Abstract
Previous studies showed that mice with genetic predisposition for high alcohol consumption as well as human alcoholics show changes in brain expression of genes related to immune signaling. In addition, mutant mice lacking genes related to immune function show decreased alcohol consumption (Blednov et al., in press), suggesting that immune signaling promotes alcohol consumption. To test the possibility that activation of immune signaling will increase alcohol consumption, we treated mice with lipopolysaccaride (LPS; 1 mg/kg, i.p.) and tested alcohol consumption in the continuous two-bottle choice test. To take advantage of the long-lasting activation of brain immune signaling by LPS, we measured drinking beginning one week or one month after LPS treatment and continued the studies for several months. LPS produced persistent increases in alcohol consumption in C57/Bl6 J (B6) inbred mice, FVBxB6F1 and B6xNZBF1 hybrid mice, but not in FVB inbred mice. To determine if this effect of LPS is mediated through binding to TLR4, we tested mice lacking CD14, a key component of TLR4 signaling. These null mutants showed no increase of alcohol intake after treatment with LPS. LPS treatment decreased ethanol-conditioned taste aversion but did not alter ethanol-conditioned place preference (B6xNZBF1 mice). Electro-physiological studies of dopamine neurons in the ventral tegmental area showed that pretreatment of mice with LPS decreased the neuronal firing rate. These results suggest that activation of immune signaling promotes alcohol consumption and alters certain aspects of alcohol reward/aversion.
Keywords: Lipopolysaccharide, Alcohol intake, CD14, Knockout mice, Alcohol olfaction, Conditioned taste aversion, Conditioned place preference, Dopamine neuron
1. Introduction
Genes encoding proteins involved in “immune/stress responses” are one of the most prominent functional groups that exhibit differential gene expression in the frontal cortex between human alcoholics and non-alcoholics (Liu et al., 2006). Expression of these genes in brain is also related to genetic predisposition for alcohol consumption in mice, indicating a role for pro-inflammatory mediators in regulating alcohol intake (Mulligan et al., 2006). Analysis of these gene-expression data sets led to the selection of six genes related to neuroimmune pathways, and behavioral testing of genes selected from these studies showed that null mutant mice lacking selected neuroimmune signaling components drink less alcohol (Blednov et al., in press). Other evidence linking brain neuroimmune or pro-inflammatory signaling to alcohol action includes the finding that long-lasting increases in levels of several pro-inflammatory cytokines were found in rat brain after chronic treatment with high doses of ethanol followed by injection of lipopolysaccharide (LPS) (Qin et al., 2008). Interestingly, one of the cytokines that increased in rat brain, MCP-1 (Ccl2), was also increased in the brain of human alcoholics (He and Crews, 2008). Recently, Kong et al. (2010) found up-regulation of genes in the Toll and Imd innate immune signaling pathways in Drosophila exposed to ethanol, extending the link between alcohol and immune mediators to invertebrates.
We hypothesized that the normal functioning of the immune system maintains drinking at low to moderate levels, whereas activation of the neuroimmune system could promote excessive alcohol consumption. We tested this by using LPS to activate neuroimmune signaling before measuring drinking. LPS is a bacterial endotoxin normally confined to the gut, but it can leak from the gut as a result of chronic alcohol abuse (Mandrekar and Szabo, 2009). LPS produces activation of the immune system when administered systemically by binding to toll-like receptor 4 (TLR4) found on macrophages, Kupffer and stellate cells in the liver, and endothelial cells (Andonegui et al., 2003; Mandrekar and Szabo, 2009; Suzumura et al., 2006; Zanoni and Granucci, 2010). TLR4 is also found in the brain on neurons, astrocytes, microglia and endothelial cells (Tang et al., 2007; Mallard et al., 2009). Some publications suggest that LPS elicits TLR4 signaling in the brain by interactions with these receptors (Chakravarty and Herkenham, 2005; Gosselin and Rivest, 2008), but others have failed to find LPS within the brain parenchyma after systemic administration (Singh and Jiang, 2004). It is clear that LPS binds to TLR4on cerebral endothelial cells and this may have a role in actions of LPS on brain function (Singh and Jiang, 2004; Verma et al., 2006).
Activation of TLR4 by LPS produces release of a number of immune mediators, including cytokines, and a “sickness” response characterized by decreased food and water intake, loss of weight, lethargy and anhedonia (Dantzer, 2001). Most of these effects of LPS are due to actions on the brain, and are generally attributed to cytokines that are released peripherally and are transported across the blood–brain barrier (Wisse et al., 2007). The increased levels of serum cytokines produced by LPS are transient (hours) and the sickness response lasts only a day or two. However, there are persistent actions of LPS exposure on brain neuroinflammatory signaling, including elevation of certain brain cytokines such as TNFα, IL-1 and CCl2 for up to 10 months (Qin et al., 2007, 2008).
To determine if inflammatory activation by LPS might produce a long-lasting increase in alcohol consumption, we gave mice one or two injections of LPS, waited one week (to allow normalization of body weight and water intake following the sickness response), then began tests of alcohol consumption. Because alcohol drinking in mice is highly dependent on genetic background, we used several different strains of mice with different levels of alcohol consumption. We also tested phenotypes that might be related to alcohol consumption or reward: conditioned place preference (CPP), conditioned taste aversion (CTA), firing activity of midbrain dopamine neurons, consumption of saccharin and quinine, and olfactory detection of ethanol.
2. Materials and methods
2.1. Animals
Studies were conducted in drug-naïve C57BL/6 J (B6), FVB/NJ (FVB), NZB/B1NJ (NZB), and F1 hybrid mice derived from these three progenitors (FVBxB6 F1, maternal strain × paternal strain; B6xNZB F1). B6, FVB, and NZB breeders as well as Cd14 (B6.129S-cd14tm1Frm/J; Stock #003726) null mice were purchased from Jackson Laboratories (Bar Harbor, ME) and mated at the age of 8 weeks in the Texas Genetic Animal Core of the Integrated Neuroscience Initiative on Alcohol (INIA) at the University of Texas at Austin. Cd14 knockout mice were backcrossed on C57Bl/6 J genetic back- ground more than 10 times. Mice (four–five per cage) were housed in standard polycarbonate shoebox cages with food (Prolab RMH 1800 5LL2 chow) and water provided ad libitum. The colony rooms and testing rooms were maintained at an ambient temperature of 21 ± 1 °C, humidity (40–60%), and centrally controlled ventilation (12–15 cycles/h with 100% exhaust). Colony rooms were on a 12:12 light/dark light cycle (lights on at 07:00 AM). All procedures were approved by the Institutional Animal Care and Use Committee and adhered to NIH Guidelines. The University of Texas facility is AAALAC accredited.
2.2. Lipopolysaccharide treatment
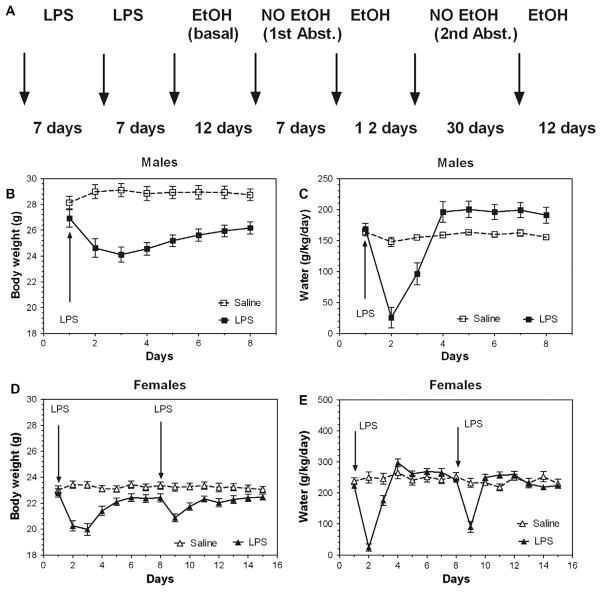
Lipopolysaccharide (LPS) (strain O111:B4, Sigma Chemical Co., St. Louis, MO) dissolved in saline was injected at a dose of 0.33 or 1 mg/kg i.p. in volume 0.1 ml/10 g of body weight. In several experiments, a second LPS injection was made one week after the first injection. After each drug injection, mice were allowed to recover from sickness response to LPS (as judged by changes in body weight and water consumption and the recovery was monitored by daily measurement of body weight and water consumption) (Fig. 1). Usually one week was sufficient for complete recovery. Therefore, one week after the last LPS injection the mice were tested for voluntary ethanol intake. The schedule for LPS injection and alcohol consumption as well as monitoring of recovery from sickness in B6 inbred mice are presented in Fig. 1.
Fig. 1.
Schedule for injection of LPS and monitoring of recovery from acute LPS effects. (A) Schedule for injection of LPS and alcohol consumption. (B and C) Monitoring of recovery from acute LPS effects in B6 male mice. (B) Daily changes in body weight. (C) Daily changes in water intake. (D and E) Monitoring of recovery from acute LPS effects (two injections) in B6 female mice. (D) Daily changes in body weight. (E) Daily changes in water intake.
