Abstract
Adenosquamous carcinoma (ADSC) of the head and neck is an aggressive variant of squamous cell carcinoma (SCC). Certain variants of head and neck SCC are human papillomavirus (HPV)-related and have better prognosis. The relationship of HPV to head and neck ADSC has not been investigated. We searched our files for the term “adenosquamous” and head and neck subsites and found cases from 1998 to 2009. The requisite histologic criteria were the presence of SCC combined with distinct gland formation and/or intracellular mucin. DNA in situ hybridization for high-risk HPV, RNA in situ hybridization for high risk HPV E6 and E7 transcripts, and immunohistochemistry for p16 and p53 were performed. The existing literature on ADSC was also reviewed. Of the 18 cases, eight were from the larynx and hypopharynx, four from the oral cavity, three from the oropharynx, and three from the nasal cavity. Three cases (16%) showed both high risk HPV E6 and E7 and p16 expression, one from the nasal cavity and two from the oropharynx. Both oropharyngeal carcinoma patients were alive and disease free at 34 and 103 months, respectively. ADSCs of the head and neck are a heterogeneous group of tumors. A small minority of cases harbor HPV and most of these, particularly those occurring at sites with known high prevalence of HPV, show active viral transcription with detectable E6 and E7 and overexpression of p16. The HPV-related oropharyngeal cases, though rare, appear to do very well clinically, while the remaining cohort of ADSC patients do quite poorly. Head and neck ADSC appears to be a mixed variant that can be further classified according to its HPV status.
Keywords: Adenosquamous carcinoma, Human papillomavirus, Head and neck, p16, p53
Introduction
Adenosquamous carcinoma (ADSC) is a rare variant of head and neck squamous cell carcinoma (SCC) that is characterized by mixed differentiation, with both SCC and true adenocarcinoma, as defined by the World Health Organization [1, 2]. It has been described in a variety of body sites, including the uterine cervix, lung, and pancreas. The existence of ADSC of the head and neck as a distinct entity was somewhat controversial for many years. It was considered to be a high grade mucoepidermoid carcinoma by some investigators [3]. ADSC of the head and neck was first defined in 1968 by Gerughty et al. in a series of 10 patients, where it was shown to be extremely aggressive and highly malignant, with 80% of the patients having proven metastases [4]. The distinctly worse prognosis of ADSC as compared to mucoepidermoid carcinoma (MEC), even high grade ones, along with morphologic and clinical differences, necessitated the separation of these entities.
ADSC has two distinct histologic components. SCC usually predominates, can be in situ or invasive, and can range from well to poorly differentiated. The adenocarcinomatous component can have a tubular, alveolar and/or glandular morphology. Mucin production is typically present, but it is not a requirement for diagnosis in the presence of true gland formation [1, 2]. The gland formation classically should consist of “punched out” spaces with smooth, rather than ragged, edges.
It has been recently shown that HPV is an important etiologic agent in head and neck carcinomas, particularly in the oropharynx and nasal cavity. These tumors may present as different variants distinct from the typical keratinizing-type SCC, including nonkeratinizing [5, 6], basaloid [7, 8], and papillary SCC [9] and also undifferentiated carcinoma [10]. It has also been documented in several studies that HPV-related carcinomas of the upper aerodigestive tract have more favorable prognosis than HPV negative ones [11–13]. Most previous studies investigated HPV in tumors using DNA-based polymerase chain reaction (PCR) [14] while in more recent years DNA-based in situ hybridization (ISH) has been used [5, 13, 15]. However, it is becoming increasingly recognized that HPV can be present without necessarily being biologically active, such that mere presence of the virus (as detected by these methods) may not indicate biological/clinical significance. The presence of virus in transcriptionally active form, as evidenced by expression of high risk HPV proteins E6 and E7, has emerged as the gold standard for biologically significant HPV in SCC [16]. Newer studies have utilized reverse transcriptase (RT) PCR [17], which is cumbersome for routine clinical use due to the complex procedures of RNA extraction from formalin-fixed paraffin-embedded tissues and RT–PCR. Here, we use a recently developed slide-based RNA ISH assay which allows for visual evaluation for E6/E7 expression directly in individual tumor cells (Fay et al. manuscript in preparation).
p16 is a tumor suppressor protein that is considered an excellent surrogate marker of HPV in SCC of the cervix and head and neck [18]. It is overexpressed in HPV-related SCC but is usually deleted or otherwise inactivated in non-HPV-related head and neck SCC [19, 20]. p16 expression by immunohistochemistry approaches 100% in HPV-positive oropharyngeal carcinomas in some studies [5, 11, 21, 22] and is indicative of transcriptionally-active HPV. HPV-negative SCCs tend to harbor p53 mutations leading to nuclear accumulation of p53 protein that is detectable by immunohistochemistry. In HPV-related SCC, p53 is frequently wild-type, being targeted for ubiquination and degradation by the HPV E6 oncoprotein, leading to little or no detectable protein by immunohistochemistry [6, 23].
