Abstract
Desmoplastic fibroblastoma is a rare, benign, soft tissue tumor affecting mainly the subcutaneous and muscle tissue. Only five cases identified in the oral cavity have been reported in prior literature. This article presents a case report of a 56-year-old man, with no previous history of trauma, who presented a slow-growing mass in the buccal mucosa. Histopathology and immunohistochemistry staining studies were performed, and a diagnosis of the desmoplastic fibroblastoma was made. The patient has been disease-free for one year.
Keywords: Collagenous fibroma, Desmoplastic fibroblastoma, Fibroma, Neoplasm of connective and soft tissue
Introduction
Desmoplastic fibroblastoma (DF), also known as collagenous fibroma, is a rare, benign, slow growing lesion [1–7], which was first described in 1995 by Evans [2]. DF occurs in the subcutaneous or intramuscular tissue, with a wide anatomic distribution, showing a strong predilection for men between the fifth and sixth decades of life [1, 3]. However, oral involvement is rarely reported (only five published cases) and has only been found in the hard palate, on the tongue, and in the alveolar bone [3, 7]. This article presents the first case of a DF identified in the buccal mucosa.
Case Report
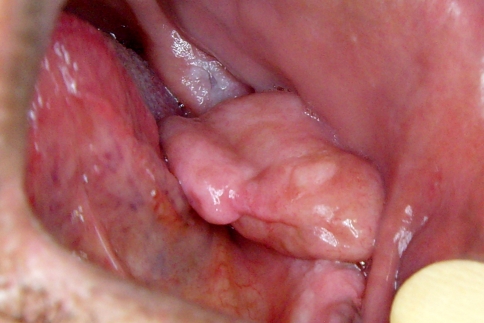
A 56-year-old man was referred to the Oral Medicine Clinic of the School of Dentistry at Universidade Federal de Minas Gerais to evaluate a mass in the left buccal mucosa. Extra-oral examination showed no abnormalities. Intra-oral inspection revealed a single, well-circumscribed, sessile, pink-colored tumor, with a firm consistency, covered by healthy mucosa, and measuring 35 mm in diameter (Fig. 1). The patient had noticed this painless lesion 3 years prior to the appointment, but it had recently increased in size, interfering in his chewing, thus causing an imprint on the surface of the lesion. He was edentulous and used no removable prosthesis. No traumatic event or habit could be associated with the lesion. His medical history included controlled hypertension; social and cultural histories were not contributory. Considering the main clinical hypothesis of a benign soft tissue tumor, a wide surgical excision, including the surrounding healthy tissue, was performed.
Fig. 1.
Clinical features of the desmoplastic fibroblastoma in the buccal mucosa. A single, well-circumscribed, sessile, pink-colored tumor measuring 35 mm. The lesion is covered by healthy mucosa and an imprint can be observed on its surface
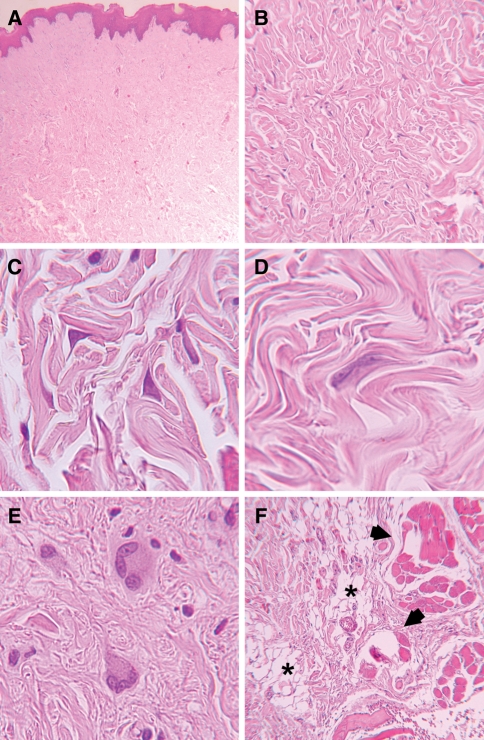
Histopathology revealed, at low power magnification, a well-defined, non-encapsulated, fibroblastic proliferation, characterized by paucicellularity within a highly collagenous matrix (Fig. 2a, b). At high power magnification, fibroblasts presented a spindle to stellate shape (Fig. 2c), with large and rounded nuclei exhibiting thin chromatin and small nucleoli. Binucleated (Fig. 2d) and multinucleated (Fig. 2e) fibroblasts were observed. Blood vessels were few and small, and neither mitosis nor necrosis was present. In the deep area of the specimen, lesional tissue trapped adipocytes and skeletal muscle fibers (Fig. 2f). Tumor fibroblasts showed intense immunopositivity for vimentin and some positive cells to Factor XIIIa. The cells were immunonegative for α-smooth muscle actin, desmin, S-100, AE1/AE3, CD34, and CD68.