2.3. Ethanol intake in two-bottle choice test
The two-bottle choice protocol was carried out as previously described (Blednov et al., 2003). Two drinking tubes were available continuously to each mouse, and tubes were weighed daily. One tube always contained water. Food was available ad libitum. During recovery, after LPS injection, the mice were weighed and water intake was measured daily. During the two-bottle choice experiment, mice were weighed every 4 d. After 7 d of water consumption (during recovery after LPS injection), mice were offered a choice between 3% ethanol (v/v) versus water for 2 d. Tube positions were changed every day to control for position preferences. The quantity of ethanol consumed (g/kg body weight/24 h) was calculated for each mouse, and these values were averaged for each concentration of ethanol. Immediately following 3% ethanol, a choice between 6% (v/v) ethanol and water was offered for 2 d, then 9% (v/v) ethanol vs. water for 2 d, then 12% (v/v) ethanol vs. water for 2 d, 15% (v/v) ethanol vs. water for 2 d, 18% (v/v) ethanol vs. water for 2 d, and finally 21% (for female mice, not for male mice). Throughout the experiment, evaporation/spillage estimates were calculated every day from two bottles placed in an empty cage, one containing water and the other containing the appropriate ethanol solution. In another experiment, mice were injected with LPS, and drinking was tested beginning 30 days after LPS administration. In this study, mice were housed singly beginning 3 d before LPS injection and throughout the remainder of the experiment.
Aaper brand (Aaper Alcohol and Chemical, Shelbyville, KY) 200-proof ethanol was used to mix solutions as v/v in tap water.
2.4. Preference for non-ethanol tastants in two-bottle choice test
Separate groups of B6 mice were also tested for saccharin, quinine, and sucrose consumption after treatment with LPS. Different groups of mice were serially offered sucrose (0.1%, 0.5%, 1.0%, 2.5%, and 5.0%), quinine hemisulfate (0.015 mM, 0.03 mM, and 0.06 mM) and saccharin (0.00165%, 0.033%, 0.066%, 0.132%, and 0.264%), and intakes for 24 h of drinking were calculated. Each concentration was offered for 2 d, with bottle positions changed every day. Within each tastant, the low concentration was always presented first, followed by the higher concentrations in increasing order.
2.5. Olfactory testing of ethanol
LPS treatment has been shown to induce neuronal apoptosis in the olfactory bulb in mice (Mori et al., 2010) and changes in the dopamine content in the anterior olfactory nucleus (Ota et al., 2008). These findings raise the concern that changes in alcohol consumption could result from a loss of olfactory perception of ethanol. Because there are few studies of ethanol olfaction, we used two different tests to assess olfactory function in the present study. A modification of the buried food pellet test was employed except that a cotton ball soaked in 20% ethanol was used instead of a Purina mouse chow pellet (Nathan et al., 2004). The buried pellet test reveals the mouse’s ability to learn to detect and find palatable food. Individually housed mice were food restricted and maintained at ~90% body weight for 5 d prior to and during testing. Food-restricted mice were given 3–4 g of mouse chow per animal per day depending on weight. To associate the odor of ethanol with the source of food, the piece of Purina chow was placed on the top of a Petri dish with a cotton ball soaked in 20% ethanol. Sixteen holes were made in the top of Petri dish. Thus, animals could sniff the ethanol but could not lick it. Conditioning sessions were carried out every day for 4 d. Every session was ended when the mouse found the piece of food. Weights were monitored each day during food restriction. Buried target testing was performed for 5 d, and the surface target control test was performed twice for one trial per day starting 1 d after the buried target. For the buried-target test, a clean mouse cage was filled with 3 cm of clean bedding. A Petri dish with a cotton ball soaked with 20% ethanol was buried along the perimeter of the cage, approximately 0.5 cm below the bedding so that it was not visible. A mouse was then placed in the center of the cage and the latency to dig up was measured using a stopwatch. If an animal did not locate the odor within 5 min, then the animal was removed, returned to its home cage, and given a score of 5 min. After the trial, each animal was returned to its home cage, and one piece of Purina chow was given. The bedding was changed between mice for all testing periods, and the cereal was buried in a different location on each test day. The surface target test was set up in a similar way, except that on the first day of testing the piece of Purina chow was placed on top of a Petri dish with a cotton ball soaked in 20% ethanol and on the second day the piece of Purina chow was placed on top of a Petri dish without ethanol. After the second day of surface target testing, the buried-target test was performed again daily for three more days. Every day, animals had to find the buried Petri dish with the cotton ball soaked in different concentrations of ethanol (5%, 10%, and 15%).
A modification of the block test was employed as previously described by Fleming et al. (2008). The block test evaluated the mouse’s ability to detect social scents and distinguish between familiar and novel scents, an ethologically important behavior in mice. In this test, individually housed animals were habituated to a stimulus by placing three small empty plastic tissue cartridges (Fisher Scientific, Pittsburgh, PA, USA) in each animal’s cage for 7 d. During this time, the cage bedding was not changed, so that the plastic cartridges took on the odor of that animal. On the day of the test, the stimulus was removed from the cage, and animals were transferred into a separate testing area where water bottles, food, and metal cage grids were removed and just the filtered cage lid remained on each cage. Animals were habituated to the room for 1 h. First, one familiar plastic cartridge was placed in the testing cage, and the animal adapted to this familiar stimulus for an additional 15 min. Second, another familiar plastic cartridge was placed in the testing cage, and behavior was recorded for 5 min. This session served as a control for placing additional (but familiar) targets in the testing area. Finally, a cotton ball soaked in 10% ethanol was placed inside the third familiar plastic cartridge. The scented cartridge was then placed in the cage, and the mouse was videotaped for another 5 min. Latency to approach the cartridge and total time for exploration (sniffing) of the target were measured.
2.6. Conditioned taste aversion
Conditioned taste aversion was carried out in the manner described by Blednov et al. (2003). After recovery from LPS pretreatment, mice were adapted to a water-restriction schedule (2 h of water per day) over a 7-d period. At 48-h intervals over the next 10 d (days 1, 3, 5, 7, 9, and 11), all mice received 1-h access to a solution of saccharin (0.15% w/v sodium saccharin in tap water). Immediately after 1-h access to saccharin, the mice received injections of saline or ethanol (2.5 g/kg) (days 1, 3, 5, 7, and 9). Mice also received 30-min access to tap water 5 h after each saccharin access period to prevent possible dehydration, and this was followed by injection of ethanol (2.5 g/kg, i.p.) (days 1, 3, 5, 7, and 9). On intervening days, the mice had 2 h of continuous access to water at standard times in the morning (days 2, 4, 6, 8, and 10).
2.7. Conditioned place preference
The conditioned place preference (CPP) protocol was carried out as previously described (Blednov et al., 2003). Four identical acrylic boxes (30 × 15 × 15 cm) were separately enclosed in ventilated light- and sound-attenuating chambers (Med Associates, St. Albans, VT). Each box has two compartments separated by a wall with a door. The two compartments each have a different type of floor (either bars – [GRID−] or wire – [GRID+]). Infrared light sources and photodetectors were mounted opposite each other at 2.5-cm intervals along the length of each box, 2.2 cm above the floor. Occlusion of the infrared light beams was used to measure general activity and location of the animal (left or right) within the box. Total activity counts and location of the animal (left or right compartment) within the box were recorded by computer. The floors and the insides of the boxes were wiped with a damp sponge and the litter paper beneath the floors was changed between animals. The main principles of CPP have been described earlier (Cunningham, 1993). Briefly, the place-conditioning study involved one habituation session, eight conditioning sessions, and one test session. No pretest sessions were used. A 2-d weekend break occurred between the first four and last four conditioning sessions. For the habituation session, mice received an injection of saline immediately before being placed in the conditioning box for 30 min on a smooth metal plate covering the floor. During the habituation session, both compartments were available for the mice. The purpose of the habituation session was to reduce the stress associated with the novelty of experimental procedures and exposure to the apparatus. Mice were not exposed to the distinctive floor textures to avoid latent inhibition. For conditioning, mice of each of two pretreated (saline or LPS) groups were randomly assigned to one of two conditioning subgroups, GRID + or GRID−, and exposed to a Pavlovian differential conditioning procedure. On alternating days, mice in the GRID + group received an injection of ethanol (2 g/kg, i.p.) immediately before a 5-min session on the grid floor (CS + sessions). On intervening days, these mice received saline immediately before exposure to the wire mesh floor (CS-sessions). Conversely, mice in the GRID− group received ethanol paired with the wire mesh floor and saline paired with the grid floor. During conditioning trials, all mice had access to only one of the two compartments of the apparatus. The dose of ethanol (2 g/kg) was chosen because it produces a strong preference for the paired tactile stimuli (Chester and Cunningham, 1998). The 5-min session duration was chosen based on previous studies showing that it produced a stronger CPP with ethanol than did longer session durations (Cunningham and Prather, 1992). For the 30-min test session, all mice received saline. Both compartments of each box were available for exploration during the test session.