To date, no one has studied the role of human papillomavirus (HPV) in the pathogenesis of ADSC of the head and neck. Since ADSC of the uterine cervix is known to be HPV-related [24], we investigated the role of HPV in 18 cases of head and neck ADSC using in situ hybridization (ISH) for high risk HPV DNA, RNA-ISH for high risk HPV E6 and E7 mRNA, and immunostaining for p16, gathered clinical follow up information, and reviewed the literature on this rare tumor.
Materials and Methods
Case Selection
After obtaining approval from the Human Research Protection Office at Washington University, the surgical pathology files at Barnes-Jewish Hospital/Washington University were searched for patients diagnosed with ADSC of the head and neck. The cases which were identified were from the period of 1998 to 2009. Hematoxylin and eosin (H and E) stained slides from biopsy and resection specimens of primary tumors that had corresponding paraffin-embedded tissue were retrieved and reviewed by two of the study pathologists (RPM and JSL). The criteria used for inclusion of cases were those of the 2004 WHO Classification of head and neck tumors [1]. Specifically, the tumors had to show definitive squamous differentiation and also well-defined, smooth edged gland formation with or without actual mucin production, in the lumina or as globules in the cytoplasm of individual cells. Most cases showed some form of surface squamous dysplasia. After review, a few cases that were initially diagnosed as ADSC were excluded due to lack of definitive, well-formed and smooth, glands.
Mucoepidermoid carcinoma (MEC) was excluded by either finding a SCC in situ component, definitive and robust squamous differentiation with or without actual keratin formation (something not seen in MEC except very focally), an adenocarcinoma component in the deep aspects of the tumors and not widely intermingled with the squamous differentiation, a haphazard rather than lobular arrangement of the nests, and/or lack of cystic change in the nests. Clinical follow up data was obtained from computerized patient files or from the accumulated paper medical records of the patients’ surgeons and/or oncologists.
DNA In Situ Hybridization for HPV
In situ hybridization (ISH) for high risk HPV DNA was performed on a Ventana Benchmark LT autostainer utilizing formalin fixed, paraffin embedded, 4 μm tissue sections and an ISH I View Blue Plus Detection Kit (Ventana Medical System, Inc., Tucson, AZ) according to the manufacturer’s instructions. The probes used in this assay hybridize with the high risk HPV (HR HPV) genotypes 16, 18, 33, 35, 45, 51, 52, 56, and 66. Ventana Red Counterstain II (Ventana Medical System, Inc., Tuscon, AZ) was used. Positive staining was identified as blue nuclear dots. Any definitive nuclear staining in the tumor cells was considered positive. Cases were classified in a binary manner as either positive or negative. Appropriate positive and negative controls were used.
RNA In Situ Hybridization for HPV
In situ hybridization for high risk HPV E6/E7 RNA was performed by hand using the RNAscope™ HPV kit (Advanced Cell Diagnostics, Inc., Hayward, CA) according to the manufacturer’s instructions. Briefly, 4 μm formalin fixed, paraffin embedded tissue sections were pretreated with heat and protease prior to hybridization with a target probe to the high risk HPV genotype 16 (performed on its own individual slide and designated “16”) and target probes to the other high risk genotypes 18, 31, 33, 35, 52, and 58 (performed as a cocktail for these six types on its own individual slide and designated “high risk”). An HRP-based signal amplification system was then hybridized to the target probes followed by color development with DAB. Positive staining was identified as brown, punctate dots present in the nucleus and/or cytoplasm. Cases were read by authors JSL and JJF and were classified in a binary manner as either positive or negative. Control probes for the bacterial gene DapB (negative control) and for the housekeeping gene ubiquitin C (positive control-evidence of adequate RNA) were also included on each case.
Immunohistochemistry for p16 and p53
Immunohistochemistry was performed on representative 4 μm sections cut from formalin-fixed, paraffin-embedded tissue blocks using monoclonal antibodies to p16 (MTM Laboratories; clone E6H4; monoclonal; 1:1 dilution) and p53 (Ventana; clone Bp-53-11; monoclonal; prediluted) on a Ventana Benchmark XT automated immunostainer (Ventana Medical Systems, Inc., Tucson AZ) according to standard protocols. Detection involved Ventana’s ultraView Universal DAB Detection Kit which utilizes a cocktail of enzyme labeled secondary antibodies that locate the bound primary antibody. The complex is then visualized with hydrogen peroxide substrate and a 3,3′–diaminobenzidine tetrahydrochloride (DAB) chromogen. No biotin is involved. Antigen retrieval, standard on the machine, utilized the Ventana CC1, EDTA-Tris, pH 8.0 solution. Known p16 and p53 expressing head and neck SCC cases were used as positive controls. Staining was nuclear and cytoplasmic for p16 and was solely nuclear for p53. The immunostains were reviewed by a single study pathologist (RPM). For p16, staining was graded in a quartile manner for its extent as follows: 0 = negative; 1+ = 1–25% of cells positive; 2+ = 26–50%; 3+ = 51–75%; 4+ = 76–100%. Results were further divided binarily into strongly positive (3 or 4+) and negative (0, 1+, or 2+) as this is the staining pattern that is generally agreed upon as correlating with the presence of biologically significant HPV. For p53, staining was graded according to the percentage of positive cells, as either negative (<25% of the nuclei staining) or positive (25% or more of the nuclei staining) nuclear staining. This scoring system for p53 was based on a concept that cases with greater than 25% of cells with nuclear staining are more likely to harbor p53 mutations [25].