Fig. 2.
Histopathology of the desmoplastic fibroblastoma. a Mucosal fragment covered by keratinized stratified squamous epithelium with lamina propria showing fibroblastic proliferation (Hematoxylin and eosin stain, original magnification ×25). b The lesion is characterized by paucicellularity within a highly collagenous matrix with scarce vascularity (Hematoxylin and eosin, original magnification ×100). The fibroblasts are c stellated; d binucleated; or e multinucleated (Hematoxylin and eosin, original magnification ×560). f Lesional tissue trapping adipocytes asterisks) and skeletal muscle fibers (arrows) (Hematoxylin and eosin, original magnification ×100)
The diagnosis was desmoplastic fibroblastoma. The patient is currently undergoing follow-up therapy and has been free of disease for one year.
Discussion
The current case presents clinical and histopathological features of a diagnosis of desmoplastic fibroblastoma. DF is a rare benign soft tissue tumor, frequently found in several locations, such as the arm and the posterior portion of the neck or upper back [1]. However, this tumor is especially rare in the mouth, with only five cases reported, all of which affected women [3–7]. Five key cases occurred: three in the palate [3–5], one on the tongue [7], and one in the alveolar bone [6]. Considering the reported cases [3–7], including the present study, oral DF shows a predilection for adults, with a mean age of 54 years. The current case is the first of its kind found in the buccal mucosa of a man. This therefore represents the sixth case in which this tumor has affected the oral cavity.
According to Evans [2], DF represents a neoplasm, given that no inciting event was clinically mentioned and no specific cause for a reactive fibrous proliferation could be microscopically identified. Furthermore, a cytogenetically abnormal clone, involving the 11q12 breakpoint, was detected in some cases, corroborating with the neoplastic nature of the DF [8]. In the current case, neither traumatic injury nor an inciting event could be identified or described by the patient. Clinically, oral DF appears as a painless, solitary, slow growing, well-circumscribed mass [3–7], with yellowish [7], reddish [6], or normally colored mucosa [3–5], with no precipitating trauma [3–7]. Once enlarged, it leads to difficulty in speaking, eating, and closing of the mouth [5]. The consistency is described as firm [3, 5, 6] to elastic [4], with a size greater than 1.0 cm [3–6]. The current case is in accordance with these clinical features.
Taking into consideration that this lesion presents of medium size and had grown slowly over a long period of time, it is possible to present some clinical differential diagnoses. Schwannoma, neurofibroma, myofibroma, and lipoma consist of smooth-surfaced, slow-growing, asymptomatic masses of variable size [9–12], representing the clinical feature of the current case. The peak of incidence in schwannoma commonly occurs between the third and sixth decades of life and tends to show a predilection for the palate [9]. Although oral neurofibroma can occur within any site, the most frequent location is the tongue. It also tends to affect patients at a mean of 31.2 years of age [10]. Oral myofibroma, which may also occur in adults, commonly affects infants [11]. Oral lipomas, which frequently appear with a yellowish tinge and a soft consistency, predominantly affect the buccal mucosa and are more common in patients between 40 and 60 years of age [12]. More remote possibilities include benign fibrous histiocytoma and granular cell tumor, which are relatively uncommon benign neoplasms [13, 14], as well as leiomyoma, which rarely affects the buccal mucosa [15]. The pleomorphic adenoma, one of the most common intraoral minor salivary gland tumor [16], should be included as a possible differential diagnosis as well.