2.8. Electrophysiology
Basal firing of dopamine (DA) neurons in the ventral tegmental area (VTA) was recorded from horizontal midbrain slices prepared 7–10 days after a single injection of LPS. The experimenter was not blind to the treatment history. Mice were killed by cervical dislocation under halothane anesthesia, and horizontal midbrain slices (210 μm) containing the VTA were prepared. Slices were cut using a vibratome (VT1000S; Leica Microsystems, Bannockburn, IL) in ice-cold cutting solution containing (in mM): 205 sucrose, 2.5 KCl, 1.25 NaH2PO4, 7.5 MgCl2, 0.5 CaCl2, 10 glucose, 25 NaHCO3, saturated with 95% O2 and 5% CO2, and then incubated at 35 °C for >1 h in physiological saline containing (in mM): 126 NaCl, 2.5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 11 glucose, 21.4 NaHCO3, saturated with 95% O2 and 5% CO2 (pH 7.4, ~295 mOsm/kg). Recordings were made at 34–35 °C in the same physiological saline perfused at 2–3 ml/min. Neurons were visualized using a 40x objective on an upright microscope (BX51WI; Olympus America, Center Valley, PA) with infrared/oblique illumination optics. Loose-patch recordings (~10–20 M Ω seal) of dopamine neuron firing were made in a voltage clamp using borosilicate glass pipettes (2.2–2.5 M Ω) filled with 150 mM NaCl. Putative dopamine neurons were identified by spontaneous pacemaker firing (0.5–5 Hz) and broad action potentials (>1.2 ms) (Ford et al., 2006). A Multi-clamp 700-A amplifier (Molecular Devices, Union City, CA) was used to record the data, which were filtered at 5 kHz, digitized at 10 kHz, and collected using AxoGraph X (AxoGraph Scientific, Sydney, Australia).
2.9. Data analysis
Data are reported as the mean ± S.E.M value. The dependent measures were weight of ethanol, water and different tastants consumed, ethanol dose (g/kg per day) consumed, preference ratio for ethanol and for the different tastants. When appropriate, a trial was included as a repeated-measures factor. To evaluate the differences between groups, analysis of variance (two-way ANOVA and one-way ANOVA with Post-hoc Bonferroni Multiple Comparison) were used. The statistics software program GraphPad Prizm (Jandel Scientific, Costa Madre, CA) was used throughout.
3. Results
3.1. Ethanol intake
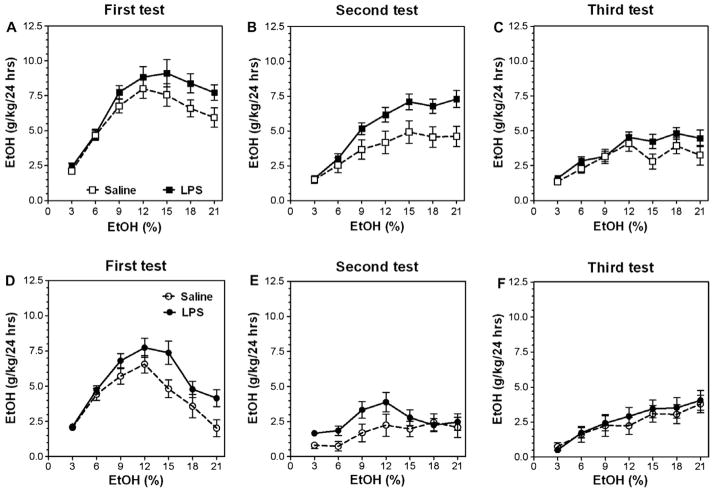
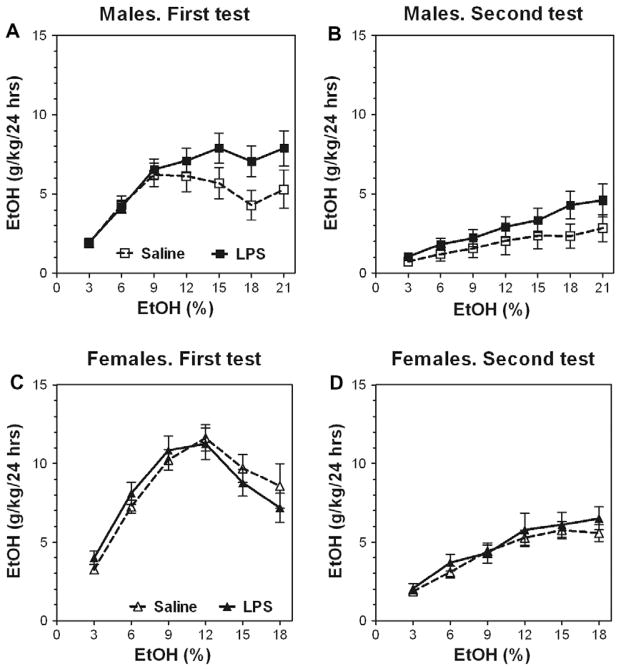
In a two-bottle, free-choice paradigm in which mice could drink either water or an ascending series of ethanol concentrations, pre-treatment with LPS significantly increased the amount of ethanol consumed in B6 male mice after one injection of LPS as well as after two LPS injections (Fig. 2A and D). A similar increase of alcohol intake was seen in both experimental groups after the first period (one week) of alcohol deprivation (Fig. 2B and E). However, after a second period (one month) of alcohol deprivation, a slight increase of alcohol intake still was seen only in B6 male mice after one injection of (Fig. 2C and F). Complete data for ethanol intake (amount of ethanol consumed, preference for ethanol, and total fluid intake) in a continuous-access, two-bottle choice test for B6 male mice are presented in Supplemental Figs. 1 and 2 (for detailed statistics see Supplementary materials in Supplemental Table 1).
Fig. 2.
LPS pretreatment produces long-lasting increase of ethanol intake in C57Bl/6 J male mice. (A–C) Ethanol intake (g/kg/24 h) after single injection of LPS (1 mg/kg). n = 13 per group. (D–F) Ethanol intake (g/kg/24 h) after two injections of LPS. n = 10–14 per group. (A and D) Initial ethanol intake (g/kg/24 h). (B and E) Ethanol intake (g/kg/24 h) after first (one week) ethanol deprivation. (C and F) Ethanol intake (g/kg/24 h) after second (one-month) ethanol-deprivation period.
Results for females were similar to those for males; pretreatment with LPS increased ethanol consumption in B6 female mice after one injection of LPS as well as after two LPS injections (Fig. 3A and D). However, after one week of alcohol deprivation the increased alcohol intake was observed only for mice given two injections of LPS (Fig. 3B and E). After one month of alcohol deprivation, the increased alcohol intake was seen more clearly in B6 female mice after two injections of LPS than after one injection of LPS (Fig. 3C and F). The schedule for two injections of LPS and testing of drinking as well as the effects of LPS on body weight and water consumption are shown in Fig. 1. LPS produced a marked decrease in body weight and water consumption which was more pronounced for the first injection compared with the second. Complete data for ethanol intake (amount of ethanol consumed, preference for ethanol, and total fluid intake) in a continuous-access, two-bottle choice test for B6 female mice are presented in Supplemental Figs. 3 and 4 (for detailed statistics see Supplementary materials in Supplemental Table 2).
Fig. 3.
LPS pretreatment produces long-lasting increase of ethanol intake in C57Bl/6 J female mice. (A–C) Ethanol intake (g/kg/24 h) after single injection of LPS (1 mg/kg). n = 10–13 per group. (D–F) Ethanol intake (g/kg/24 h) after two injections of LPS. n = 11–13 per group. (A and D) Initial ethanol intake (g/kg/24 h). (B and E) Ethanol intake (g/kg/24 h) after first (one-week) ethanol-deprivation period. (C and F) Ethanol intake (g/kg/24 h) after second (one-month) ethanol-deprivation period.
These experiments showed that LPS increased alcohol intake in male mice injected with LPS once or twice, but in females two injections of LPS led to more stable and more long-lasting increase of alcohol intake compared with a single administration of LPS. Therefore, in all subsequent experiments male mice were pretreated with a single injection of LPS, and female mice received two injections.
The studies presented above used a dose of 1 mg/kg LPS; to determine if a lower dose would increase drinking, 0.33 mg/kg was injected one week before testing drinking. This treatment did not change the ethanol intake, preference for ethanol, or total fluid intake (Supplemental Fig. 5). Because this dose was ineffective for increasing drinking, all subsequent studies were carried out with 1 mg/kg LPS.