We also reviewed the literature on ADSC of the head and neck, including any manuscript showing ADSC of the head and neck with diagnostic criteria as per the WHO.
Results
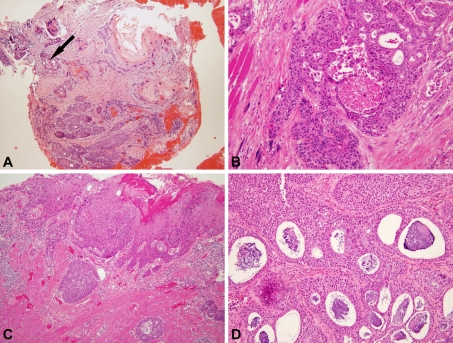
The morphology of the tumors was very much in line with the previous literature, with the squamous component of each tumor ranging from well to poorly differentiated (Fig. 1a, b). The adenocarcinoma component consisted of well-formed glands, tubules or individual cells with intracytoplasmic mucin. Many cases had surface squamous dysplasia/carcinoma in situ (Fig. 1c). Although not requisite for the diagnosis, all of the cases histologically showed intra-cellular or intra-luminal mucin (Fig. 1d).
Fig. 1.
Histologic features of adenosquamous carcinoma. a Biopsy specimen showing a mixture of mature, keratinizing-type SCC and adenocarcinoma characterized by cells with higher nuclear to cytoplasmic rations and scattered gland formation (arrow) with basophilic mucin (100× magnification). b Punched out, rounded glandular spaces devoid of mucin (200× magnification). c Surface, keratinizing-type squamous cell carcinoma in situ and submucosal nests of cells with gland formation (100× magnification). d Florid gland formation with round, smooth edges and basophilic intraluminal and also intracytoplasmic mucin (200× magnification)
Clinical and pathologic data, along with HPV status and immunohistochemistry for p16 and p53, are shown for all patients in Table 1. Patients’ ages ranged from 42 to 87 years (mean 59.7 years; median 60.5 years). Sixteen of the eighteen patients (88.9%) were men. For the 15 patients where status could be obtained, 11 (73.3%) were lifetime tobacco users (nine smokers and two oral tobacco). The larynx was the most common site (8/18–44.5%), followed by the oral cavity (4/18–22.2%), nasal cavity (3/18–16.7%) and oropharynx (3/18–16.7%). Tumor size ranged from 0.5 to 6.5 cm. Angiolymphatic invasion was present in five cases and perineural invasion was present in six. Lymph node metastases were present in eight patients (44.5%) at diagnosis/presentation. Two patients (cases 5 and 6) developed local recurrence 5 months and 1 month after surgery, respectively. Two patients (cases 2 and 15) developed regional recurrence 16 and 24 months post surgery, respectively. Distant metastases developed in four patients (case 4—neck skin, 30 months; case 5—lung and liver, 5 months; case 7—lung, 128 months; case 15—lung, 23 months). Most tumors were stage IV (10/18), with one Stage III, two Stage II and three Stage I tumors. Staging information was not available for two patients. Seventeen patients (94.4%) were treated by surgical excision, supplemented in nine patients with postoperative radiation. Chemotherapy was additionally given in three patients. Treatment information for one patient (case 1) was not available. Follow up data was available for 16 patients and ranged from 1.5 to 128 months (median 30 months). Six patients died of disease and one patient died of unrelated causes. Seven patients were alive with no evidence of disease and two patients were alive with disease. For the group of 10 patients with sufficient follow up data, the 3 years overall survival rate was 50%.
Table 1.
