.9, 30
For HDAd to serve as an optimal investigational and therapeutic tool, it should have minimal effects on vasomotor function. In chow-fed rabbits, several groups reported that FGAd impairs endothelium-dependent relaxation.6, 31, 32 In cholesterol-fed rabbits, a single study showed baseline, diet-related impairment of endothelium-dependent relaxation that was not worsened by FGAd infusion.33 This impairment was, however, reversed by infusion of an FGAd expressing endothelial nitric oxide synthase. In the present study, also performed in cholesterol-fed rabbits, both FG- and HDAd reduced maximal endothelium-mediated dilation (Figure 5). This result suggests that the Ad capsid (common to FG- and HD-Ad) impairs endothelium-mediated vasodilation, consistent with a report of impaired endothelium-mediated vasodilation only 6 hr after FGAd infusion.6 We considered why in our study, in contrast to the study in cholesterol-fed rabbits cited above,33 both FG- and HDAd decreased maximal endothelium-dependent relaxation. This inconsistency might be because in the present study a shorter duration of cholesterol feeding (6 versus 11 – 12 wk) and a lower percentage of dietary cholesterol (0.25% versus 0.5 – 1.0 %) cause less impairment of endothelial function. With less diet-related impairment of endothelial function, a detrimental effect of Ad on endothelial function may be unmasked. The apparent inhibitory effect of HDAd on maximal endothelium-mediated vasodilation (Figure 5) seems to be a limitation shared by both HDAd and FGAd, and could likely be avoided either by lowering the vector dose or by including an endothelial nitric oxide synthase expression cassette in the vectors.6, 33
The lack of significant intimal lesion growth after HDAd infusion at a submaximal dose (2×1011 vp/mL; Supplemental Figure IV) and the relatively small lesions in 2 – 4-wk arteries infused at a higher dose (7.5 ×1011 vp/mL; Figures 2, 4, and 6) bode well for HDAd as an investigational and therapeutic tool. However, this result has a problematic aspect because the intimal lesions that form in this model after Ad infusion are the substrate on which Ad-encoded transgenes are tested for atherogenic or atheroprotective activities.17, 18 Atherogenic activities of transgenes expressed from HDAd will likely still be revealed because they would increase lesion growth above a low background; however, atheroprotective activities of transgenes might not be detected. Fortunately, we found that both continuing the experiment for an additional 4 wk and spiking HDAd with FGAdNull increased intimal lesion size (Figure 6). Both approaches improve the suitability of this model for detecting atheroprotective transgene effects.
In conclusion, in atherosclerosis-prone arteries HDAd is superior to FGAd for both experimental and therapeutic purposes because it expresses transgenes more durably than FGAd (Figure 1 and Supplemental Figure I) and causes less lesion growth, lipid and macrophage accumulation, and vascular inflammation (Figures 2, 3, and 6). Future work will be directed at addressing the remaining limitations of HDAd for vascular gene transfer, including an initially low level of transgene expression, mild impairment of maximal endothelium-mediated vasodilation, and possible atherogenic effects at later time points.
Supplementary Material
Acknowledgments
We thank AdVec, Inc. for permission to use the HDAd reagents, Dan Minter, Mia Jaffe, and Jingwan Zhang for excellent technical assistance, and Margo Weiss for administrative assistance.
Sources of Funding
This study was supported by grants HL076226 (Dr. Dichek) from the National Institutes of Health, a postdoctoral fellowship award (09POST2050061) from the American Heart Association (Dr. Jiang), and the John L. Locke Jr. Charitable Trust.