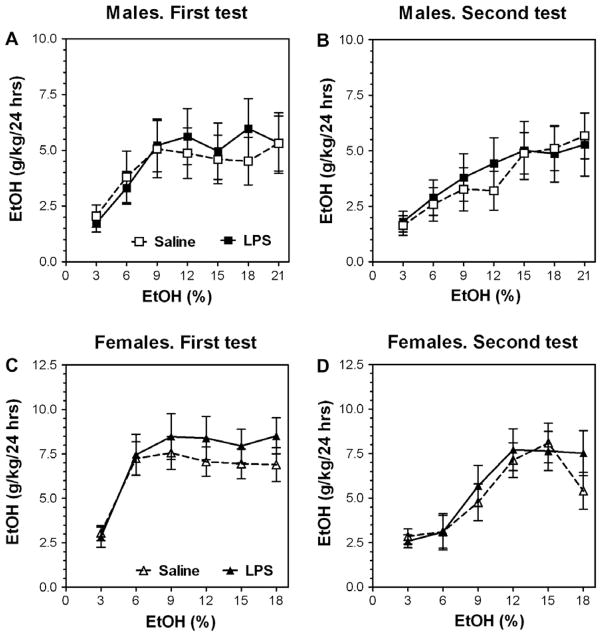
To test whether the increased alcohol consumption required signaling through the TLR4 system, we deleted CD14, which is a key accessory protein for TLR4 function (Peri et al., 2010). After injection of LPS, these mice also showed some reduction of body weight accompanied by reduction of water intake (Supplemental Figs. 6 and 7), but these reductions were much smaller than were seen in control B6 mice (Fig. 1). Mice lacking CD14 showed no increase of alcohol consumption after pretreatment (Fig. 4). Complete data for ethanol intake (amount of ethanol consumed, preference for ethanol and total fluid intake) in a continuous-access, two-bottle choice test for CD14 null mice of both sexes are presented in Supplemental Figs. 6 and 7 (for detailed statistics see Supplementary materials in Supplemental Table 3).
Fig. 4.
CD14 null mutant mice do not show increased ethanol consumption after pretreatment with LPS. (A and B) CD14 null male mice. n = 7–10 per group. (C and D) CD14 null female mice. n = 10 per group. (A and C) Initial ethanol intake (g/kg/24 h). (B and D) Ethanol intake (g/kg/24 h) after first (one-week) ethanol deprivation.
In the previous experiments, we began testing of alcohol consumption one week after LPS injection. In the next experiment we asked if the increased drinking would be seen if we waited for one month. LPS was administered to B6 males (one injection) and B6 females (two injections) and one month after the last LPS administration mice were tested in a two-bottle choice paradigm. The increase of alcohol intake was still present in the LPS pre-treated group in male mice (Fig. 5A), and this increased consumption was sustained after one week of alcohol deprivation (Fig. 5B). In contrast, no differences in alcohol consumption between saline and LPS-pretreated groups were found in B6 female mice (Fig. 5C and D). Thus, LPS increases alcohol consumption in both male and female mice, but the male mice appear more vulnerable (require only a single injection, females require two treatments) and the effect is more persistent in males than females. Complete data for ethanol intake (amount of ethanol consumed, preference for ethanol and total fluid intake) in a two-bottle choice test for B6 inbred mice of both sexes are presented in Supplemental Figs. 8 and 9 (for detailed statistics see Supplementary materials in Supplemental Table 4).
Fig. 5.
C57Bl/6 J male mice shows the increase of ethanol intake even one month after LPS injection. (A and B) B6 male mice. n = 10–13 per group. (C and D) CD14 null female mice. n = 10–12 per group. (A and C) Initial ethanol intake (g/kg/24 h). (B and D) Ethanol intake (g/kg/24 h) after first (one-week) ethanol deprivation.
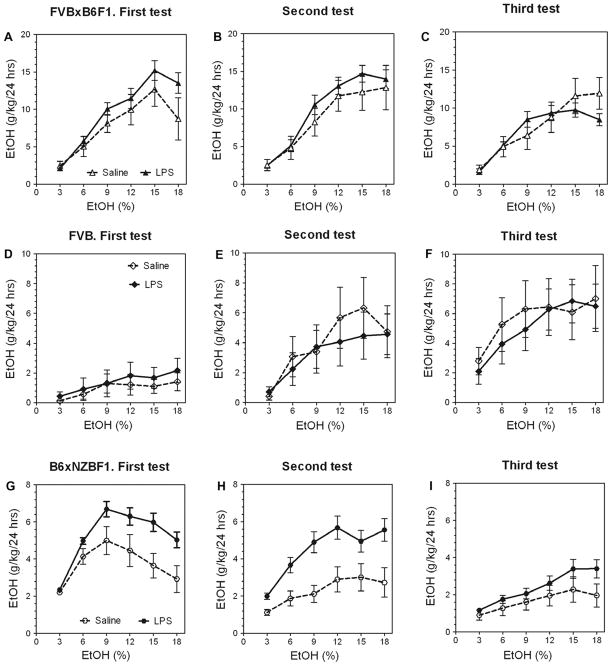
Alcohol consumption in the two-bottle choice test is extremely sensitive to genetic background (Blednov et al., 2010b). The B6 mouse has the highest alcohol consumption of inbred strains, but we found that an FVBxB6F1 hybrid has even higher alcohol consumption and that B6xNZBF1 shows moderate consumption, whereas the FVB strain demonstrates very low consumption (Blednov et al., 2005b; Yoneyama et al., 2008). In addition to the initial level of consumption, we showed that the effect of a period of abstinence or deprivation on subsequent consumption varies among genotypes, with some showing sustained alcohol consumption and others showing reduced alcohol consumption (Blednov et al., 2010a). Therefore, we asked if LPS pretreatment could increase alcohol consumption in the very-high-consuming hybrid mice, and we tested related hybrids with lower alcohol consumption and with different patterns of consumption after abstinence. The effect of abstinence described above was demonstrated in female mice, and we used only females (with two injections of LPS) for these experiments.
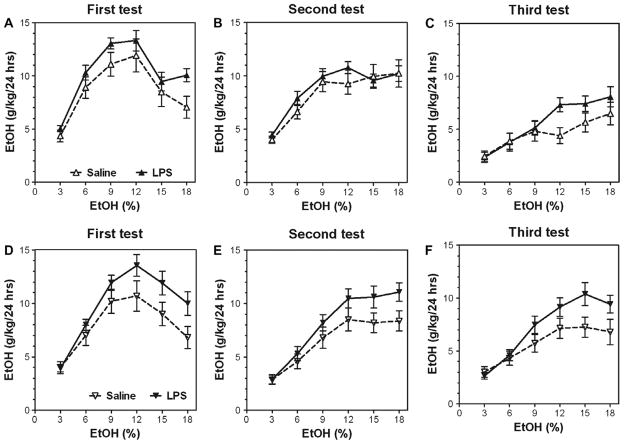
Pretreatment with LPS significantly increased ethanol consumption during the initial period of intake in FVBxB6F1 mice as well as in B6xNZBF1 hybrids but not in FVB inbred mice (Fig. 6A, B and G). After alcohol deprivation, the increased ethanol intake was observed only in B6xNZBF1 hybrid mice (Fig. 6H and I). No differences between LPS- and saline-pretreated groups of FVB and FVBxB6F1 mice were found during the second period of drinking. Complete data for ethanol intake (amount of ethanol consumed, preference for ethanol and total fluid intake) in a two-bottle choice test for FVB, FVBxB6F1, and B6xNZBF1 female mice are presented in Supplemental Figs. 10–12 (for detailed statistics see Supplementary materials in Supplemental Table 5).
Fig. 6.
Ability of LPS to increase the alcohol intake depends on genetic background in female mice. (A–C) FVBxB6F1 female mice. n = 10–12 per group. (D–F) FVB inbred female mice. n = 10–14 per group. (G–I) B6xNZBF1 female mice. n = 8–17 per group. (A, D and G) Initial ethanol intake (g/kg/24 h). (B, E and H) Ethanol intake (g/kg/24 h) after first (one-week) ethanol deprivation. (C, F and I) Ethanol intake (g/kg/24 h) after second (one-month) ethanol deprivation.
3.2. Sensory perception of ethanol
Because the alcohol intake in the two-bottle choice paradigm depends on taste (Bachmanov et al., 2003), in the next experiments the effect of LPS pretreatment on consumption of sweet/noncaloric (saccharin) solutions, bitter (quinine) solutions, and sweet/caloric (sucrose) solutions were tested. Also, the possibility that LPS treatment may change the olfactory recognition of ethanol was tested. B6 mice of both sexes were used for all these experiments.
Pretreatment with LPS did not change the preference for saccharin solutions in B6 male mice at any time periods. On the contrary, in female B6 mice pretreatment with LPS reduced the preference for saccharin.