Adenosquamous carcinoma of the head and neck: clinicopathologic data and immunohistochemical and in situ hybridization results
| Case | Location | Age/Sex | T and N-stage | Overall stage | Distant metastases? (site) | Follow-up (months) | HPV DNA | HPV RNA (Type) | p16 | p53 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Paranasal sinus | 82/M | NA | NA | Unknown | DWD (2) | N | N | 2+ | P |
| 2 | Tongue | 42/M | T2/N0 | II | Yes (mediastinum) | DWD (34) | N | N | N | P |
| 3 | Tongue | 65/F | T1/N0 | I | No | NA | N | N | 1+ | P |
| 4 | Soft palate/Tonsil | 62/M | T2/N2 | IV | Yes (neck skin) | DWD (36) | N | N | N | P |
| 5 | Larynx | 59/M | T4/N0 | IV | Yes (lung and liver) | DWD(8) | N | N | N | P |
| 6 | Tonsil | 63/M | T2/N2 | IV | No | AWOD(103) | P | P (16) | 4+ | P |
| 7 | Larynx | 43/M | T2/N0 | II | Yes (lung) | AWOD (128) | N | NS | 4+ | N |
| 8 | Larynx | 60/M | T2/N0 | II | No | AWOD (54) | N | N | N | N |
| 9 | Tongue | 48/M | T2/N1 | III | No | AWOD (3.5) | N | N | 4+ | N |
| 10 | Larynx | 69/M | T2/N2 | IV | No | DWD (1.5) | N | N | N | P |
| 11 | Hypopharynx | 52/M | T3/N2 | IV | No | DWOD (65) | P | N | N | P |
| 12 | Tonsil | 46/M | T1/N2 | IV | No | AWOD (34) | P | P (16) | 4+ | P |
| 13 | Larynx | 51/M | T1/N0 | I | No | AWOD (42) | N | N | N | N |
| 14 | Nose/hard palate/maxilla | 52/M | T4/N0 | IV | No | DWD (4.5) | P | P (HR) | 4+ | P |
| 15 | Larynx | 66/M | T2/N0 | II | No | AWD (26) | N | NS | 4+ | P |
| 16 | Larynx | 58/M | T4/N0 | IV | No | NA | P | N | N | P |
| 17 | Tongue | 87/F | T2/N2 | IV | No | AWD(9) | N | N | N | P |
| 18 | Nasal cavity | 62/M | T1/N0 | I | No | AWOD(26) | N | NS | 3+ | P |
M Male; F Female; NA Not available; DWD Died with disease; DWOD Died without disease; AWD Alive with disease; AWOD Alive without disease; P Positive; N Negative; HPV Human papillomavirus; DNA Deoxyribonucleic acid; RNA Ribonucleic acid; NS Not scoreable; 16 Human papillomavirus 16; HR human papillomavirus, non-type 16 high risk type
Bold text in follow up column indicates patients who died in follow up; bold text in the HPV DNA, HPV RNA, and p16 columns indicate those cases demonstrating HPV
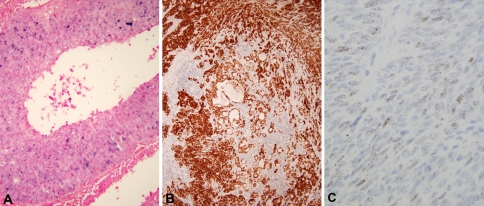
HPV status by ISH and immunohistochemistry (Fig. 2) results according to the location of the tumors are shown in Table 2. The majority of cases were p53 positive (14/18, 78%) above the threshold we utilized to indicate likely mutation. Immunoreactivity was diffuse and intense in all of these cases and was observed in both the squamous and glandular components. Five tumors (27.7%) were HPV positive by DNA ISH and 13 (72.3%) were HPV negative. HPV DNA ISH staining ranged from focal to diffuse. Three tumors (20.0%) were positive by RNA ISH for high risk HPV E6 and E7 expression, and the staining was strong and diffuse in all of them. Three tumors could not be evaluated because, due to RNA degradation, there was inadequate control ubiquitin C staining or in one case there was no tumor remaining in the block. Nine tumors (50.0%) were positive for p16 by IHC. However, strong, diffuse staining with p16 is frequently considered necessary for p16 as a surrogate marker of HPV. As such, seven tumors (38.9%) were p16 positive with greater than 50% of cells staining including all cases which were high risk HPV RNA ISH positive.
Fig. 2.
Immunohistochemical and in situ hybridization studies of adenosquamous carcinoma. a Positive DNA in situ hybridization showing granular, blue nuclear staining of tumor cells (200× magnification). b Positive p16 immunohistochemistry showing strong, diffuse nuclear and cytoplasmic staining of both squamous and glandular components of the tumor (100× magnification). c Positive RNA in situ hybridization showing granular, brown nuclear and cytoplasmic staining (400× magnification)
Table 2.
Site-specific distribution by HPV and p16 status
| Location | HPV DNA+ | HPV DNA− | HPV RNA+ | HPV RNA− | p16+ | p16− |
|---|---|---|---|---|---|---|
| Larynx (n = 8) | 2 | 6 | 0 | 6 | 2 | 6 |
| Oral cavity (n = 4) | 0 | 4 | 0 | 4 | 1 | 3 |
| Nasal cavity (n = 3) | 1 | 2 | 1 | 1 | 2 | 2 |
| Oropharynx (n = 3) | 2 | 1 | 2 | 1 | 2 | 1 |
HPV Human papillomavirus; DNA Deoxyribonucleic acid; RNA Ribonucleic acid
Only three cases (16%) were positive for transcriptionally-active HPV (i.e. positive by all three assays). One arose in the nasal cavity (case 14) and two in the oropharynx (cases 6 and 12). Of these three cases, two were from smokers (one oropharynx and one nasal cavity) and one (oropharynx) was a non-smoker. Interestingly, all three of these cases showed overexpression of p53.