Microscopically, DF is characterized by a paucicellular proliferation of spindle to stellate-shaped cells, embedded in a highly hyalinized collagenous stroma with or without focal fibromyxoid degeneration [4]. Binucleation [3, 6] and a few multinucleated giant cells can be observed in some reports [1, 3, 20–22], as was the case in the present study. However, future research concerning the exact origin of multinucleated giant cells is warranted. Other common histological findings include adjacent skeletal muscle or fat tissue entrapment [3] as well as an atypical finding of focal calcifications [7, 21, 26]. Oral DF with entrapment has been observed in three reported cases [3, 4, 7] as well as in the present study.
Oral DF must be histologically differentiated from other soft tissue lesions, mainly inflammatory fibrous hyperplasia (IFH), traumatic fibroma (TF), and giant cell fibroma (GCF). IFH and TF rarely exhibit binucleated or stellate-shaped cells, but do show a greater level of cellularity and contain associated inflammatory infiltrate. GCF also contains a higher level of cellularity, and multinucleated fibroblasts remain relatively invisible [27]. Additionally, oral DF commonly shows the entrapment of adipocytes and muscle fibers [3, 4]. Mesquita et al. [3] demonstrated that collagen distribution may also aid in distinguishing these entities. Another histological differential diagnosis is the solitary fibrous tumor, which is a spindle-cell lesion characterized by a variety of growth patterns. Areas of extensive sclerosis or hyalinization, similar to that of oral DF, can be seen in solitary fibrous tumor however, hypercellular areas can only be found in solitary fibrous tumor [20]. Juvenile hyaline fibromatosis consists predominantly of spindle-ovoid-shaped, fibroblast-like cells, but it does contain a deposition of amorphous hyaline material, which represents a key diagnostic criterion [17]. Providing a microscopic differential diagnosis of fibromatosis is quite difficult, due to the histological similarity between fibromatosis and DF. However, fibromatosis is consistently and diffusely more cellular than is DF [18]. Distinguishing DF from fibromatosis is important, as the latter commonly presents an aggressive clinical behavior [18].
The immunohistochemistry profiles of DF are demonstrated in Table 1. DF is strong and diffusely positive to vimentin [1, 3, 5–7, 19–25] and preferably negative to S-100 protein [1, 3, 5–7, 20, 22–26], CD34 [1, 3, 5, 7, 19–26], desmin [1, 3, 19, 22–26], cytokeratins [1, 19, 21, 24, 25], epithelial membrane antigen [21, 23–25], and CD68 [1, 3, 20, 23]. The majority of studies containing α-smooth muscle actin have shown that lesional cells are focally positive [3, 5, 6, 19–21, 23, 24]. However, as occurred in the present research, other studies have also reported negative α-smooth muscle actin [1, 22, 25, 26]. Factor XIIIa proved to be positive in only a few selected cells [3, 6, 26], while negative staining could be observed in one study [1]. The immunohistochemistry profiles of DF in most of these reports reveal that the tumor cells are predominantly fibroblastic, although focal myofibroblastic differentiation may also be well-developed, as illustrated by its immunopositivity to α-smooth muscle actin and to HHF-35 [19, 23]. Moreover, concerning this wide range of immunoprofiles, it is important to emphasize that immunohistichemistry is not mandatory in DF diagnoses, but it can aid in the exclusion of other diagnoses. Hence, lesions with negative S-100, CD34, or EMA/cytokeratins proteins, neural, vascular, or epithelial origins, are ruled out.
Table 1.