The preference for bitter solutions of quinine was increased (reduced avoidance) in B6 male mice during the initial period of measurement. Post-hoc analyses showed significant differences between saline- and LPS-pretreated groups for concentration of quinine 0.06 mM. A small reduction in preference for quinine in B6 male mice was observed also after the second (one-month) period of deprivation (from quinine). No differences were found after the first (one-week) period of deprivation. Females of the B6 inbred strain did not show any differences in preference for quinine during the initial period of measurement. However, after deprivation a slight reduction (increased avoidance) in preference for quinine in LPS pretreated animals was found.
No effect of LPS was observed during the initial period of sucrose consumption or after first deprivation (from sucrose) (one week). After the second deprivation (one month), a slight reduction in preference for sucrose in LPS pretreated B6 male mice was found. Females did not change the preference for sucrose in any of the periods of measurement. Complete data for intake of different tastants (preference for saccharin, quinine, and sucrose, as well as total fluid intake) in a continuous-access, two-bottle choice test are presented in Supplemental Figs. 13–18 (for detailed statistics see Supplementary materials in Supplemental Table 5). Increased alcohol consumption could be produced by increased perception of sweet taste or decreased perception of bitter taste, but such changes in taste perception do not appear to result from LPS treatments.
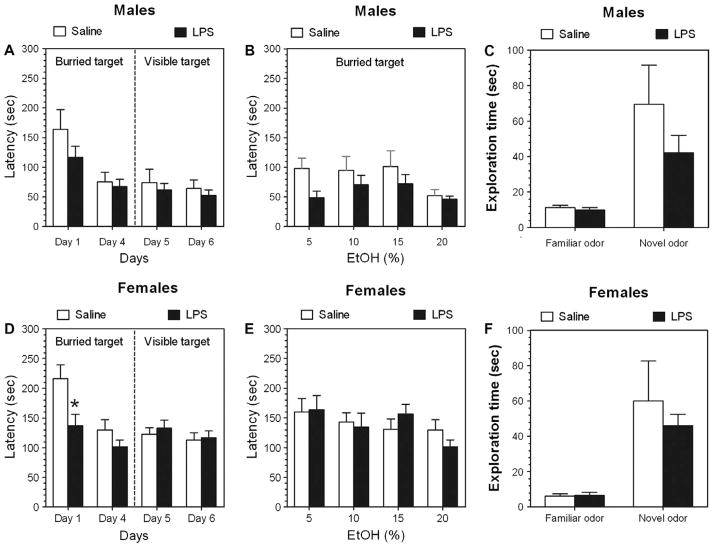
Altered consumption of ethanol could potentially result from altered olfactory sensitivity. We used a buried-target test, to compare the latency to locate a Petri dish with odor of ethanol buried beneath the bedding or placed on its surface. The ability to locate the “buried odor” of ethanol improved over days of observation [F(1, 18) = 10.5, p < .01, main effect of time]. However, no effect of LPS pretreatment was found in B6 male mice (Fig. 7A). Also, no differences between groups were found in their ability to locate the visible (with or without ethanol) target. The ability to locate the odor of ethanol overall depended on concentration of ethanol [F(3, 54) = 4.8, p < .01, main effect of concentration] but did not depend on pretreatment (Fig. 7B). Both groups of B6 male mice were able to discriminate the novel (10% ethanol) odor from familiar (own) odor [F(1, 34) = 14.9, p < .001, main effect of novelty] without differences between groups (Fig. 7C). In contrast, in B6 female mice their ability to uncover the “buried odor” of ethanol was dependent on treatment [F(1, 40) = 8.8, p < .01] and time [F(1, 40) = 11.3, p < .01]. Post-hoc analyses showed that females pretreated with LPS located the ethanol scent significantly faster but only on the first day of the experiment (p < .01) (Fig. 7D). Also, no differences between groups were found in their ability to locate the visible (with or without ethanol) target. No differences in ability to discriminate between novel (ethanol) or familiar (own) scents were found in female mice [F(1, 36) = 18.8, p < .001, main effect of novelty] (Fig. 7F). Thus, the increased alcohol consumption cannot be attributed to altered olfactory function.
Fig. 7.
LPS pretreatment produces no changes in olfactory recognition of ethanol in C57Bl/6 J mice. (A–C) B6 male mice. (D–F) B6 female mice. (A and D) Latency to uncover the buried or visible target (odor of 20% of ethanol). (B and E) Latency to uncover the buried target (odor of different concentrations of ethanol). (C and F) Time spent exploring the target in the “block” test. *p < .05, statistically significant difference between LPS- and saline-pretreated groups (Bonferroni’s post-hoc analyses).
3.3. Motivational properties of alcohol
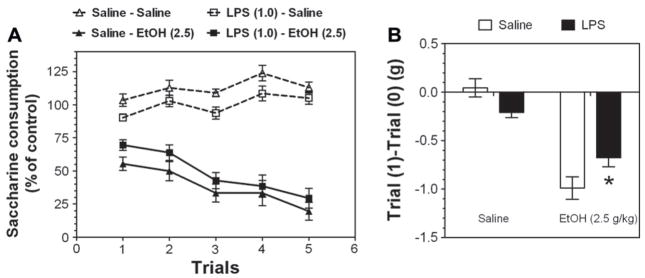
Conditioned taste aversion (CTA) is traditionally used to evaluate the aversive properties of ethanol (Liu et al., 2009). There was no difference in consumption of saccharin on trial 0 (before conditioning) between saline- and LPS-pretreated mice (66.2 ± 2.1 g/kg body weight and 61.9 ± 2.7 g/kg body weight, respectively). However, to correct for any initial fluctuations in intake of tastant, intake was calculated as a percentage of the trial 0 consumption for each subject by dividing the amount of saccharin solution consumed on subsequent conditioning trials by the amount of saccharin solution consumed on trial 0 (before conditioning). Ethanol-saccharin pairings produced reduction in saccharin intake across trials compared with saline-saccharin pairings, indicating the development of conditioned taste aversion (CTA) in saline-pre-treated [F(1, 115) = 169; p < .001, effect of treatment; F(4, 115) = 2.6; p < .05, effect of trial; F(4, 115) = 3.9; p < .01, trial × treatment interaction] as well as for LPS-pretreated mice [F(1, 115) = 292; p < .001, effect of treatment; F(4, 115) = 6.5; p < .001, trial × treatment interaction] (Fig. 8A). However, in saline-treated groups LPS-pretreated mice consumed less saccharin than saline-pre-treated mice [F(1, 90) = 17.9, p < 0.001, effect of treatment; F(4, 90) = 5.2, p < 0.001, effect of trial]. Comparison mice from ethanol-treated groups also showed that LPS-pretreated mice consumed less saccharin than saline-pretreated mice [F(1, 140) = 5.9, p < .05, effect of treatment; F(4, 90) = 10.4, p < .001, effect of trial].
Fig. 8.
LPS pretreatment reduces the aversive effect of ethanol in B6xNZB F1 female mice. (A) Consumption of saccharin, the conditioned stimulus, is presented as percent of saccharin intake at day 0 (before injection) for all conditioning days. (B) Consumption of saccharin, the conditioned stimulus, is presented as differences in saccharin intake between day 1 (consumption after first injection) and day 0 (consumption at day before injection). n = 10 for both groups of saline-treated mice and n = 15 for both groups of ethanol-treated mice. *p < .05, statistically significant difference between LPS- and saline-pretreated groups (Bonferroni’s post-hoc analyses).
It should be noted that the correlation between ethanol-induced conditioned taste aversion and ethanol intake in a two-bottle choice paradigm was shown for the initial (after first injection of ethanol) changes in saccharin consumption (Broadbent et al., 2002). Therefore, changes in saccharin intake for trial 1 compared with Trial 0 were calculated, and the results are presented in Fig. 8B. Although these data showed no main effect of pretreatment but only a main effect of dose [F(1, 46) = 55.4, p < .001] and pretreatment × dose interaction [F(1, 46) = 7.8, p < .01], post-hoc analyses showed significant differences between both ethanol-injected groups of mice (p < .05), indicating a smaller reduction in saccharin intake after pretreatment with LPS. Taken together, these results indicate that LPS pretreatment reduces ethanol CTA.