Footnotes
Disclosure
None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindemann D, Schnittler H. Genetic manipulation of endothelial cells by viral vectors. Thromb Haemost. 2009;102:1135–1143. doi: 10.1160/TH09-10-0724. [DOI] [PubMed] [Google Scholar]
- 2.Melo LG, Gnecchi M, Pachori AS, Kong D, Wang K, Liu X, Pratt RE, Dzau VJ. Endothelium-targeted gene and cell-based therapies for cardiovascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1761–1774. doi: 10.1161/01.ATV.0000142363.15113.88. [DOI] [PubMed] [Google Scholar]
- 3.Yla-Herttuala S, Martin JF. Cardiovascular gene therapy. Lancet. 2000;355:213–222. doi: 10.1016/S0140-6736(99)04180-X. [DOI] [PubMed] [Google Scholar]
- 4.Gaffney MM, Hynes SO, Barry F, O’Brien T. Cardiovascular gene therapy: current status and therapeutic potential. Br J Pharmacol. 2007;152:175–188. doi: 10.1038/sj.bjp.0707315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman KD, Dunn PF, Owens JW, Schulick AH, Virmani R, Sukhova G, Libby P, Dichek DA. Adenovirus-mediated gene transfer into normal rabbit arteries results in prolonged vascular cell activation, inflammation, and neointimal hyperplasia. J Clin Invest. 1995;96:2955–2965. doi: 10.1172/JCI118367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Channon KM, Qian H, Youngblood SA, Olmez E, Shetty GA, Neplioueva V, Blazing MA, George SE. Acute host-mediated endothelial injury after adenoviral gene transfer in normal rabbit arteries: impact on transgene expression and endothelial function. Circ Res. 1998;82:1253–1262. doi: 10.1161/01.res.82.12.1253. [DOI] [PubMed] [Google Scholar]
- 7.Gruchala M, Bhardwaj S, Pajusola K, Roy H, Rissanen TT, Kokina I, Kholova I, Markkanen JE, Rutanen J, Heikura T, Alitalo K, Bueler H, Yla-Herttuala S. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. J Gene Med. 2004;6:545–554. doi: 10.1002/jgm.535. [DOI] [PubMed] [Google Scholar]
- 8.Baker AH. Development and use of gene transfer for treatment of cardiovascular disease. J Card Surg. 2002;17:543–548. doi: 10.1046/j.1540-8191.2002.01011.x. [DOI] [PubMed] [Google Scholar]
- 9.Parks RJ, Chen L, Anton M, Sankar U, Rudnicki MA, Graham FL. A helper-dependent adenovirus vector system: Removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc Natl Acad Sci U S A. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandig V, Youil R, Bett AJ, Franlin LL, Oshima M, Maione D, Wang F, Metzker ML, Savino R, Caskey CT. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc Natl Acad Sci U S A. 2000;97:1002–1007. doi: 10.1073/pnas.97.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki M, Cela R, Clarke C, Bertin TK, Mourino S, Lee B. Large-scale production of high-quality helper-dependent adenoviral vectors using adherent cells in cell factories. Hum Gene Ther. 2010;21:120–126. doi: 10.1089/hum.2009.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer DJ, Ng P. Helper-dependent adenoviral vectors for gene therapy. Hum Gene Ther. 2005;16:1–16. doi: 10.1089/hum.2005.16.1. [DOI] [PubMed] [Google Scholar]
- 13.Oka K, Belalcazar LM, Dieker C, Nour EA, Nuno-Gonzalez P, Paul A, Cormier S, Shin JK, Finegold M, Chan L. Sustained phenotypic correction in a mouse model of hypoalphalipoproteinemia with a helper-dependent adenovirus vector. Gene Ther. 2007;14:191–202. doi: 10.1038/sj.gt.3302819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brunetti-Pierri N, Stapleton GE, Law M, Breinholt J, Palmer DJ, Zuo Y, Grove NC, Finegold MJ, Rice K, Beaudet AL, Mullins CE, Ng P. Efficient, long-term hepatic gene transfer using clinically relevant HDAd doses by balloon occlusion catheter delivery in nonhuman primates. Mol Ther. 2009;17:327–333. doi: 10.1038/mt.2008.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen S, Graf S, Massey PG, Dichek DA. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110:1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. [DOI] [PubMed] [Google Scholar]