Immunohistochemistry profiles of desmoplastic fibroblastoma
| Vimentin | α-smooth muscle actin | HHF-35 | Desmin | Factor XIIIa | S-100 | CD34 | Cytokeratins | Epithelial membrane antigen | CD68 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Watanabe et al. [1] n = 1 | + | − | NP | − | − | − | − | − | NP | − |
| Mesquita et al. [3] n = 1 | ++ | + | NP | − | + | − | − | NP | NP | − |
| Simoyama et al. [5] n = 1 | ++ | + | NP | NP | NP | − | − | NP | NP | NP |
| Cazal et al. [6] n = 1 | ++ | + | NP | NP | + | − | NP | NP | NP | NP |
| Nonaka et al. [7] n = 1 | ++ | ++ | NP | NP | NP | − | − | NP | NP | NP |
| Nielsen et al. [19] n = 6 | ++ (6/6) |
++ (1/6) + (2/6) |
++ (1/6) + (1/6) |
− (6/6) |
NP | + (2/6) |
− (6/6) |
− (6/6) |
NP | NP |
| Ide et al. [20] n = 1 | ++ | + | NP | NP | NP | − | − | NP | NP | − |
| Hasegawa et al. [21] n = 4 | ++ (4/4) |
+ (1/4) |
− (4/4) |
+ (1/4) |
NP | − (4/4) |
− (4/4) |
− (4/4) |
− (4/4) |
NP |
| Jang et al. [22] n = 1 | ++ | − | NP | − | NP | − | NR | NP | NP | NP |
| Miettinen et al. [23] n = 26 | ++ (*/26) |
+ (*/26) |
+ (*/26) |
− (26/26) |
NP | − (26/26) |
− (26/26) |
+ (10% of cases) |
− (26/26) |
− (26/26) |
| Huang et al. [24] n = 3 | ++ (3/3) |
+ (2/3) |
− (3/3) |
− (3/3) |
NP | − (3/3) |
− (3/3) |
− (3/3) |
− (3/3) |
NP |
| Dagli et al. [25] n = 1 | ++ | − | NP | − | NP | − | − | − | − | NP |
| Weisberg et al. [26] n = 1 | NP | − | − | − | + | − | − | NP | NP | NP |
| Current case n = 1 | ++ | − | NP | − | + | − | − | − | NP | − |
++, Diffuse positivity; +, Positivity in some cells; −, Negative; NP, Not performed
* The authors did not describe the number of positive cases
Treatment of DF is performed by complete excision and, although to date neither recurrences nor metastasis have been reported [1–7, 19–26], it is important to know the characteristics of this rare condition in an attempt to prove differential diagnoses when compared to other oral lesions.
Acknowledgments
This study was supported by grants from the National Council for Scientific and Technological Development (CNPq) (# 301490/2007-4). Mesquita RA and Aguiar MCF are research fellows of CNPq.
References
- 1.Watanabe H, Ishida Y, Nagashima K, et al. Desmoplastic fibroblastoma (collagenous fibroma) J Dermatol. 2008;35:93–97. doi: 10.1111/j.1346-8138.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- 2.Evans HL. Desmoplastic fibroblastoma. A report of seven cases. Am J Surg Pathol. 1995;19:1077–1081. doi: 10.1097/00000478-199509000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Mesquita RA, Okuda E, Jorge WA, et al. Collagenous fibroma (desmoplastic fibroblastoma) of the palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:80–84. doi: 10.1067/moe.2001.109638. [DOI] [PubMed] [Google Scholar]
- 4.González-Moles MA, Ruiz-Avila I, Gil-Montoya JA. Collagenous fibroma (desmoplastic fibroblastoma) of the palate associated with Marfan’s syndrome. Oral Oncol Extra. 2004;40:39–42. doi: 10.1016/j.ooe.2003.12.004. [DOI] [Google Scholar]
- 5.Shimoyama T, Horie N, Ide F. Collagenous fibroma (desmoplastic fibroblastoma): a new case originating in the palate. Dentomaxillofac Radiol. 2005;34:117–119. doi: 10.1259/dmfr/22428083. [DOI] [PubMed] [Google Scholar]
- 6.Cazal C, Etges A, Almeida FCS, et al. Collagenous fibroma (desmoplastic fibroblastoma) of alveolar bone: a case report. J Bras Patol Med Lab. 2005;41:185–188. doi: 10.1590/S1676-24442005000300008. [DOI] [Google Scholar]
- 7.Nonaka CFW, Carvalho MV, Moraes M, et al. Desmoplastic fibroblastoma (collagenous fibroma) of the tongue. J Cutan Pathol. 2010;37:911–914. doi: 10.1111/j.1600-0560.2009.01467.x. [DOI] [PubMed] [Google Scholar]
- 8.Dernal K, Nelson M, Neff JR, et al. Translocation (2;11)(q31;q12) is recurrent in collagenous fibroma (desmoplastic fibroblastoma) Cancer Genet Cytogenet. 2004;149:161–163. doi: 10.1016/S0165-4608(03)00298-X. [DOI] [PubMed] [Google Scholar]
- 9.López-Jornet P, Bermejo-Fenoll A. Neurilemmoma of the tongue. Oral Oncol Extra. 2005;41:154–157. doi: 10.1016/j.ooe.2005.03.007. [DOI] [Google Scholar]