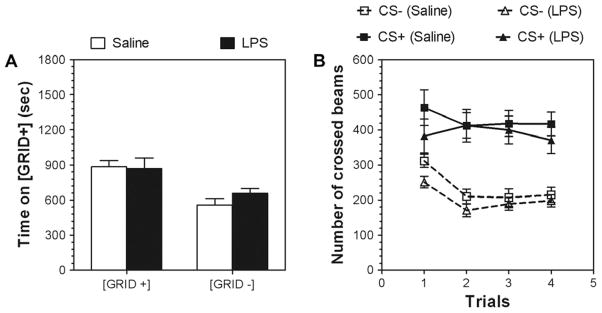
Conditioned place preference (CPP) is often used as a measure of drug reward, and we asked if the hybrids differed in ethanol-induced CPP (Tzschentke 1998). Analysis of test day data indicates that both saline- and LPS-pretreated groups of mice develop a similar ethanol-induced CPP, indicating a similar sensitivity to the rewarding properties of ethanol (Fig. 9A). Saline groups did not exhibit a floor type preference on the test day (data not shown). The data were analyzed by two-way ANOVA {group (GRID + and GRID−) × pretreatment (saline and LPS)}. There was no group × pretreatment interaction and no main effect of pre-treatment was seen. However, there was a main effect of group [F(1, 36) = 17.2, p < .001]. Both pretreated groups of mice spent more time on the floor when it was paired with ethanol injections (GRID + group), and both groups spent less time on the floor when it was paired with saline (GRID-group). Mean activity during each 5-min ethanol (CS+) and saline (CS−) conditioning trial are depicted in Fig. 9B. Activity on saline trials was decreased across trials in both groups of mice (saline- and LPS-pretreated) [F(3, 147) = 8.8, p < .001, effect of trial], with slightly reduced activity in LPS-pretreated mice [F(3, 147) = 5.9, p < .05, effect of pre-treatment]. Ethanol produced an increase in activity relative to saline in both groups of mice. However, this stimulation of motor activity was not affected by LPS treatment.
Fig. 9.
LPS pretreatment does not change the rewarding properties of ethanol in a conditioned place preference (CPP) test in NZBxB6 F1 female mice. (A) Ethanol-induced CPP. Time (s) spent on Grid + floor (circles) during 30-min test session in ethanol-conditioned (Grid + and Grid−) groups. (B) Motor activity of mice (Grid + and Grid− groups) reported as total distance traveled (cm) during 5-min conditioning trials for CS + and CS− trials. n = 10 per each group.
3.4. Basal firing of dopamine neurons in VTA
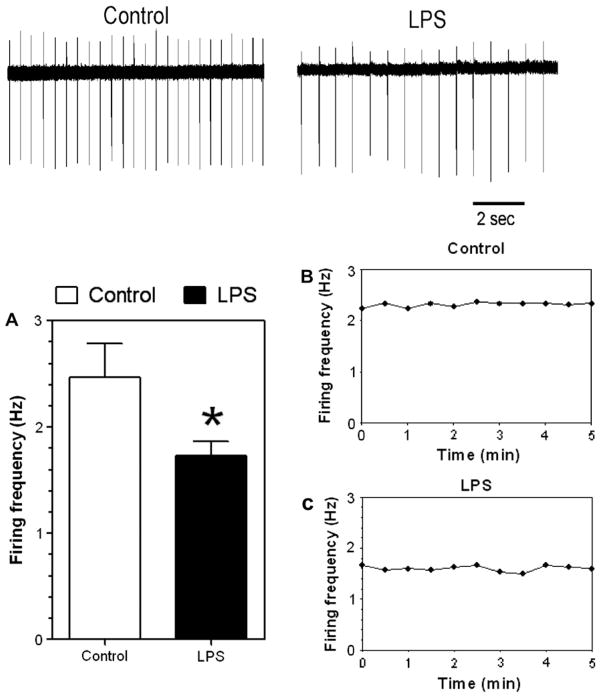
We asked if the firing activity of VTA dopaminergic neurons is affected by LPS treatment. Midbrain slices containing the VTA were prepared from saline- and LPS-treated mice (one week after one LPS injection in B6 male mice). We found that the basal firing frequency of dopamine neurons was reduced in LPS-treated mice [t(22) = 2.45, p < .05, unpaired t-test] (Fig. 10A).
Fig. 10.
LPS pretreatment significantly reduced the basal firing of DA neurons in VTA of C57Bl/6 male mice. Upper panel: Example traces of action potential firing in dopamine neurons from saline- and LPS-treated mice. (A) Average firing frequencies in saline- and LPS-treated groups. (B and C) Time graphs of firing frequency during 5-min recording periods for the neurons shown in the upper panel. These graphs illustrate the stability of firing recording in individual neurons. *p < 0.05, Student’s t-test. n = 9 and 15 in saline and LPS groups, respectively.
3.5. Effects of LPS on body weight and relation to drinking
LPS induces a transient sickness behavior, and we estimated the severity of this sickness by changes in body weight and water intake (Fig. 1). We asked if the severity of this sickness behavior is related to later changes in alcohol intake. We calculated the correlation between severity of sickness and ethanol intake for all mice used in this study. The severity of sickness was calculated as an area under the curve for daily monitoring of body weight after the first injection of LPS. Body weight the day before LPS injection was taken as 100%, and body weight for each of the next seven days was expressed as a percentage of the initial body weight. This parameter reflects not only the peak severity of sickness but also the course of recovery. Ethanol intake was calculated as an area under the curve for ethanol intake from 3% of ethanol solution up to 18% of ethanol (using amount of ethanol consumed, g/kg/d). No correlations between these parameters were found (Fig. 11), which suggests that increased ethanol consumption produced by LPS is not a consequence of the sickness response.
Fig. 11.
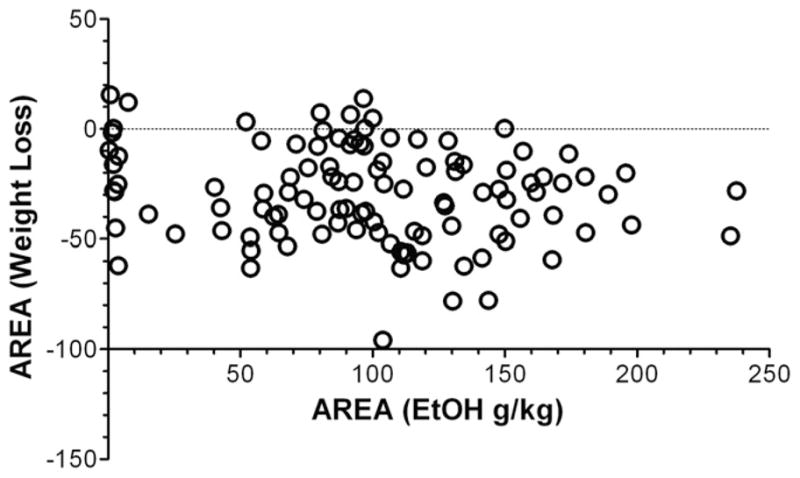
Lack of correlation between ethanol intake and severity of sickness developed after LPS injection. Y-axis: The severity of sickness was calculated as an area under the curve for daily monitoring of body weight after the first injection of LPS. Body weight the day before LPS injection was taken as 100% and body weight for each of the next seven days were expressed as a percentage of the initial body weight. This parameter reflects not only the peak severity of sickness but also the course of recovery. X-axis: Ethanol intake was calculated as an area under the curve for ethanol intake from 3% of ethanol solution up to 18% of ethanol (using amount of ethanol consumed, g/kg/d).
4. Discussion
Our results clearly show that inflammatory activation (treatment with LPS) can produce long-lasting (up to 3 months, including several periods of alcohol deprivation) increases of ethanol intake, although there are differences in the size and persistence of the effect among different genetic backgrounds and between male and female mice. Both B6 and FVBxB6F1 mice showed the increased consumption. It should be noted that B6 show the highest level of ethanol preference and intake among all available inbred mouse strains (Belknap et al., 1993) and that the FVBxB6F1 show the highest alcohol intake of any mouse line (Blednov et al., 2005b). Thus, it is notable that even a single injection of LPS was able to produce a long-lasting increase in consumption in these high-drinking mice. This is consistent with our finding of reduced alcohol consumption in null mutant mice lacking key components of immune signaling (Blednov et al., 2005a, in press). These studies showed that mice lacking several chemokines and cytokines showed decreased alcohol consumption in the two-bottle choice test.
Lipopolysaccharide (LPS) is the major lipid constituent of the cell wall of gram-negative bacilli and is known to trigger the acute-phase immune response. Inflammatory processes are characterized by the release of pro-inflammatory cytokines such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, anti-inflammatory cytokines like IL-10 and endogenous IL-1 receptor antagonist (IL-1ra) (Feghali and Wright, 1997). These cytokines not only control local inflammatory processes in peripheral tissues and mediate immune responses but also affect central nervous system activity. They coordinate physiological responses such as fever, activate the hypothalamic–pituitary–adrenal axis, and induce behavioral changes, such as decreased intake of food and water, social withdrawal, anhedonia, and disturbed sleep, which are adaptive responses collectively termed “sickness behavior” (Dantzer, 2001). Some aspects of the acute sickness behavior such as anorexia can be produced by doses of LPS as low as 0.05 mg/kg (Wisse et al., 2007), whereas we found that at least 1 mg/kg LPS was required for the persistent increase in alcohol consumption. In addition, we saw no correlation between initial weight loss produced by LPS and increased alcohol consumption, suggesting that the later effects of LPS are independent of the acute responses. LPS doses in the range of 0.8–5 mg/kg are commonly used to produce longer-lasting effects of LPS such as depressive-like behavior and neurodegeneration (O’Connor et al., 2009; Qin et al., 2008). Marsh et al. (2009) found that systemic administration of 1 mg/kg LPS repro-grams TLR4-expressing cells within the brain. It appears that the persistent actions of LPS require a higher dose than is needed for the acute effects, although studies with dose–response data are lacking.