Both transcriptionally-active HPV-related oropharyngeal ADSC patients were alive and disease free at 103 and 34 months, respectively, while, after excluding those with minimal clinical follow up information, six of the remaining 14 patients (42.8%) had died of their tumors. All six patients with no evidence of HPV, lack of p16 expression, and positive overexpression of p53, regardless of primary anatomic site, had disease recurrence, with five dying of disease and the sixth having regional disease recurrence at 7 months.
Review of the Literature
A review of the literature revealed 75 reported cases of ADSC of the head and neck region. Some cases were excluded from our review due to lack of sufficient information. Table 3 summarizes the clinicopathologic data of these 75 cases as well as the 18 cases from our study (for a total of 93 cases). Considering all cases together, ADSC is an aggressive tumor type relative to typical head and neck SCC with a high rate of distant metastasis (24.7%).
Table 3.
Clinical and pathologic characteristics of 93 cases of adenosquamous carcinoma of the head and neck*
| Age (years) (N = 93) | |
| Range | 22–87 |
| Gender (N = 93) | |
| Male | 80 |
| Female | 13 |
| Male: female ratio | 6:1 |
| Location (N = 93) | |
| Larynx | 45 (48.4%) |
| Oral cavity | 28 (30.1%) |
| Nasal cavity/paranasal sinuses | 9 (9.7%) |
| Oropharynx | 8 (8.6%) |
| Hypopharynx | 2 (2.1%) |
| Pharynx, NOS | 1 (1%) |
| Outcome (N = 93) | |
| Alive with disease | 13 |
| Alive with no evidence of disease | 27 |
| Died of disease | 34 |
| Died of other causes | 4 |
| Not reported | 15 |
| Local recurrence (N = 78) | 28 (35.9%) |
| Regional metastases (N = 78) | 37 (47.4%) |
| Distant metastases (N = 77) | 19 (24.7%) |
| Lung | 13 |
| Skin | 4 |
| Liver | 4 |
| Kidney | 2 |
| Bone | 1 |
| Brain | 1 |
| Bone marrow | 1 |
| Adrenal gland | 1 |
| Colon | 1 |
| Pericardium | 1 |
| Axillary lymph nodes | 1 |
Only five cases of ADSC of the oropharynx have been reported previously in the literature[26–28]. Four cases had minimal follow-up data (ranging from 2 to 78 months). Three patients were alive with disease and one died of other causes. Two patients had local recurrence, two had regional metastases, and two had distant metastases (lung and skin). No HPV specific or surrogate marker testing was performed on them.
Discussion
Adenosquamous carcinoma of the head and neck is a rare variant of squamous cell carcinoma. Little is known about its etiopathogenesis, mainly due to its rarity and lack of larger, controlled studies. However, there is nearly unanimous agreement among authors about its aggressive behavior and propensity for locoregional and distant metastases. A strong male predilection (M:F > 6:1) is observed, and the tumors occur over a broad age range (21–87 years). The larynx is the most common site (48.4%), followed by the oral cavity (30%). Most tumors are high stage at the time of presentation. In our series of 18 cases, 10 patients (55.6%) had local recurrence or nodal metastases and 4 (22.2%) developed distant metastases. In our combined review, 49 patients (52.7%) developed local recurrence or nodal metastases and 19 (20.4%) developed distant metastases [4, 24, 26–41]. Also, in a previous review of 58 cases of head and neck ADSC, Keelawat et al. reported 65% regional metastases, 23% distant metastases, and 43% of patients dying of disease [26]. The lung is the most common site for distant metastases. Thus, our current data and the overall literature support the notion that ADSC is more clinically aggressive than typical head and neck SCC, for which only 10–15% of patients develop distant metastases [42, 43], and which has a 5 years relative overall survival of approximately 50% [44].
Recent studies have demonstrated that head and neck SCC patients, particularly of the oropharynx, have a much better prognosis when their tumors are associated with transcriptionally-active HPV than when they are not [13]. High risk HPV types, particularly type 16, have been detected in as many as 80% of oropharyngeal SCC [11]. The virus contributes to carcinogenesis by expressing E6 and E7 oncoproteins, which bind to p53 and retinoblastoma (Rb), respectively. These latter proteins are critical for apoptosis and cell cycle regulation and, when disrupted, cause cells to proliferate and fail to undergo apoptosis appropriately. Inactivation of Rb, in particular, leads to marked overexpression of the tumor suppressor protein p16, for which transcription is normally repressed by Rb. This marked overexpression, which is very uncommon in non-HPV-related SCC, makes p16 a good surrogate marker of HPV, but more importantly, a good marker of transcriptionally-active HPV [18], because while HPV DNA can be found in a relatively high numbers of head and neck SCC of all anatomic subsites, the ones with better clinical outcomes have only been those where HPV is present in the tumor and in transcriptionally active form [12]. It is generally accepted that if one finds evidence of high risk HPV in a tumor that coexistent overexpression of p16 and/or significant expression of HPV E6 and E7 transcripts, this indicates biologically and clinically significant role of HPV in the tumor. Occasionally, p16 is overexpressed but no HPV can be detected. These tumors, in the oropharynx, still appear to have the same good prognosis, but their biology is still unknown. It is possible that in most such tumors, HPV is present but the tests don’t detect it. But one can also speculate that tumors can truly overexpress p16 in the absence of HPV. This is something yet to be clearly defined. For non-oropharyngeal SCC, the significance of p16 overexpression is not clear. It does not seem to be a good marker of prognosis or necessarily a good surrogate for HPV. We had a few non-oropharyngeal, p16 overexpressing ADSC cases and these did not harbor HPV. Although they appeared to do well clinically, the number of cases is too small to make any meaningful conclusions.