- 10.Depprich R, Singh DD, Reinecke P, et al. Solitary submucous neurofibroma of the mandible: review of the literature and report of a case. Head Face Med. 2009;24:31–34. doi: 10.1186/1746-160X-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vered M, Allon I, Bchner A, et al. Clinico-pathologic correlations of myofibroblastic tumors of the oral cavity.II. Myofibroma and myofibromatosis of the oral soft tissues. J Oral Pathol Med. 2007;36:304–314. doi: 10.1111/j.1600-0714.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 12.Furlong MA, Fanburg-Smith JC, Childers ELB. Lipoma of the oral and maxillofacial region: site and subclassification of 125 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:441–450. doi: 10.1016/j.tripleo.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 13.Alves FA, Vargas PA, Siqueira SAC, et al. Benign fibrous histiocytoma of the buccal mucosa: case report with immunohistochemical features. J Oral Maxillofac Surg. 2003;61:269–271. doi: 10.1053/joms.2003.50026. [DOI] [PubMed] [Google Scholar]
- 14.Bomfin LE, Alves FA, Almeida OP, et al. Multiple granular cell tumors of the tongue and parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:10–11. doi: 10.1016/j.tripleo.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Natiella JR, Neiders ME, Greene GW. Oral leyomioma. J Oral Pathol. 1982;11:353–365. doi: 10.1111/j.1600-0714.1982.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 16.Pires FR, Pringle GA, Almeida OP, et al. Intra-oral minor salivary gland tumors: a clinicopathological study of 546 cases. Oral Oncol. 2007;43:463–470. doi: 10.1016/j.oraloncology.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Altug HA, Günal A, Günham O, Sencimen M. Juvenile hyaline fibromatosis of the mandible with bone involvement: report of a rare case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:59–63. doi: 10.1016/j.tripleo.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Fowler CB, Hartman KS, Brannon RB, et al. Fibromatosis of the oral and paraoral regions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1994;77:373–386. doi: 10.1016/0030-4220(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen GP, O’Connel JX, Dickersin GR, et al. Collagenous fibroma (desmoplastic fibroblastoma): a report of seven cases. Mod Pathol. 1996;44:1945–1954. [PubMed] [Google Scholar]
- 20.Ide F, Shimoyama T, Horie N, et al. Collagenous fibroma (desmoplastic fibroblastoma) presenting as a parotid mass. J Oral Pathol MedS. 1999;28:465–468. doi: 10.1111/j.1600-0714.1999.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa T, Shimoda T, Hirohashi S, et al. Collagenous fibroma (desmoplastic fibroblastoma): report of four cases and review of the literature. Arch Pathol Lab Med. 1998;122:455–460. [PubMed] [Google Scholar]
- 22.Jang JG, Jung HH, Suh KS, et al. Desmoplastic fibroblastoma (collagenous fibroma) Am J Dermatopathol. 1999;21:256–258. doi: 10.1097/00000372-199906000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Miettinen M, Fetsch JF. Collagenous fibroma (desmoplastic fibroblastoma): a clinicopathologic analysis of 63 cases of a distinctive soft tissue lesion with stellate-shaped fibroblasts. Human Pathol. 1998;29:676–682. doi: 10.1016/S0046-8177(98)90275-1. [DOI] [PubMed] [Google Scholar]
- 24.Huang HY, Sung MT, Eng HL, et al. Superficial collagenous fibroma. APMIS. 2002;110:283–289. doi: 10.1034/j.1600-0463.2002.100402.x. [DOI] [PubMed] [Google Scholar]
- 25.Dagly M, Eryilmaz A, Acar A, et al. Collagenous fibroma (desmoplastic fibroblastoma) Yonsei Med J. 2004;45:941–943. doi: 10.3349/ymj.2004.45.5.941. [DOI] [PubMed] [Google Scholar]
- 26.Weisberg NK, Dicaudo DJ, Meland NB. Collagenous fibroma (desmoplastic fibroblastoma) J Am Acad Dermatol. 1999;41:292–294. doi: 10.1016/S0190-9622(99)70367-1. [DOI] [PubMed] [Google Scholar]
- 27.Neville B, Damm DD, Allen CM, Bouquot J, editors. Oral and maxillofacial pathology. Philadelphia, USA: Elsevier; 2009. [Google Scholar]