LPS treatment alters some behaviors, which potentially may modify the alcohol consumption. Acutely, LPS decreases preference for saccharin, increases intake of quinine and reduces fluid intake (Aubert and Dantzer, 2005). However, it does not change sucrose palatability (Cross-Mellor et al., 1999). Cross-Mellor et al. (2009) showed that LPS-induced immune system activation significantly impairs the rapid acquisition of a conditioned taste aversion to LiCl–sucrose mixture in taste reactivity tests. In contrast, Larson (2006), using a CPP procedure, showed that LPS and IL-1β decreased sucrose intake (sucrose used as a primary reinforcer) but had no effect on the expression of a sucrose-induced place preference (sucrose-paired environment as a conditioned reinforcer), indicating a differential effect of immune system activation on appetitive behaviors maintained by primary and conditioned reinforcers (Larson, 2006). It is important to note that all of these studies explored the immediate effect of LPS injection and are thus studies of the acute “sickness” behavior. In contrast, in all of our experiments mice were allowed to recover from these effects of LPS injection before testing.
Taste perception is a critical factor in determining ethanol consumption in two-bottle choice paradigm. The existence of a positive relationship between ethanol and sweet intake has been known for more than 40 years (Rodgers et al., 1963). Deletion of several genes involved in detection of sweet taste leads to a substantial reduction of alcohol intake without any changes in pharmacological actions of ethanol (Blednov et al., 2008). However, pretreatment with LPS did not produce changes in preference for sweet solutions of saccharin, which could explain the increased ethanol consumptions.
It is thought that the avoidance of more concentrated ethanol solutions can be related to increased bitterness (Kampov-Polevoy et al., 1990; Goodwin et al., 2000). Although increased consumption of bitter solutions of quinine found in B6 male mice after LPS are consistent with the increased alcohol intake, other changes in quinine consumption were not correlated with changes in alcohol intake. For example, males and females showed similar differences in alcohol drinking, but females did not show any differences in quinine intake after LPS treatment.
Alcohol is not only a pharmacological substance but also a nutrient with energy content (Forsander, 1998; Kiefer, 1995). However, in agreement with earlier findings (Cross-Mellor et al., 1999), pretreatment with LPS did not change the intake of highly caloric solutions of sucrose. Therefore, the increased alcohol intake could not be a result of changes of alcohol palatability.
Alcohol-related olfactory cues may be powerful appetitive cues, particularly because they are present both before (nasally) and during drinking (retronasally). This would make them effective conditioned cues for alcohol’s immediate reward (Grüsser et al., 2000; Rohsenow et al., 1997). It is known that peripheral acute injection of LPS induces neuronal apoptosis in the olfactory bulb in mice (Mori et al., 2010) and changes the dopamine content in the anterior olfactory nucleus (Ota et al., 2008). Therefore, these degenerative changes might modify the olfactory perception of ethanol and change ethanol intake. However, LPS injection did not alter the perception of ethanol smell in different olfactory tests. Taken together, these data show that LPS effects on sensory perception of ethanol cannot explain the excessive alcohol consumption.
We investigated other possible mechanisms for increased ethanol intake in the two-bottle choice paradigm. For example, mice can increase consumption of ethanol because of reduced aversive properties of alcohol as well as an increase (or decrease) in ethanol reward (Chester and Cunningham, 2002; Green and Grahame, 2008). Our studies of CPP showed no changes in rewarding properties of ethanol after LPS pretreatment but showed a reduction of the aversive properties of ethanol as measured by CTA. This is consistent with a large literature relating reduced CTA to increased alcohol consumption (Green and Grahame, 2008). In addition, we found that deletion of some chemokine genes increased alcohol CTA (and decreased alcohol consumption) (Blednov et al., 2005a), providing additional evidence that immune signaling is important for the aversive properties of alcohol.
Our data demonstrate that previous LPS exposure leads to a reduction in the basal firing activity of VTA dopamine neurons. Low basal dopamine levels in the nucleus accumbens (NAc), the major dopamine neuron projection area, have been associated with high ethanol preference and consumption in both mice and rats (George et al., 1995; McBride et al., 1995). Furthermore, profound decreases in VTA dopamine neuron activity and NAc dopamine release have been reported in rats withdrawn from repeated ethanol exposure (Diana et al., 1993). Interestingly, these ethanol-dependent rats consume ethanol until NAc dopamine levels are restored to control levels (Weiss et al., 1996). Therefore, the reduced dopamine neuron activity observed after LPS treatment may mimic the ethanol-dependent state that drives excessive ethanol consumption. In this regard, it is of note that reduced dopamine release in the striatum has also been reported in detoxified alcoholics (Martinez et al., 2005; Volkow et al., 2007).
Regarding the mechanism of action of LPS to increase drinking, it appears to be mediated by toll-like receptors (TLR), because deletion of the CD14 adapter protein was sufficient to prevent such an increase of ethanol consumption. CD14 has been shown to be important in TLR4 as well as in TLR2 signaling (see Palsson-McDermott and O’Neill, 2004 for rev.) However, Naert et al. (2009) showed that TLR2 is not involved in the immune response to LPS. Therefore, TLR4-signaling is the most promising molecular mechanism for mediation of increase of alcohol consumption. There is evidence that peripherally injected LPS cannot permeate into the blood–brain barrier (Singh and Jiang, 2004). The peripheral cytokines released by LPS can signal the brain through a number of routes, including the activation of vagal afferent fibers projecting to the nucleus of the solitary tract and higher viscerosensory centers, cytokine-specific transport molecules expressed on the brain endothelium, and circumventricular organs (Besedovsky and del Rey, 1996; McAfoose and Baune, 2009; Quan, 2008). Upon reaching the brain, cytokine signals can be amplified through a central cytokine network that has profound effects on neurotransmitter metabolism, neuroendocrine function, synaptic plasticity, and behavior (Dantzer et al., 2008). Thus, it seems likely that LPS acts on TLR4 on peripheral tissues, such as macrophages, liver Kupffer cells or endothelial cells to transiently release cytokines which then act on their receptors in the brain to produce long-lasting changes in neuroimmune function (Qin et al., 2007, 2008). However, there is some evidence that LPS elicits TLR4 signaling in the brain independent of peripheral cytokine responses (Chakravarty and Herkenham, 2005; Gosselin and Rivest, 2008). Regardless of the site of action of LPS, it is likely that this persistent perturbation of neuroimmune signaling is responsible for the changes in dopamine cell firing, CTA, and alcohol consumption that we observed.
Regulation of alcohol consumption in mice is highly dependent on the genetic background (Belknap et al., 1993: Yoneyama et al., 2008; Blednov et al., in press), and thus it is not surprising that the effects of LPS also depended upon the mouse strain. However, there is no obvious relationship between genetic differences in initial drinking of ethanol and the effect of LPS. For example, the largest effect of LPS was seen in the moderately drinking B6xNZBF1 hybrid, but the low-drinking FVB strain did not show any effect of LPS. It is interesting that the B6xNZBF1 mice reduced their alcohol consumption after periods of deprivation, whereas the FVB mice increased consumption. It is possible that genetic differences in LPS actions are related to these positive or negative alcohol- deprivation effects.
In summary, these results show that a single injection of LPS produces long-lasting increases in alcohol consumption as well as changes in alcohol-conditioned taste aversion and firing of dopamine neurons in the VTA. Previous results showed altered expression of genes related to neuroimmune signaling in human alcoholics (Liu et al., 2006), rodents with genetic predisposition for alcohol consumption (Mulligan et al., 2006; Saba et al., 2006), and mice and drosophila treated with ethanol (Qin et al., 2008; Kong et al., 2010), and that deletion of immune signaling components decreases alcohol consumption (Blednov et al., in press). Taken together, these results provide strong evidence for neuroimmune regulation of alcohol intake and alcohol aversion as well as the activity of dopaminergic neurons. In view of the ability of alcohol abuse to increase leakage of LPS from the gut and to produce widespread changes in human immune function (Brown et al., 2006; Nelson and Kolls, 2002; Wang et al., 2010), it is possible that activaton of immune signaling may have a role in promoting excessive alcohol consumption in humans.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute of Alcohol Abuse and Alcoholism (AA U01 13520 - INIA West Project, AA06399, AA015521). The authors would like to thank Danielle Walker and Virginia Bleck for excellent technical assistance.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbi.2011.01.008.