Several histologic variants of SCC, including nonkeratinizing SCC, basaloid SCC, papillary SCC, and undifferentiated carcinoma [7–10], have been shown to harbor high risk HPV when arising in the oropharynx. Papillary SCC, in particular, can have transcriptionally-active HPV in non-oropharyngeal locations such as the larynx [9]. Where clinical outcomes have been investigated in these variants, HPV-positive patients have had better survival. The relationship of head and neck ADSC to HPV has not been previously investigated. In the uterine cervix [24], as many as 75% of cases harbor HPV [45], and in the single series of lung cases, most harbored HPV as well [46].
In our series of 18 ADSC cases, we utilized a novel, slide-based high risk HPV RNA ISH assay along with the traditional p16 IHC and Ventana DNA ISH assays. This new RNA-based assay for transcriptionally-active HPV allows evaluation for viral E6 and E7 directly in tissue sections. This is considered by many to be the “gold standard” for biologically relevant tumor HPV. Only a small minority of ADSC harbored HPV (2 oropharynx, 1 nasal cavity, 2 larynx) with fewer still (2 oropharynx, 1 nasal cavity) harboring transcriptionally active HPV and showing overexpression of p16. As mentioned earlier, the oropharynx and the nasal cavity are sites with known high-prevalence of HPV-related carcinomas. We have added 18 cases of ADSC to the existing literature. Our cases had a similar prognosis to those in the literature review, supporting the notion that ADSC of the head and neck is more aggressive than typical squamous cell carcinoma. ADSC may be relatively even more aggressive if one excludes good prognosis, HPV-related oropharyngeal cases and evaluates the remaining group.
Two of the five HPV DNA ISH positive cases lacked both p16 overexpression and high risk HPV RNA expression. These are likely false positives due to high, nonspecific background blue staining on the DNA ISH masquerading as true staining. Or, alternatively, this may represent the presence of high risk HPV DNA which does not have enough transcriptional activity to demonstrate E6 and E7 expression above background. As is well known, high risk HPV DNA can be found in a large percentage of non-oropharyngeal SCC from PCR assays, but we know from functional assays that there isn’t evidence of transcriptional activity in most non-oropharyngeal SCC. These ADSC cases could simply represent this phenomenon.
It is generally accepted that the majority of HPV-related tumors harbor an unmutated wild type p53 protein. In contrast, we observed a high rate of p53 protein overexpression as evidenced by p53 immunostaining in the majority of our cases, including the HPV-related ones. Similar findings were also reported by Alos et al. in their series of 12 patients [30]. This observation suggests that p53 mutations may play an important role in tumorigenesis of ADSC. The four patients in our series with p53 negative tumors were alive without disease with follow up ranging from 3.5 to 123 months (cases 7, 8, 9, 13). Interestingly, however, all six patients with p16 and HPV negative tumors also showing overexpression of p53 suffered disease recurrence, with most dying with disease.
In conclusion, ADSC of the head and neck are relatively heterogeneous with regard to localization and HPV status. Most of the cases show p53 protein overexpression/mutation, as documented by immunostaining. Only a small minority of cases are HPV-related, and these occur in the oropharynx and nasal cavity, sites with a known high prevalence of HPV-related tumors. The HPV-related oropharyngeal cases, in particular, appear to do very well clinically, akin to other SCC variants in the oropharynx that are HPV-related. The remaining cohort of ADSC patients do quite poorly. While the numbers are small, our study suggests that ADSC of the head and neck may be a mixed variant that may not be uniformly associated with poor clinical outcome.
Acknowledgments
We would like to thank Jianping Li for her excellent work performing the p16 immunostaining for this project. We would also like to thank Bridgette Sims, RN, for helping us to gather clinical information on the patients.