- 16.Feldman LJ, Steg PG, Zheng LP, Chen D, Kearney M, McGarr SE, Barry JJ, Dedieu JF, Perricaudet M, Isner JM. Low-efficiency of percutaneous adenovirus-mediated arterial gene transfer in the atherosclerotic rabbit. J Clin Invest. 1995;95:2662–2671. doi: 10.1172/JCI117968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider DB, Vassalli G, Wen S, Driscoll RM, Sassani AB, DeYoung MB, Linnemann R, Virmani R, Dichek DA. Expression of Fas ligand in arteries of hypercholesterolemic rabbits accelerates atherosclerotic lesion formation. Arterioscler Thromb Vasc Biol. 2000;20:298–308. doi: 10.1161/01.atv.20.2.298. [DOI] [PubMed] [Google Scholar]
- 18.Falkenberg M, Tom C, DeYoung MB, Wen S, Linnemann R, Dichek DA. Increased expression of urokinase during atherosclerotic lesion development causes arterial constriction and lumen loss, and accelerates lesion growth. Proc Natl Acad Sci U S A. 2002;99:10665–10670. doi: 10.1073/pnas.162236599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vassalli G, Agah R, Qiao R, Aguilar C, Dichek DA. A mouse model of arterial gene transfer. Antigen-specific immunity is a minor determinant of the early loss of adenovirus-mediated transgene expression. Circ Res. 1999;85:e25–e32. [PubMed] [Google Scholar]
- 20.Flynn R, Buckler JM, Tang C, Kim F, Dichek D. Helper-dependent adenoviral vectors are superior in vitro to first-generation vectors for endothelial cell-targeted gene therapy. Mol Ther. 2010;18:2121–2129. doi: 10.1038/mt.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Anton M, Graham FL. Production and characterization of human 293 cell lines expressing the site-specific recombinase Cre. Somat Cell Mol Genet. 1996;22:477–488. doi: 10.1007/BF02369439. [DOI] [PubMed] [Google Scholar]
- 22.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cybulsky MI, Gimbrone MA., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 24.Cozen AE, Moriwaki H, Kremen M, DeYoung MB, Dichek HL, Slezicki KI, Young SG, Veniant M, Dichek DA. Macrophage-targeted overexpression of urokinase causes accelerated atherosclerosis, coronary artery occlusions, and premature death. Circulation. 2004;109:2129–2135. doi: 10.1161/01.CIR.0000127369.24127.03. [DOI] [PubMed] [Google Scholar]
- 25.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Luttrell IP, Swee M, Starcher B, Parks WC, Chitaley K. Erectile dysfunction in the type II diabetic db/db mouse: impaired venoocclusion with altered cavernosal vasoreactivity and matrix. Am J Physiol Heart Circ Physiol. 2008;294:H2204–2211. doi: 10.1152/ajpheart.00027.2008. [DOI] [PubMed] [Google Scholar]
- 27.Du L, Dronadula N, Tanaka S, Dichek DA. A helper-dependent adenoviral vector achieves prolonged, stable expression of IL-10 in rabbit carotid arteries but does not limit early atherogenesis. Hum Gen Ther. 2011 doi: 10.1089/hum.2010.175. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ooboshi H, Rios CD, Chu Y, Christenson SD, Faraci FM, Davidson BL, Heistad DD. Augmented adenovirus-mediated gene transfer to atherosclerotic vessels. Arterioscler Thromb Vasc Biol. 1997;17:1786–1792. doi: 10.1161/01.atv.17.9.1786. [DOI] [PubMed] [Google Scholar]
- 29.Dronadula N, Du L, Flynn R, Buckler JM, Kho J, Jiang Z, Tanaka S, Dichek DA. Construction of a novel expression cassette for increasing transgene expression in vivo in endothelial cells of large blood vessels. Gene Ther. 2010 doi: 10.1038/gt.2010.173. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Li Q, Ertl HCJ, Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lafont A, Loirand G, Pacaud P, Vilde F, Lemarchand P, Escande D. Vasomotor dysfunction early after exposure of normal rabbit arteries to an adenoviral vector. Hum Gene Ther. 1997;8:1033–1040. doi: 10.1089/hum.1997.8.9-1033. [DOI] [PubMed] [Google Scholar]
- 32.Qian HS, Channon K, Neplioueva V, Wang Q, Finer M, Tsui L, George SE, McArthur J. Improved adenoviral vector for vascular gene therapy: Beneficial effects on vascular function and inflammation. Circ Res. 2001;88:911–917. doi: 10.1161/hh0901.090926. [DOI] [PubMed] [Google Scholar]
- 33.Sato J-I, Mohacsi T, Noel A, Jost C, Gloviczki P, Mozes G, Katusic ZS, O’Brien T. In vivo gene transfer of endothelial nitric oxide synthase to carotid arteries from hypercholesterolemic rabbits enhances endothelium-dependent relaxations. Stroke. 2000;31:968–975. doi: 10.1161/01.str.31.4.968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.