References
- Andonegui G, Bonder CS, Green F, Mullaly SC, Zbytnuik L, Raharjo E, Kubes P. Endothelium-derived toll-like receptor-4 is the key molecule in LPS- induced neutrophil sequestration into lungs. J Clin Invest. 2003;111:1011–1020. doi: 10.1172/JCI16510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A, Dantzer R. The taste of sickness: lipopolysaccharide-induced finickiness in rats. Physiol Behav. 2005;84:437–444. doi: 10.1016/j.physbeh.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Kiefer SW, Molina JC, Tordoff MG, Duffy VB, Bartoshuk LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences, and consumption. Alcohol Clin Exp Res. 2003;27:220–231. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Alva H, Creech K, Findlay G, Harris RA. GABAA receptor alpha 1 and beta 2 subunit null mutant mice: behavioral responses to ethanol. J Pharmacol Exp Ther. 2003;305:854–863. doi: 10.1124/jpet.103.049478. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA. Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res. 2005a;165:110–125. doi: 10.1016/j.bbr.2005.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6JxFVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res. 2005b;29:1949–1958. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ozburn AR, Walker D, Ahmed S, Belknap JK, Harris RA. Hybrid mice as genetic models of high alcohol consumption. Behav Genet. 2010a;40:93–110. doi: 10.1007/s10519-009-9298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Walker DL, Iyer SV, Homanics G, Harris RA. Mice lacking Gad2 show altered behavioral effects of ethanol, flurazepam and gabaxadol. Addict Biol. 2010b;15:45–61. doi: 10.1111/j.1369-1600.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. doi: 10.1111/j.1369-1600.2010.00284.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–148. [PubMed] [Google Scholar]
- Brown LA, Cook RT, Jerrells TR, Kolls JK, Nagy LE, Szabo G, Wands JR, Kovacs EJ. Acute and chronic alcohol abuse modulate immunity. Alcohol Clin Exp Res. 2006;30:1624–1631. doi: 10.1111/j.1530-0277.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cels sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. Modulation of corticosterone does not affect the acquisition or expression of ethanol-induced conditioned place preference in DBA/2J mice. Pharmacol Biochem Behav. 1998;59:67–75. doi: 10.1016/s0091-3057(97)00320-1. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. GABA(A) receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–143. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Cross-Mellor SK, Kent WD, Ossenkopp KP, Kavaliers M. Differential effects of lipopolysaccharide and cholecystokinin on sucrose intake and palatability. Am J Physiol. 1999;277:R705–R715. doi: 10.1152/ajpregu.1999.277.3.R705. [DOI] [PubMed] [Google Scholar]
- Cross-Mellor SK, Foley KA, Parker LA, Ossenkopp KP. Lipopolysaccharide dose dependently impairs rapid toxin (LiCl)-induced gustatory conditioning: a taste reactivity examination of the conditioned taste aversion. Brain Behav Immun. 2009;23:204–216. doi: 10.1016/j.bbi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Prather LK. Conditioning trial duration affects ethanol-induced conditioned place preference in mice. Anim Learn Behav. 1992;20:187–194. [Google Scholar]
- Cunningham CL. Pavlovian drug conditioning. In: van Haaren F, editor. Methods in Behavioral Pharmacology. Elsevier; New York: 1993. pp. 349–381. [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci USA. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF. Olfactory deficits in mice overexpressing human wildtype alpha- synuclein. Eur J Neurosci. 2008;28:247–256. doi: 10.1111/j.1460-9568.2008.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsander OA. Dietary influences on alcohol intake: a review. J Stud Alcohol. 1998;59:26–31. doi: 10.15288/jsa.1998.59.26. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Ng GY, Jung SY, O’Dowd BF, Naranjo CA. Low endogenous dopamine function in brain predisposes to high alcohol preference and consumption: reversal by increasing synaptic dopamine. J Pharmacol Exp Ther. 1995;273:373–379. [PubMed] [Google Scholar]
- Goodwin FL, Bergeron N, Amit Z. Differences in the consumption of ethanol and flavored solutions in three strains of rats. Pharmacol Biochem Behav. 2000;65:357–362. doi: 10.1016/s0091-3057(99)00222-1. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Rivest S. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol Psychiatr. 2008;13:480–497. doi: 10.1038/sj.mp.4002122. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüsser SM, Heinz A, Flor H. Standardized stimuli to assess drug craving and drug memory in addicts. J Neural Transm. 2000;107:715–720. doi: 10.1007/s007020070072. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol. 1990;7:83–85. doi: 10.1016/0741-8329(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Kiefer SW. Alcohol, palatability, and taste reactivity. Neurosci Biobehav Rev. 1995;19:133–141. doi: 10.1016/0149-7634(94)00027-x. [DOI] [PubMed] [Google Scholar]
- Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, Wolf FW. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol Clin Exp Res. 2010;34:302–316. doi: 10.1111/j.1530-0277.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SJ. Lipopolysaccharide and interleukin-1beta decrease sucrose intake but do not affect expression of place preference in rats. Pharmacol Biochem Behav. 2006;84:429–435. doi: 10.1016/j.pbb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Liu J, Lewohl JM, Harris RA, Iyer VR, Dodd PR, Randall PK, Mayfield RD. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31:1574–1582. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- Liu C, Showalter J, Grigson PS. Ethanol-induced conditioned taste avoidance: reward or aversion? Alcohol Clin Exp Res. 2009;33:522–530. doi: 10.1111/j.1530-0277.2008.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard C, Wang X, Hagberg H. The role of toll-like receptors in perinatal brain injury. Clin Perinatol. 2009;36:763–772. doi: 10.1016/j.clp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Bodart B, Lumeng L, Li TK. Association between low contents of dopamine and serotonin in the nucleus accumbens and high alcohol preference. Alcohol Clin Exp Res. 1995;19:1420–1422. doi: 10.1111/j.1530-0277.1995.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Mori K, Kaneko YS, Nakashima A, Nagatsu T, Nagatsu I, Ota A. Peripherally injected lipopolysaccharide induces apoptosis in the subventricular zone of young adult mice. Neurosci Lett. 2010;481:126–130. doi: 10.1016/j.neulet.2010.06.070. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci USA. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G, Laflamme N, Rivest S. Toll-like receptor 2-independent and MyD88-dependent gene expression in the mouse brain. J Innate Immun. 2009;1:480–493. doi: 10.1159/000225990. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behav Brain Res. 2004;150:1–7. doi: 10.1016/S0166-4328(03)00219-5. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–259. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatr. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota A, Mori K, Kaneko YS, Nakashima A, Nagatsu I, Nagatsu T. Peripheral lipopolysaccharide administration affects the olfactory dopamine system in mice. Ann NY Acad Sci. 2008;1148:127–135. doi: 10.1196/annals.1410.071. [DOI] [PubMed] [Google Scholar]
- Palsson-McDermott NM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri F, Piazza M, Calabrese V, Damore G, Cighetti R. Exploring the LPS/TLR4 signal pathway with small molecules. Biochem Soc Trans. 2010;38:1390–1395. doi: 10.1042/BST0381390. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, He J, Hanes RN, Pluzarev O, Hong JS, Crews FT. Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. J Neuroinflamm. 2008;5:1–17. doi: 10.1186/1742-2094-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N. Immune-to-brain signaling: how important are the blood–brain barrier-independent pathways? Mol Neurobiol. 2008;37:142–152. doi: 10.1007/s12035-008-8026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers DA, McClearn GE, Bennett EL, Hebert M. Alcohol preference as a function of its caloric utility in mice. J Comp Physiol Psychol. 1963;56:666–672. doi: 10.1037/h0040350. [DOI] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Colby SM, Gulliver SB, Sirota AD, Niaura RS, Abrams DB. Effects of alcohol cues on smoking urges and topography among alcoholic men. Alcohol Clin Exp Res. 1997;21:101–107. [PubMed] [Google Scholar]
- Saba L, Bhave SV, Grahame N, Bice P, Lapadat R, Belknap J, Hoffman PL, Tabakoff B. Candidate genes and their regulatory elements: alcohol preference and tolerance. Mamm Genome. 2006;17:669–688. doi: 10.1007/s00335-005-0190-0. [DOI] [PubMed] [Google Scholar]
- Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201:197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Suzumura A, Takeuchi H, Zhang G, Kuno R, Mizuno T. Roles of glia- derived cytokines on neuronal degeneration and regeneration. Ann NY Acad Sci. 2006;1088:219–229. doi: 10.1196/annals.1366.012. [DOI] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Verma S, Nakaoke R, Dohgu S, Banks WA. Release of cytokines by brain endothelial cells: a polarized response to lipopolysaccharide. Brain Behav Immun. 2006;20:449–455. doi: 10.1016/j.bbi.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304–1313. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse BE, Ogimoto K, Tang J, Harris MK, Jr, Raines EW, Schwartz MW. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology. 2007;148:5230–5237. doi: 10.1210/en.2007-0394. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni I, Granucci F. Differences in lipopolysaccharide-induced signaling between conventional dendritic cells and macrophages. Immunobiology. 2010;215:709–712. doi: 10.1016/j.imbio.2010.05.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.