References
- 1.Cardesa A, Zidar N, Alos L. Adenosquamous carcinoma. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. Lyon: IARC Press; 2005. pp. 130–131. [Google Scholar]
- 2.Slootweg PJ, Richardson M. Squamous cell carcinoma of the upper aerodigestive system. In: Gnepp DR, editor. Diagnostic surgical pathology of the head and neck. Philadelphia: Saunders Elsevier; 2009. pp. 88–91. [Google Scholar]
- 3.Damiani JM, Damiani KK, Hauck K, et al. Mucoepidermoid-adenosquamous carcinoma of the larynx and hypopharynx: a report of 21 cases and a review of the literature. Otolaryngol Head Neck Surg. 1981;89(2):235–243. doi: 10.1177/019459988108900218. [DOI] [PubMed] [Google Scholar]
- 4.Gerughty RM, Hennigar GR, Brown FM. Adenosquamous carcinoma of the nasal, oral and laryngeal cavities. A clinicopathologic survey of ten cases. Cancer. 1968;22(6):1140–1155. doi: 10.1002/1097-0142(196811)22:6<1140::AID-CNCR2820220610>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Chernock RD, El-Mofty SK, Parvin CA, Lewis JS, Jr, Chernock RD. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head and Neck Pathol. 2009;3(3):186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Mofty SK, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Chernock RD, Lewis JS, Jr, Zhang Q, et al. Human papillomavirus-positive basaloid squamous cell carcinomas of the upper aerodigestive tract: a distinct clinicopathologic and molecular subtype of basaloid squamous cell carcinoma. Hum Pathol. 2010;41(7):1016–1023. doi: 10.1016/j.humpath.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Begum S, Westra WH. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am J Surg Pathol. 2008;32(7):1044–1050. doi: 10.1097/PAS.0b013e31816380ec. [DOI] [PubMed] [Google Scholar]
- 9.Jo VY, Mills SE, Stoler MH, et al. Papillary squamous cell carcinoma of the head and neck: frequent association with human papillomavirus infection and invasive carcinoma. Am J Surg Pathol. 2009;33(11):1720–1724. doi: 10.1097/PAS.0b013e3181b6d8e6. [DOI] [PubMed] [Google Scholar]
- 10.Singhi AD, Stelow EB, Mills SE, et al. Lymphoepithelial-like carcinoma of the oropharynx: a morphologic variant of HPV-related head and neck carcinoma. Am J Surg Pathol. 2010;34(6):800–805. doi: 10.1097/PAS.0b013e3181d9ba21. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JS, Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2010;34(8):1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National cancer institute state of the science meeting, November 9–10, 2008, Washington, DC. Head and Neck. 2009;31(11):1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 13.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32(Suppl 1):S59–S66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28(27):4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15(22):6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 17.Shi W, Kato H, Perez-Ordonez B, et al. Comparative prognositc value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27(36):6213–21. [DOI] [PubMed]
- 18.Westra W. The changing face of head and neck cancer in the twenty-first Century: the impact of HPV on the epidemiology and pathology of oral cancer. Head and Neck Pathol. 2009;3:78–81. doi: 10.1007/s12105-009-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strati K, Pitot HC, Lambert PF. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc Natl Acad Sci USA. 2006;103(38):14152–14157. doi: 10.1073/pnas.0606698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer. 2008;122(12):2656–2664. doi: 10.1002/ijc.23458. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Koo BS, Kang S, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120(7):1418–1425. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 22.Klussmann JP, Gultekin E, Weissenborn SJ, et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162(3):747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Mofty SK, Lu DW. Prevalence of human papillomavirus type 16 DNA in squamous cell carcinoma of the palatine tonsil and not the oral cavity, in young patients: a distinct clinicopathologic and molecular disease entity. Am J Surg Pathol. 2003;27(11):1463–1470. doi: 10.1097/00000478-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida T, Sano T, Oyama T, et al. Prevalence, viral load, and physical status of HPV 16 and 18 in cervical adenosquamous carcinoma. Virchows Arch. 2009;455(3):253–259. doi: 10.1007/s00428-009-0823-x. [DOI] [PubMed] [Google Scholar]
- 25.Cruz I, Snijders PJ, Houten V, et al. Specific p53 immunostaining patterns are associated with smoking habits in patients with oral squamous cell carcinomas. J Clin Pathol. 2002;55(11):834–840. doi: 10.1136/jcp.55.11.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keelawat S, Liu CZ, Roehm PC, et al. Adenosquamous carcinoma of the upper aerodigestive tract: a clinicopathologic study of 12 cases and review of the literature. Am J Otolaryngol. 2002;23(3):160–168. doi: 10.1053/ajot.2002.123462. [DOI] [PubMed] [Google Scholar]
- 27.Sheahan P, Toner M, Timon CV. Clinicopathological features of head and neck adenosquamous carcinoma. ORL J Otorhinolaryngol Relat Spec. 2005;67(1):10–15. doi: 10.1159/000083008. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Madrigal F, Baden E, Casiraghi O, et al. Oral and pharyngeal adenosquamous carcinoma. A report of four cases with immunohistochemical studies. Eur Arch Otorhinolaryngol. 1991;248(5):255–258. doi: 10.1007/BF00176748. [DOI] [PubMed] [Google Scholar]
- 29.Abdelsayed RA, Sangueza OP, Newhouse RF, et al. Adenosquamous carcinoma: a case report with immunohistochemical evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(2):173–177. doi: 10.1016/S1079-2104(98)90422-X. [DOI] [PubMed] [Google Scholar]
- 30.Alos L, Castillo M, Nadal A, et al. Adenosquamous carcinoma of the head and neck: criteria for diagnosis in a study of 12 cases. Histopathology. 2004;44(6):570–579. doi: 10.1111/j.1365-2559.2004.01881.x. [DOI] [PubMed] [Google Scholar]
- 31.Ferlito A. A pathologic and clinical study of adenosquamous carcinoma of the larynx. Report of four cases and review of the literature. Acta Otorhinolaryngol Belg. 1976;30(4):379–389. [PubMed] [Google Scholar]
- 32.Fujino K, Ito J, Kanaji M, et al. Adenosquamous carcinoma of the larynx. Am J Otolaryngol. 1995;16(2):115–118. doi: 10.1016/0196-0709(95)90042-X. [DOI] [PubMed] [Google Scholar]
- 33.Izumi K, Nakajima T, Maeda T, et al. Adenosquamous carcinoma of the tongue: report of a case with histochemical, immunohistochemical, and ultrastructural study and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(2):178–184. doi: 10.1016/S1079-2104(98)90423-1. [DOI] [PubMed] [Google Scholar]
- 34.Aden KK, Niehans G, Adams GL, et al. Adenosquamous carcinoma of the larynx and hypopharynx with five new case presentations. Trans Am Laryngol Assoc. 1988;216–21.
- 35.Leung KL, Yang KY, Chen CJ. Adenosquamous carcinoma of the buccal mucosa: a case report. Taiwan J Oral Maxillofac Surg. 2009;20:19–28. [Google Scholar]
- 36.Minic AJ, Stajcic Z. Adenosquamous carcinoma of the inferior turbinate: a case report. J Oral Maxillofac Surg. 1994;52(7):764–767. doi: 10.1016/0278-2391(94)90497-9. [DOI] [PubMed] [Google Scholar]
- 37.Napier SS, Gormely JS, Newlands C, et al. Adenosquamous carcinoma. A rare neoplasm with an aggressive course. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(5):607–611. doi: 10.1016/S1079-2104(05)80103-9. [DOI] [PubMed] [Google Scholar]
- 38.Sanderson RJ, Rivron RP, Wallace WA. Adenosquamous carcinoma of the hypopharynx. J Laryngol Otol. 1991;105(8):678–680. doi: 10.1017/S0022215100117013. [DOI] [PubMed] [Google Scholar]
- 39.Scully C, Porter SR, Speight PM, et al. Adenosquamous carcinoma of the mouth: a rare variant of squamous cell carcinoma. Int J Oral Maxillofac Surg. 1999;28(2):125–128. doi: 10.1016/S0901-5027(99)80203-3. [DOI] [PubMed] [Google Scholar]
- 40.Sheahan P, Fitzgibbon J, Lee G, et al. Adenosquamous carcinoma of the tongue in a 22 years old female: report of a case with immunohistochemistry. Eur Arch Otorhinolaryngol. 2003;260(9):509–512. doi: 10.1007/s00405-003-0616-9. [DOI] [PubMed] [Google Scholar]
- 41.Siar CH, Ng KH. Adenosquamous carcinoma of the floor of the mouth and lower alveolus: a radiation-induced lesion? Oral Surg Oral Med Oral Pathol. 1987;63(2):216–220. doi: 10.1016/0030-4220(87)90315-X. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Di B, Shang Y, et al. Clinicopathologic risk factors for distant metastases from head and neck squamous cell carcinomas. Eur J Surg Oncol. 2009;35(12):1348–1353. doi: 10.1016/j.ejso.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Al-Othman MO, Morris CG, Hinerman RW, et al. Distant metastases after definitive radiotherapy for squamous cell carcinoma of the head and neck. Head Neck. 2003;25(8):629–633. doi: 10.1002/hed.10275. [DOI] [PubMed] [Google Scholar]
- 44.Skarsgard DP, Groome PA, Mackillop WJ, et al. Cancers of the upper aerodigestive tract in Ontario, Canada, and the United States. Cancer. 2000;88(7):1728–1738. doi: 10.1002/(SICI)1097-0142(20000401)88:7<1728::AID-CNCR29>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Ogura K, Ishi K, Matsumoto T, et al. Human papillomavirus localization in cervical adenocarcinoma and adenosquamous carcinoma using in situ polymerase chain reaction: review of the literature of human papillomavirus detection in these carcinomas. Pathol Int. 2006;56(6):301–308. doi: 10.1111/j.1440-1827.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- 46.Tsuhako K, Nakazato I, Hirayasu T, et al. Human papillomavirus DNA in adenosquamous carcinoma of the lung. J Clin Pathol. 1998;51(10):741–749. doi: 10.1136/jcp.51.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]